Abstract
Receptor-interacting protein (RIP) has been implicated in the induction of death receptor-mediated, nonapoptotic cell death. However, the mechanisms remain to be elucidated. Here we show that tumor necrosis factor alpha induced RIP-dependent inhibition of adenine nucleotide translocase (ANT)-conducted transport of ADP into mitochondria, which resulted in reduced ATP and necrotic cell death. The inhibition of ADP/ATP exchange coincided with the loss of interaction between ANT and cyclophilin D and the inability of ANT to adopt the cytosolic conformational state, which prevented cytochrome c release. Neither overexpression of Bcl-xL nor inhibition of reactive oxygen species prevented necrosis. In contrast, the ectopic expression of ANT or cyclophilin D was effective at preventing cell death. These observations demonstrate a novel mechanism initiated through death receptor ligation and mediated by RIP that results in the suppression of ANT activity and necrosis.
Receptor Interacting Protein 1 (RIP) is essential for both tumor necrosis factor alpha (TNF-α)- and Fas-mediated NF-κB activation (19, 21), which may protect against apoptotic cell death by the expression of survival genes such as FLIP (36). However, in certain cell types, when caspase activation is prevented, even when NF-κB activation is not suppressed, the ligation of TNFR1 or Fas results in necrotic cell death by a mechanism that involves RIP (16, 25). Although reactive oxygen species (ROS) have been implicated (25), the mechanism(s) by which RIP may mediate necrotic cell death remains to be elucidated. Characterizing the mechanisms which regulate this process is important, since necrosis is observed in a variety of pathogenic infections, ischemia, and inflammatory conditions (38) and since caspase inhibition resulted in TNF-α-induced shock (6). This understanding is particularly important for monocytic cells and macrophages, which express both Fas and Fas ligand (36) and produce abundant TNF-α.
Modulation of the life-supporting functions of mitochondria, such as maintaining internal homeostasis and ATP synthesis, may be involved in regulation of caspase-dependent and caspase-independent cell death (13, 17, 35). Mitochondrial synthesis of ATP requires ADP transport from cytosol into mitochondria by the inner mitochondrial membrane ADP/ATP carrier adenine nucleotide translocase (ANT) (11). ADP/ATP exchange depends on transition between two conformational states of ANT. In the cytosolic state (c-state) the hydrophilic loop of the ANT nucleotide binding site faces the cytosol, while in the m-state, this binding site faces the matrix (11, 30). Additionally, ANT function may depend on other mitochondrial molecules, including VDAC, hexokinase, and cyclophilin D (47). The interaction of ANT with cyclophilin D and VDAC is important in regulating mitochondrial permeability transition pore (MPTP). Although recent studies have demonstrated that ANT may not be essential for MPTP formation (20), cyclophilin D-deficient mice provide strong evidence that cyclophilin D plays a critical role not only in MPTP formation but also in the induction of necrotic cell death (2, 34). Characterizing the mechanism(s) regulating ANT function and the role of the interaction between ANT and cyclophilin D may be important not only in the elucidation of pathways contributing to necrosis but also in understanding the pathogenesis of various diseases such as breast, ovarian, and uterine cancers (40), viral cardiomyopathy (41), and certain myopathies (12) which may result from the altered expression or function of either ANT or cyclophilin D.
Here we demonstrate that a death receptor-initiated pathway, mediated through RIP, disrupted the interaction between cyclophilin D and ANT and permitted the binding of zVAD.fmk (zVAD) to ANT. Cys56 was critically important in the binding of zVAD to ANT. This interaction prevented ANT from adopting the conformational c-state, which resulted in inhibition of ADP/ATP exchange, the reduction of cellular ATP, and necrotic cell death.
MATERIALS AND METHODS
Materials.
Caspase inhibitors zVAD and biotinylated-VAD.fmk (biotinylated-VAD) were obtained from Enzyme Systems Products (Aurora, OH). Fluorescein isothiocyanate (FITC)-VAD.fmk was obtained from Promega, Madison, WI. Atractyloside and geldanamycin (GA) were from Calbiochem (San Diego, CA), and Lipofectamine and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA). Propidium iodide (PI) was from Roche Molecular Biochemicals (Indianapolis, IN), and rhodamine 123 (Rh123), tetramethylrhodamine methyl ester (TMRM), 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA), and Mitotracker Red were purchased from Molecular Probes (Eugene, OR).
Cell culture.
Human myelomonocytic U937 cells and THP-1 cells as well as human embryonic kidney 293T cells were obtained from ATCC (Rockville, MD). U937 cells stably overexpressing Bcl-xL or vector control cells were kindly provided by Vincent Cryns, Northwestern University Feinberg School of Medicine. U937 and THP-1 cells were maintained in complete RPMI medium containing 10% heat-inactivated fetal bovine serum, l-glutamine (2 mM), and a 1% penicillin-streptomycin solution. Prior to initiation of the experiments, cells were incubated overnight in serum-free medium and then resuspended in RPMI medium containing 0.5% fetal bovine serum. In some assays, antioxidants were added 60 to 90 min prior to the addition of TNF-α/zVAD. THP-1 cells were treated with ascorbic acid (2 mM), glutathione (9 mM), or N-acetyl-cysteine (5 mM), and then TNF-α/zVAD or control medium was added for an additional 12 to 24 h, as indicated, prior to examination of viability, ROS, and ATP. For some experiments, in vitro-differentiated human macrophages were prepared as described previously (27, 36). In some experiments, to reduce the level of RIP, THP-1 cells were incubated with GA (0.5 μg/ml) for 14 h prior to initiation of the described experiment.
Cell transfection.
In vitro-differentiated macrophages or THP-1 cells were transfected with either nonspecific (NS) or RIP interfering RNA (RNAi) or with cyclophilin D RNAi (Dharmacon, Inc, Lafayette, CO) (final concentration, 100 nM) by use of Lipofectamine 2000 according to the manufacturer's directions (Invitrogen). The cells were then incubated for 96 h prior to immunoblot analysis or PI exclusion assay. For transient transfection of 293T cells, 2 × 106 cells were washed and resuspended in Opti-MEM (Gibco, Gaithersburg, MD) and then transfected for 6 h by use of Lipofectamine 2000 (1:2.5 ratio of DNA/Lipofectamine 2000). Plasmids expressing human Myc-ANT1 (4) (kindly provided by J. F. Peyron, Faculte de Medecine Pasteur, France) or Myc-cyclophilin D (30) (1.5 μg) and a GAPDH-containing vector were employed to maintain the total plasmid amount at 3 μg of DNA per transfection. After transfection, the cells were incubated for 30 h prior to use.
Viability assays.
PI exclusion was employed to assess cell death by flow cytometry (27, 36). Alternatively, viability assays were performed to determine the protective effect of ANT and cyclophilin D by use of the murine macrophage cell line RAW264.7. For transient transfection, the cells (2 × 106) were washed and resuspended in Opti-MEM (Gibco, Gaithersburg, MD) and then transfected for 6 h employing Lipofectamine (1:3 ratio of DNA/Lipofectamine). Plasmids expressing human ANT1 or cyclophilin D (0.4 to 1.6 μg) plus enhanced green fluorescence protein (EGFP) (1 μg) were employed, and a GAPDH-containing vector was used to maintain the total plasmid amount at 4 μg of DNA per transfection. After transfection, cultures were washed, incubated for 24 h, and then treated with zVAD and TNF-α for an additional 24 h; the results are reported as the numbers of EGFP-positive cells per 1.5 × 105 cells.
Determination of subdiploid DNA content.
At the indicated time points, cultures were harvested in 0.02% EDTA and fixed in 70% ethyl alcohol overnight. Cells were then stained with PI as described previously (36). Apoptosis was determined by DNA fragmentation (<2 N DNA).
Electron microscopy analysis.
U937 cells cultured in the presence or absence of TNF-α and zVAD were harvested, and cell pellets were fixed overnight at 4°C in a 0.2 M sodium cacodylate buffer (pH 7.4) containing 2% glutaraldehyde. Samples were then postfixed in cacodylate-buffered 1% osmium tetroxide, dehydrated, embedded in Epon 812 (Nacalai Tesque, Osaka, Japan) for ultrathin sectioning, and then stained with uranyl acetate and lead citrate and examined with a JEOL 1220 electron microscope.
Confocal microscopy.
THP-1 cells were treated with TNF-α (10 ng/ml) for 8 h and then incubated with Mitotracker Red (50 nM) in the presence or absence of VAD-FITC-fmk (Promega, Madison, WI) (10 μM) for 30 min. Cells were harvested onto slides employing a cytocentrifuge and then fixed in 2% formaldehyde in phosphate-buffered saline. For determination of intracellular RIP localization, THP-1 cells were incubated with mouse anti-RIP followed by FITC-labeled anti-mouse heavy and light chains. Images were taken with a Zeiss LSM 510 confocal microscope using a 100×-objective lens.
Analysis of mitochondrial function.
Mitochondrial transmembrane potential (ΔΨm) was assessed utilizing cationic lipophilic orange fluorochrome TMRM (150 nM) (5). Briefly, monocytic cells (1 × 106) were incubated with TMRM for 25 min at 37°C. Then, cells were subjected to flow cytometry without prior washing. Alternatively, ΔΨm was measured by green fluorochrome Rh123, as described previously (27, 36).
Reactive oxygen species were determined by assessing H2O2 levels employing DCFDA (37). To assess H2O2 levels, cells were incubated for 15 min at 37°C with DCFDA (1 μM); green fluorescence (excitation at 525 nm) was assessed by flow cytometry.
ATP was detected employing a bioluminescent somatic cell assay kit according to the manufacturer's directions (Sigma). Following treatment, cells were washed in phosphate-buffered saline, lysed in 0.5% Triton X-100-10 mM Tris-HCl (pH 7.5)-1 mM EDTA, and incubated for 10 min on ice. After removal of cell debris by centrifugation (10,000 × g, 15 min, 4°C), the ATP content was measured employing the luciferin method in a ML2200 luminometer (Dynatech, Denkendorf, Germany). The absolute values of ATP content were determined using an internal ATP standard.
Mitochondrial and cytosol-containing fractions were isolated from THP-1 cells in a buffer containing HEPES (20 mM) (pH 7.5), KCl (10 mM), MgCl2 (1.5 mM), EDTA (1 mM), EGTA (1 mM), and dithiothreitol (1 mM) as described previously (20). Briefly, cell membranes were disrupted by rapid (10-s) homogenization employing a pellet pestle (Kontes Corp., Vineland, NJ). The nuclei and cell membranes were removed by centrifugation at 670 × g for 5 min at 4°C. Mitochondria were pelleted from the resulting cytosolic supernatants by centrifugation at 10,000 × g (20 min, 4°C). The mitochondrial and cytosolic fractions were employed as described below.
Adenine nucleotide transport activity was assessed by measuring the transport of ADP into mitochondria, as described previously (33). Briefly, following treatment of THP-1 cells with TNF-α or control medium, mitochondria were isolated and incubated in 10 mM Tris-MOPS (morpholinepropanesulfonic acid) (pH 7.4)-250 mM sucrose-5 mM succinate-10 μM EGTA plus zVAD (400 μM) or dimethyl sulfoxide (DMSO) control for 15 min at 37°C. After the addition of 20 μM [14C]ADP (Sigma) (185 kBq/μmol), the mitochondria were incubated for an additional 20 s at 0°C. The reaction was terminated by the addition of 100 μM atractyloside. The mitochondria were washed and then solubilized in 0.2 ml of 1% sodium dodecyl sulfate, and the radioactivity of the lysate was determined by liquid scintillation counting (Beckman LS3801 liquid scintillation counter; Beckman Coulter, Inc., Fullerton, CA).
Cytochrome c release from isolated mitochondria in response to atractyloside was employed to examine the transition of ANT from the conformational m- to c-state (11). Mitochondrial fractions from THP-1 cells, treated with TNF-α or control medium for 8 h, were incubated with zVAD or DMSO for 15 min, prior to the addition of atractyloside (2 mM) for 40 min at 30°C. Following centrifugation, the supernatants were analyzed for cytochrome c release by immunoblot analysis, and the pellets were examined for COX IV (BD PharMingen, San Diego, CA) as the control for input of mitochondria.
Immunoblot analysis and coprecipitation analysis.
Whole-cell extracts were prepared from THP-1 or U937, and polyacrylamide gel electrophoresis was performed as described previously (27, 29, 36). The antibodies used were mouse monoclonal anti-cytochrome c and Cox IV (BD PharMingen, San Diego, CA), mouse monoclonal anti-RIP (Clontech), monoclonal antiporin (VDAC; Molecular Probes), goat polyclonal anti-ANT (Santa Cruz), and rabbit polyclonal anti-cyclophilin D, anti-Bcl-xL, rabbit anti-β-actin, and mouse antitubulin (Calbiochem). The secondary antibodies employed were donkey anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, N.J.).
Coprecipitation assays were performed as described previously (29). Briefly, lysates containing 1 mg of protein were precleared with 50 μl of protein G-agarose or streptavidin-agarose (Calbiochem) at 4°C for 1 h. Supernatants were collected and further incubated with either an anti-ANT antibody (5 μg) or biotinylated VAD (7 μg) for 1 h at 4°C or for 15 min at 37°C, respectively, followed by the addition of 50 μl protein G-agarose or streptavidin-agarose and were incubated for 1 h at 4°C. After washing, the precipitates were examined by immunoblot analysis, and probed with antibodies to cyclophilin D, ANT, RIP, caspase 8, and cytochrome c.
Mutagenesis.
Mutant ANT was generated by site-directed mutagenesis of ANT, in which cysteine56 residues had been replaced by alanine residues (15). The expression vector construct was confirmed by DNA sequencing.
Statistical analysis.
Results were expressed as the mean ± 1 standard error (SE). Significance was determined by Student t test for paired samples.
RESULTS
Induction of necrotic cell death.
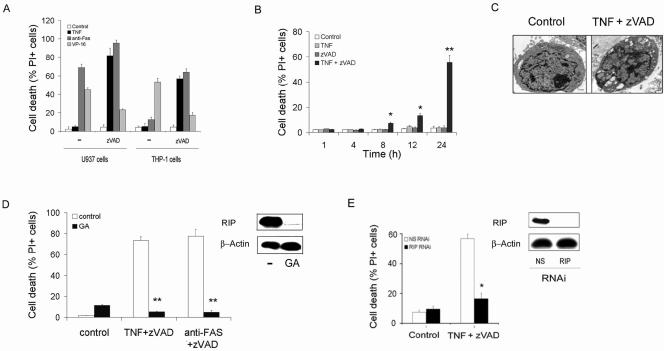
To characterize the mechanism(s) contributing to the induction of necrosis following TNFR or Fas ligation, we employed myelomonocytic U937 and THP-1 cells, since they differ in their resistance to Fas-induced cell death (Fig. 1A). In contrast to L929 murine fibrosarcoma cell results (43), TNF-α alone did not induce cell death in either of the cell lines (Fig. 1A). Further, etoposide (VP-16) induced cell death in both cell lines (Fig. 1A). As expected, the addition of zVAD, a broad-specificity caspase inhibitor, protected both THP-1 and U937 cells from VP-16-induced cell death. However, zVAD not only failed to protect U937 cells but also sensitized THP-1 cells to agonistic anti-Fas antibody-mediated cell death (Fig. 1A and B). Additionally, zVAD sensitized both cell types to TNF-α-induced cell death, starting at 8 h (Fig. 1A and B), in a concentration-dependent manner (data not shown). In contrast to treatment with VP-16, which resulted in DNA fragmentation, treatment with zVAD plus TNF-α resulted in no evidence of apoptosis, as determined by DNA fragmentation (data not shown), and the morphological features of necrosis were present (Fig. 1C). HeLa cells and 293T cells were resistant to TNF-α/zVAD-induced cell death, suggesting that the results were not due to nonspecific toxicity. These observations demonstrate that necrotic cell death is induced in myelomonocytic cells by TNF-α or Fas ligation in the presence of zVAD.
FIG. 1.
RIP is required for necrotic cell death. (A to C) The induction of necrotic cell death. (A) Cell death of U937 and THP-1 cells. Cells were cultured for 24 h with TNF-α (10 ng/ml), anti-Fas (1 μg/ml), VP-16 (40 μM), or control medium in the presence or absence of zVAD (100 μM). Loss of viability was determined by a PI exclusion test. (B) Time course for TNF-α/zVAD-induced death of THP-1 cells. TNF-α was employed at 10 ng/ml and zVAD at 100 μM. (C) TNF-α/zVAD induces necrosis. U937 cells cultured in control medium (left panel) or TNF-α/zVAD (right panel) were examined by electron microscopy to identify the features of necrosis: membrane rupture, vacuolization of organelles, intact nucleus. Scale bar, 1 μm. (D and E) TNF-α/zVAD-induced cell death is prevented by the reduction of RIP. (D) Reduction of RIP with GA (0.5 μg/ml) protects against TNF-α/zVAD- or anti-Fas-induced cell death. (E) RIP suppression protects macrophages form TNF-α/zVAD-induced cell death. In vitro-differentiated human macrophages were transfected with NS or RIP RNAi for 96 h. Cell lysates were examined by immunoblot analysis for RIP (right panels). The cells were treated with TNF-α/zVAD and cell death defined by inability to exclude PI. The values in panels A, B, D, and E are the means ± 1 SE of three or four experiments, each performed in duplicate. **, P < 0.001; *, P < 0.05 (compared to medium alone).
The effects of zVAD were not entirely specific, since zDEVD.fmk, another broad-specificity caspase inhibitor, sensitized THP-1 cells to TNF-α-induced cell death (data not shown). In contrast, caspase inhibitors with more-restricted specificity, such as the caspase 1 and 2 inhibitors, zYVAD.fmk, and zVDVAD.fmk, did not have any effect on THP-1 viability in the presence or absence of TNF-α. However, use of the broad caspase inhibitor-containing CHO group in place of the fluoromethyl ketone (fmk), cpm-VAD-CHO, failed to induce cell death in the presence of TNF-α (data not shown). These observations suggest that both broad specificity and the fmk groups were necessary to sensitize to cell death.
RIP is required for necrosis.
RIP may be crucial for some forms of Fas- and TNF-α-induced, caspase-independent cell death, including necrosis and autophagy (16, 25, 44, 50). To examine the role of RIP, geldanamycin, which disrupts the interaction between HSP90 and RIP, resulting in RIP degradation (16, 23), was employed. With THP-1 cells, geldanamycin resulted in a decrease of RIP but not β-actin (Fig. 1D, panel insert) or ANT (data not shown). THP-1 cells treated with geldanamycin became resistant to both TNF-α/zVAD- and anti-Fas/zVAD-induced cell death (Fig. 1D). In addition, RIP RNAi reduced the expression of RIP (Fig. 1E, panel insert) and TNF-α/zVAD-induced cell death of in vitro-differentiated macrophages (Fig. 1E) and THP-1 cells (data not shown). These findings demonstrate that TNF-α- and anti-Fas-induced necrosis of monocytic cells is controlled by RIP, consistent with observations of other cell types (16, 44, 50).
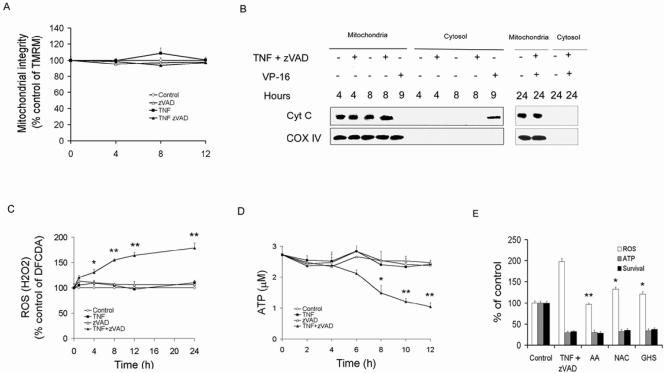
Mitochondrial outer membrane permeabilization (MOMP) and loss of ΔΨm are not essential for necrosis.
Maintaining mitochondrial integrity is essential for cell survival. Opening of the MPTP may result in dissipation of mitochondrial transmembrane potential (ΔΨm) and swelling of the inner matrix, resulting in MOMP and release of proapoptotic proteins such as cytochrome c (13). MOMP may also be induced by proapoptotic Bcl-2 family members, through action on the outer membrane, prior to the loss of ΔΨm. Therefore, the effects of TNF-α/zVAD on ΔΨm and MOMP were examined. Analysis of the gated cell population (high side-scatter population) revealed that unlike VP-16, which induced depolarization by 4 h (data not shown), TNF-α/zVAD did not result in a loss of ΔΨm at 4, 8, and 12 h (Fig. 2A). Notably, analysis of the entire cell population revealed that TNF-α/zVAD-induced loss of ΔΨm was due to the loss of viability, which progressively increased through 24 h (data not shown). Therefore, loss of ΔΨm was not detectable prior to TNF-α/zVAD-induced cell death, as was observed with VP-16-induced cell death (data not shown). To examine the effect of TNF-α/zVAD on MOMP, cytochrome c release was examined. In contrast to the effect of VP-16, treatment of U937 cells with TNF-α/zVAD failed to induce release of cytochrome c from the mitochondria (Fig. 2B). Additionally, in U937 cells, ectopically expressed Bcl-xL, which may protect against Bax/Bak-induced MOMP (7), completely abrogated VP-16-induced cell death but failed to protect against TNF-α/zVAD (see Fig. S1 in the supplemental material). These observations suggest that neither MOMP nor the loss of ΔΨm was responsible for the cell death induced by TNF-α/zVAD.
FIG. 2.
Role of mitochondria in TNF-α/zVAD-induced necrosis. (A, B) Neither mitochondrial outer membrane permeabilization nor loss of ΔΨm is involved. (A) THP-1 cells were treated with zVAD, TNF-α, or control medium, and ΔΨm was determined by TMRM retention. (B) Cytochrome c is not released from mitochondria by TNF-α/zVAD. Mitochondrial and cytosolic fractions were isolated from THP-1 cells treated with TNF-α/zVAD or VP-16 for the times indicated. The mitochondrial fractions were assessed for cytochrome c and COX IV by immunoblot analysis. (C to E) Increased ROS production and ATP depletion in necrosis. (C) ROS/H2O2 induction by ΤNF-α/zVAD. THP-1 cells were treated as described for Fig. 1 and examined for ROS/H2O2 employing DCFDA as described in Materials and Methods. (D) ΤNF-α/zVAD results in ATP depletion. THP-1 cells were treated as described for panel A, and ATP levels were determined as described in Materials and Methods. (E) Antioxidants do not prevent TNF-α/zVAD-induced cell death or ATP depletion. THP-1 cells were pretreated with ascorbic acid (AA), glutathione (GSH), or N-acetyl-cysteine (NAC) prior to addition of TNF-α/zVAD or control medium. ROS and ATP were measured as described for panels A and B, and survival was determined according to the ability to exclude PI. The cells and supernatants were harvested 24 h after addition of TNF-α/zVAD. The results presented in panels A and C to E are the means ± 1 SE of three or four experiments, each performed in duplicate. The results in panel B are representative of three independent experiments. **, P < 0.001; *, P < 0.05 (compared to the TNF-α/zVAD-treated control).
Induction of ROS and ATP depletion during necrosis.
Increases in ROS and ATP depletion have been implicated in necrotic cell death (25). In THP-1 cells, TNF-α/zVAD resulted in a progressive increase of ROS, first identified at 4 h (Fig. 2C). Additionally, ATP was significantly reduced by 8 h (Fig. 2D). The increased ROS and reduced ATP results were present by the time cell death was first documented at 8 h (Fig. 1B). Therefore, antioxidants were employed to determine whether the ROS were responsible for the reduced ATP and cell death. Even though the antioxidants ascorbic acid, N-acetyl-cysteine, and glutathione monoethyl ester effectively suppressed the accumulation of ROS, they failed to prevent either ATP depletion or cell death (Fig. 2E). These findings demonstrate that the increase of ROS in response to TNF-α/zVAD was not necessary for the depletion of ATP or cell death.
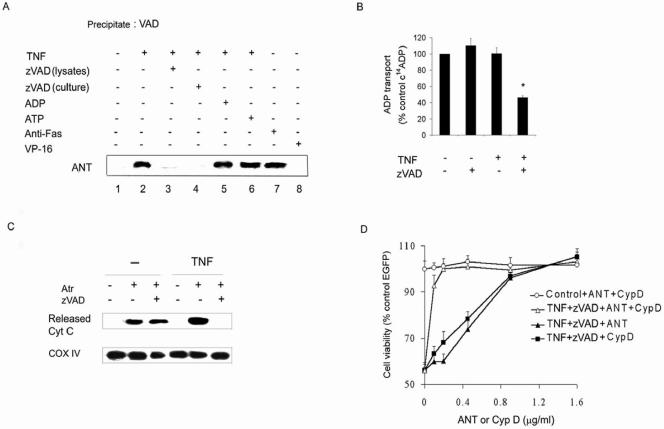
Inhibition of ANT in TNF-α/zVAD-induced necrosis.
The reduction of ATP suggested that the effects of TNF-α/zVAD were mediated through mitochondria. Since confocal microscopy suggested that FITC-VAD colocalized with Mitotracker in cells treated with TNF-α (data not shown), studies were preformed to determine whether ANT, responsible for the exchange of ADP/ATP across the inner mitochondrial membrane (11), is a target for zVAD. To examine this possibility, cell lysates were incubated with biotinylated-VAD, which was precipitated with streptavidin-agarose beads. The precipitates were washed and examined by immunoblot analysis for the presence of ANT. Negligible ANT was coprecipitated with biotinylated-VAD when cells were incubated in medium alone (Fig. 3A, lane 1). However, when THP-1 cells were pretreated with TNF-α, ANT readily coprecipitated with the biotinylated-VAD (Fig. 3A, lane 2). The addition of unlabeled zVAD to the cultures with TNF-α, or to the lysates of cells that had been incubated with TNF-α, prevented ANT precipitation (Fig. 3A, lanes 3 and 4). In contrast, pretreatment of lysates with ANT ligand ADP or ATP did not affect the binding of biotinylated-VAD to ANT (Fig. 3A, lanes 5 and 6). Biotinylated-VAD also coprecipitated with ANT in THP-1 cells incubated with anti-Fas antibody (Fig. 3A, lane 7) but not in cells treated with VP-16 (Fig. 3A, lane 8). As controls, the blots were also probed with antibodies specific for caspase 8, RIP, cytochrome c, VDAC, and cyclophilin D, which were not detected (data not shown). Therefore, the interaction of zVAD appeared to be specific for ANT and did not occur with other mitochondrial membrane proteins with which ANT interacts.
FIG. 3.
Role of ANT in necrotic cell death. (A) TNF-α and Fas ligation promote binding of VAD to ANT. THP-1 cells were treated with TNF-α, anti-Fas antibody, VP-16, or zVAD for 8 h and harvested, and the lysates were incubated with zVAD, ADP, or ATP prior to biotinylated-VAD. The streptavidin-agarose precipitates were examined by immunoblot analysis for ANT. (B) TNF-α/zVAD inhibits ANT activity. Mitochondrial fractions from TNF-α-treated or control-treated (8 h) THP-1 cells were employed to determine the effect of zVAD on C14ADP transport into mitochondria as described in Materials and Methods. (C) TNF-α/zVAD inhibits cytochrome c release. Mitochondrial fractions of TNF-α-treated or control-treated (8 h) THP-1 cells were incubated with zVAD or control medium prior to adding atractyloside (2 mM) for 40 min. Following centrifugation at 10,000 × g, supernatants were analyzed for released cytochrome c and the pellets were analyzed for COX IV. (D) ANT and cyclophilin D protect against TNF-α/zVAD-induced death. RAW264.7 cells were transfected with control or cyclophilin D- or ANT- plus EGFP-expressing vectors at the indicated concentrations. After 24 h, cells were treated with TNF-α/zVAD or control medium for an additional 24 h and viability was assessed according to EGFP-positive cells results. The data in panels B and D are the means ± 1 SE of three orfour experiments performed in duplicate. The data presented in panels A and C are representative of three or four independent experiments. *,P < 0.05 versus control.
To determine whether zVAD inhibits ANT activity, the transport of [14C]ADP into mitochondria was examined. TNF-α or zVAD alone did not affect the transport of [14C]ADP into isolated mitochondria (Fig. 3B). However, the addition of zVAD to the mitochondrial fraction of TNF-α-treated cells significantly inhibited the [14C]ADP uptake into mitochondria. Together, these observations demonstrate that TNF-α-dependent binding of zVAD to ANT results in decreased ADP transport that is essential for mitochondrial ATP synthesis.
The transition of ANT from the m- to c-state conformation is necessary for ADP/ATP exchange. The interaction of ANT with atractyloside induces its c-state conformation, which results in the release of cytochrome c from mitochondria (11). In contrast, cytochrome c release does not occur when ANT is forced to adopt m-state conformation (11, 30). Therefore, we examined whether zVAD affects the atractyloside-induced release of cytochrome c from isolated mitochondria. The addition of atractyloside to mitochondria obtained from cells cultured in medium alone caused cytochrome c release into the supernatant, and this was not affected by zVAD (Fig. 3C). In contrast, employing TNF-α-treated cells, the addition of zVAD to isolated mitochondria prevented the atractyloside-induced cytochrome c release (Fig. 3C). Probing of the mitochondrial pellets with antibodies to COX IV demonstrated comparable quantities of mitochondria in each arm of the experiment (Fig. 3C). These observations suggest that in the presence of TNF-α, zVAD forces ANT to adopt the m-state of conformation, preventing the release of cytochrome c and suppressing ADP/ATP exchange (11). This interpretation is consistent with the observation that cytochrome c was not released from the mitochondria in vivo (Fig. 2B), even in the presence of marked cell death (Fig. 1B).
To further document the role of ANT in necrotic cell death, we employed a death assay utilizing the murine macrophage line RAW264.7 cotransfected with a vector expressing EGFP. TNF-α/zVAD decreased the number of EGFP-positive cells by over 40% (Fig. 3D). Cotransfection of cells with a plasmid expressing ANT reduced cell death in a concentration-dependent manner compared to vector control results (Fig. 3D). Cyclophilin D interacts with and stabilizes ANT, inhibiting cytochrome c release and apoptosis (24, 40). Consistent with its cooperation with ANT, cotransfection with a plasmid expressing cyclophilin D suppressed TNF-α/zVAD-induced cell death (Fig. 3D). Moreover, cotransfection of vectors expressing ANT and cyclophilin D protected against TNF-α/zVAD-induced cell death at concentrations that were ineffective when employed individually. These observations suggest that ANT and cyclophilin D are critical mediators of TNF-α/zVAD-induced cell death.
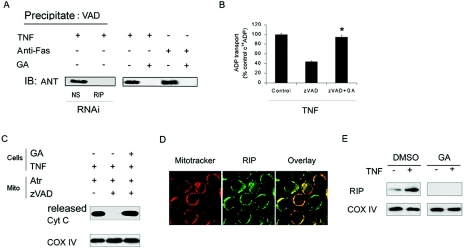
RIP is required for inhibition of ANT activity.
Since death receptor/zVAD-induced cell death requires RIP, the effect of RIP depletion on the interaction of VAD with ANT was examined. Pretreatment with geldanamycin or RIPi reduced the level of RIP (Fig. 1D and E) and abolished the binding of biotinylated-VAD to ANT, which was induced by either TNF-α or anti-Fas (Fig. 4A). The reduction of RIP also blocked TNF-α/zVAD-induced suppression of [14C]ADP uptake into isolated mitochondria (Fig. 4B). Additionally, following RIP depletion, TNF-α/zVAD failed to suppress the atractyloside-induced release of cytochrome c from mitochondria (Fig. 4C), suggesting that RIP was necessary for preventing the conformational transition of ANT that is required for ADP/ATP exchange. Next, we determined whether RIP localizes to the mitochondria in the presence of TNF-α. In TNF-α-treated THP-1 cells RIP colocalized with Mitotracker (Fig. 4D). To confirm this observation, the mitochondrial fraction was isolated from TNF-α-treated THP-1 cells. TNF-α enhanced the RIP level in mitochondrial fraction (Fig. 4E), which was not observed following treatment with geldanamycin. These observations support an important role for RIP in mitochondrial events that contribute to death receptor-mediated necrosis.
FIG. 4.
RIP is required for inhibition of ANT activity. (A) The reduction of RIP prevents the association of VAD with ANT. Macrophages were transfected with nonspecific RNAi (NS) or with RNAi for RIP (each for 96 h; left panel), or THP-1 cells were incubated with control medium or GA for 14 h (0.5 μg/ml, right panel). The cells were then treated with TNF-α or anti-Fas for 8 h, and then cell lysates were obtained and precipitated with biotinylated-VAD, as described for Fig. 5B. The precipitates were probed with antibody to ANT. IB, immunoblot. (B) The reduction of RIP by GA prevents the suppression of ANT activity induced by TNF-α/zVAD. THP-1 cells were incubated with GA (0.5 μg/ml) or control medium for 14 h and then treated with TNF-α for 8 h. The fractions containing the mitochondria were then isolated and treated with zVAD or DMSO (control) for 15 min. ADP transport activity was determined as described in Fig. 3B. (C) The reduction of RIP abrogates the inhibition of cytochrome c release by TNF-α/zVAD. THP-1 cells were treated with GA or control medium (14 h) and then TNF-α (8 h), prior to the isolation of the mitochondrial fractions, as described for panel B. The mitochondria were then incubated with zVAD for 15 min and then with atractyloside for 40 min. The supernatants and pellet were separated by centrifugation and examined for cytochrome c and COX IV, respectively. (D) RIP colocalizes with Mitotracker following treatment with TNF-α. (E) TNF-α-induced colocalization of RIP to the mitochondria was abrogated by reduction of RIP. Mitochondria were isolated from TNF-α-treated cells and analyzed for RIP by immunoblot analysis. The values in panel B represent the means ± 1 SE of three experiments performed in duplicate. The results presented in each panel are representative of three independent experiments. *, P < 0.05 versus zVAD alone.
Regulation of necrosis by cyclophilin D.
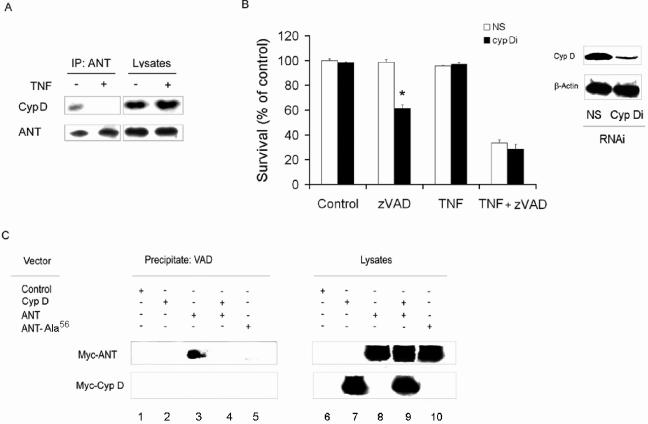
Since cyclophilin D may interact with ANT and the ectopic expression of cyclophilin D protected against TNF-α/zVAD-induced cell death, experiments were performed to define the effects of TNF-α on the interaction between ANT and cyclophilin D. When untreated THP-1 cells were employed, an interaction between cyclophilin D and ANT was observed, since cyclophilin D coimmunoprecipitated with an antibody to ANT (Fig. 5A). In contrast, when TNF-α-treated THP-1 cells were employed in the absence of zVAD, cyclophilin D was no longer detected following immunoprecipitation of ANT (Fig. 5A). The importance of cyclophilin D in TNF-α/zVAD-induced cell death was confirmed by reduction of cyclophilin D employing RNAi. Cyclophilin D RNAi reduced the expression of cyclophilin D in in vitro-differentiated macrophages (Fig. 5B). Following the reduction of cyclophilin D, the macrophages were sensitized to cell death induced by treatment with zVAD alone, in the absence of TNF-α (Fig. 5B). In the presence of TNF-α/zVAD the enhanced cell death was not observed following the reduction of cyclophilin D (Fig. 5B). These findings support the role of the interaction of cyclophilin D with ANT to maintain cell viability in the presence of zVAD.
FIG. 5.
Regulation of necrosis by cyclophilin D (CypD). (A) TNF-α suppresses ANT-cyclophilin D interaction. THP-1 cells were treated with TNF-α or control medium, and the cell lysates were used for immunoprecipitation (IP) with anti-ANT. The blots were probed for cyclophilin D and ANT. (B) Reduction of cyclophilin D facilitates zVAD-induced cell death. THP-1 cells were transfected with NS or cyclophilin D RNAi (Cyp Di) for 96 h. Some cells were harvested and examined for the expression of cyclophilin D by immunoblot analysis (right panel). The remaining cells were treated with zVAD and/or TNF-α as indicated for 24 h and were then examined by for survival (left panel) as determined by the ability to exclude PI. (C) Cyclophilin D reduces VAD/ANT interaction. 293Tcells were transfected with control, Myc-cyclophilin D (Myc-Cyp D)-, Myc-ANT-, or Myc-ANT-Ala56-expressing vectors, as described in Materials and Methods. After 24 h, the cells were harvested and the lysates incubated with biotinylated-VAD and then streptavidin-agarose. The precipitates were examined by immunoblot analysis for Myc. The values in panel B represent the means ± 1 SE of three experiments performed in duplicate. The results presented in each panel are representative of three independent experiments. *, P < 0.05 for cyclophilin D RNAi versus NS RNAi treatment.
The observation that cyclophilin D protects from zVAD-induced cell death raises the possibility that cyclophilin D may affect binding of zVAD to ANT. To examine this hypothesis, a vector expressing Myc-tagged ANT was transfected to 293T cells with or without one expressing Myc-tagged cyclophilin D. After 30 h, cell lysates were prepared and incubated with biotinylated-VAD, which was precipitated with streptavidin-agarose beads. The precipitates were washed and examined by immunoblot analysis for c-Myc, and the contribution of ANT and cyclophilin D was determined by their respective molecular weight results. No ANT or cyclophilin D was coprecipitated with biotinylated-VAD when cells were transfected with the control vector (Fig. 5C, lane 1) or cyclophilin D alone (Fig. 5C, lane 2). However, when 293T cells were transfected with the ANT-expressing vector, ANT readily coprecipitated with the biotinylated-VAD (Fig. 5C, lane 3). In contrast, cotransfection with ANT and cyclophilin D resulted in marked reduction of ANT coprecipitation (Fig. 5C, lane 4). Together, these observations suggest that the interaction of cyclophilin D with ANT suppresses the binding of zVAD to ANT.
ANT possesses three cysteine (Cys) residues. However, it is Cys56 that appears to be important in cyclophilin D binding to ANT (14). We therefore examined whether Cys56 is implicated in binding of zVAD to ANT. To this end, Cys56 was mutated to alanine (ANT-Cys-Ala56). 293T cells were transfected with Myc-ANT wild type or ANT-Cys-Ala56. Unlike the wild-type ANT, a negligible amount of ANT-Cys-Ala56 was coprecipitated with biotinylated VAD (Fig. 5C, lane 5). Theses observations demonstrate that the Cys56 of ANT is important not only for the interaction of ANT with cyclophilin D but also for the binding of zVAD to ANT.
DISCUSSION
RIP has been implicated in autophagic and necrotic caspase-independent cell death (16, 25, 50). Here, we document that RIP is required for the induction of TNF-α/zVAD-induced necrotic cell death in monocytic cells, characterizing a novel mechanism in which zVAD directly participates in the induction of cell death. TNF-α promoted the binding of zVAD to ANT involving Cys56, and this suppressed the ability of ANT to change conformational states, which is necessary for ADP/ATP exchange. These events resulted in increased ROS and decreased cellular ATP levels. Antioxidants failed to protect against cell death or the reduction of ATP, suggesting that ROS production is not essential for this form of necrosis. The interaction between cyclophilin D and ANT suppressed the binding of zVAD to ANT, and cyclophilin D cooperated with ANT in protecting monocytic cells from TNF-α/zVAD-induced cell death. Previously, zVAD alone was shown to induce autophagy in U937 cells (16, 25, 50). In our experiments, U937 cell death was not observed in response to the presence of zVAD-fmk alone. The reason for the apparent disparity between the two studies is most likely differences in experimental conditions, since our experiments were performed in low-serum conditions and for a shorter duration of time. Additionally, when in vitro-differentiated normal macrophages were employed, zVAD at concentrations >20 μM induced cell death (data not shown). Our observations demonstrate that in monocytic cells following death receptor ligation, although zVAD is capable of preventing the apoptotic phenotype, it may directly participate in death receptor-induced necrosis though its interaction with ANT.
Although RIP was found by us and others (16, 25) to be implicated in mitochondrial signaling initiated by death receptor ligation, it remains unclear whether RIP interacts directly with mitochondrial proteins or whether a second messenger downstream of RIP might translocate to mitochondria mediating RIP-induced cell death. In our study, TNF-α increased RIP associated with mitochondria. Nevertheless, no evidence of a direct interaction between RIP and cyclophilin D or ANT was documented (data not shown), suggesting that the effects of RIP might be mediated though an additional signal. An earlier study demonstrated that the RIP kinase domain was important for the induction of necrotic cell death (16). Further, RIP may be involved in activation of caspase 2 (1), mitogen-activated protein kinases (9), and Akt-1 (48), and their activity might be crucial in modulation of mitochondrial function (8, 22, 32). However, inhibitors of caspase 2, the PI3K/Akt-1 pathway, p38, ERK, and Jun N-terminal protein kinase had no effect on TNF-α/zVAD-induced cell death (data not shown). Also, the process does not appear to involve the inhibition of NF-κB, since zVAD did not suppress NF-κB activation (31). Further studies are required to characterize the mechanism by which RIP signals to the mitochondria to mediate TNF-α/zVAD-induced necrotic cell death.
The opening of MPTP may result in dissipation of ΔΨm, swelling of the inner matrix, and rupture of the mitochondrial outer membrane, resulting in MOMP and release of proapoptotic proteins such as cytochrome c (13). MOMP may also be induced by proapoptotic Bcl-2 family members, through action on the outer membrane, prior to the loss of ΔΨm. Although we did not directly examine MPTP, no reduction of ΔΨm was observed prior to cell death. As expected, depolarization coincided with massive cell death after 24 h, when the total population of cells was examined. Additionally, cytochrome c release was not observed at any time point, even though it was readily detected when cell death was induced with VP-16. Further, TNF-α/zVAD-induced cell death was not prevented by the ectopic expression of Bcl-xL, which was effective at preventing VP-16-induced cell death. Prior studies demonstrated that Bcl-xL and Bcl-2 were capable of binding a complex that contained ANT, facilitating ANT activity and protecting cells from apoptosis induced through the mitochondrial pathway (3, 45). Therefore, even though Bcl-xL may be capable of binding ANT, it was not capable of protecting against TNF-α/zVAD-induced necrosis. Overall, our findings suggest that neither depolarization nor increased MOMP was responsible for TNF-α/zVAD-induced necrosis.
Increased ROS and ATP depletion have been implicated in apoptotic and necrotic cell death (25, 35). Indeed, TNF-α/zVAD resulted in a progressive increase of ROS and loss of ATP. Nevertheless, antioxidants failed to suppress the loss of ATP or necrotic cell death even though they strongly reduced the ROS levels, suggesting that ROS were not essential for the induction of either ATP depletion or cell death. Rather, our results suggest that the accumulation of ROS was due to the inability of the mitochondria to synthesize and transport ATP. Our observations contrast with the results seen with mouse embryonic fibroblasts treated with cycloheximide plus TNF-α/zVAD, in which antioxidants protected against necrosis (26). These differences may be due to the conditions used, since we did not employ the protein synthesis inhibitor cycloheximide, which may suppress proteins synthesized in response to NF-κB activation such as superoxide dismutase and ferritin heavy chain, which protect against ROS (18, 37). A recent study demonstrated that protein synthesis persisted during necrotic cell death (39). Therefore, the cells employed in our experiments may have been protected from the effects of ROS, since we did not inhibit NF-κB activation or protein synthesis. Further, our experiments were performed with monocytic cells, which may be enriched in antioxidant molecules, since this cell type is programmed for phagocytosis, which induces ROS production (28). However, the fibrosarcoma cell line L929, in contrast to monocytic cells, was sensitive to TNF-α-induced necrosis, and this sensitivity was enhanced by caspase inhibition and suppressed by inhibition of ROS (46). Although not examined in our study, in addition to RIP, TRAF2 and FADD may be important in the induction of death receptor-mediated necrosis (25, 44). Therefore, cell type-specific differences in the level of expression of molecules implicated in the protection against ROS or in the signaling pathways involved may be important in determining the sensitivity to death receptor-induced necrosis.
Mitochondrial ATP synthesis requires ADP transport into mitochondria, which is mediated by ANT (35). ANT regulates both ADP/ATP exchange and release of cytochrome c from mitochondria. We found that zVAD translocated to the mitochondria and interacted with ANT when cells were pretreated with TNF-α or anti-Fas, a result which was not observed in RIP-depleted cells, suggesting that RIP was required for interaction between ANT and zVAD. Likewise, zVAD-induced inhibition of ADP transport into the mitochondria was not observed in RIP-depleted cells. ADP/ATP exchange is highly dependent on transition of ANT between the m- and the c-conformational states (11). In our study, ANT was not capable of adopting the c-conformational state, defined by the atractyloside-induced release of cytochrome c, when mitochondria from TNF-α-stimulated cells were exposed to zVAD, and this inhibition of the transition of ANT by TNF-α/zVAD was not observed following the reduction of RIP. Supporting the importance of the conformational transition of ANT for cell survival, atractyloside added to Jurkat cells forced ANT into the c-state conformation, resulting in the release of cytochrome c and apoptotic cell death (49). In monocytic cells treated with TNF-α/zVAD the m-state conformation was imposed, cytochrome c was not released, and the mode of cell death was necrotic. The inability of ANT to adopt the c-state may explain the observation that cytochrome c was not released from the mitochondria during TNF-α/zVAD-induced cell death.
ANT, which is situated in the inner layer of the mitochondrial membrane, may be a component of a multiprotein complex with other mitochondrial proteins such as VDAC, cyclophilin D, peripheral benzodiazepine receptor, and hexokinase, and these interactions may affect ANT function. For example, cyclophilin D suppressed the apoptosis induced by the overexpression of ANT (40), an observation which we confirmed with RAW cells (data not shown). Recent studies of cyclophilin D knockout mice demonstrated that cyclophilin D plays an important role in regulating the MPTP and necrotic cell death induced by Ca+ overload or oxidative stress but not apoptosis (2, 34). In our study, cyclophilin D decreased the sensitivity to necrotic cell death and suppressed the ability of ANT to bind with biotinylated-VAD. Moreover, the ectopic expression of cyclophilin D with ANT had a synergistic effect on reduction of necrosis, while the reduction of cyclophilin D sensitized the cells to zVAD-induced cell death in absence of TNF-α. These observations demonstrate that the interactions between cyclophilin D and ANT may be critical in regulating certain forms of necrosis.
Cys56 of ANT was previously reported to be involved in interaction between ANT and cyclophilin D (14). The substitution of Cys56 with alanine inhibited the interaction of biotinylated VAD with ANT, suggesting that zVAD and cyclophilin D may compete for the same binding site on ANT. Supporting this interpretation, TNF-α promoted the dissociation of cyclophilin D from ANT, indicating that cyclophilin D may be a target of death receptor-initiated signaling. Importantly, cyclophilin D is specifically up-regulated in human tumors of the breast, ovary, and uterus (40), which may block cell death or promote resistance to therapy in these diseases. Although a variety of mitochondrial proteins may form a complex with ANT (47), neither VDAC nor cyclophilin D was coprecipitated with ANT bound to biotinylated VAD, further supporting the interpretation that the effect of zVAD was due to binding to ANT and not another protein complexed with ANT. Our observations suggest that the modulation of the interaction of cyclophilin D with ANT may facilitate the targeting of therapies to modulate mitochondrial cell death.
The caspase inhibitor zVAD binds covalently to the catalytic cysteine of active caspases through the fmk group (10). Further, enzymatically active full-length caspase 8 interacts with zVAD (42). Although Cys56 of ANT was critical for the binding of zVAD to ANT, our data did not document the direct binding of zVAD to Cys56. It is not clear whether or not the binding is covalent and which, if any, enzymatic activity might be necessary for the binding of zVAD to ANT. In transient transfection assays, neither TNF-α nor anti-Fas antibody was required to induce the binding of biotinylated VAD when ANT was overexpressed, suggesting that the death receptor pathway was not necessary for this interaction. In our study caspase 8 did not associate with biotinylated VAD, and TNF-α alone did not induce caspase 8 activity in human macrophages (data not shown). Moreover, the ectopic expression of the caspase 8 viral inhibitor Crm A did not protect macrophages from necrosis (data not shown) indicating that active caspase 8 was not involved in interaction between zVAD to ANT or in induction of necrosis. The fmk group was essential but not sufficient for ANT inhibition, since not all fmk-containing caspase inhibitors induced necrosis in combination with TNF-α and since cpm-VAD-CHO, a broad-specificity caspase inhibitor, failed to affect cell viability. Nevertheless, the molecular mechanism by which zVAD binds to ANT remains to be characterized.
Overall, our findings elucidate a novel mechanism by which RIP may initiate necrotic cell death by a pathway effecting ADP/ATP exchange. It is possible that at inflammatory sites, where the expression of TNF-α and Fas ligand might be enhanced, molecules expressed by pathogenic organisms may promote necrosis (38), perhaps by affecting ANT function. Further, these observations suggest that whenever employed in vivo or in vitro, zVAD or other broad-specificity caspase inhibitors possessing the fmk group may induce necrosis by interfering with ANT function rather than protecting against apoptotic cell death. These observations also provide insights that may be utilized in developing new strategies to promote cell death in cancer and chronic inflammatory conditions or to protect against necrotic cell death that may occur following ischemia or trauma.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants AR049217 and AR048269 to R.M.P.
We thank M. Paniaqua and L. Reynolds from the Robert Lurie Cancer Center for assistance with flow cytometry and electron microscopy and N. Chandel and K. Machida for helpful discussions.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahmad, M., S. M. Srinivasula, L. Wang, R. V. Talanian, G. Litwack, T. Fernandes-Alnemri, and E. S. Alnemri. 1997. CRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIP. Cancer Res. 57:615-619. [PubMed] [Google Scholar]
- 2.Baines, C. P., R. A. Kaiser, N. H. Purcell, N. S. Blair, H. Osinska, M. A. Hambleton, E. W. Brunskill, M. R. Sayen, R. A. Gottlieb, G. W. Dorn, J. Robbins, and J. D. Molkentin. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658-662. [DOI] [PubMed] [Google Scholar]
- 3.Belzacq, A. S., H. L. Vieira, F. Verrier, G. Vandecasteele, I. Cohen, M. C. Prevost, E. Larquet, F. Pariselli, P. X. Petit, A. Kahn, R. Rizzuto, C. Brenner, and G. Kroemer. 2003. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 63:541-546. [PubMed] [Google Scholar]
- 4.Bottero, V., F. Rossi, M. Samson, M. Mari, P. Hofman, and J. F. Peyron. 2001. Ikappa b-alpha, the NF-kappa B inhibitory subunit, interacts with ANT, the mitochondrial ATP/ADP translocator. J. Biol. Chem. 276:21317-21324. [DOI] [PubMed] [Google Scholar]
- 5.Castedo, M., K. Ferri, T. Roumier, D. Metivier, N. Zamzami, and G. Kroemer. 2002. Quantitation of mitochondrial alterations associated with apoptosis. J. Immunol. Methods 265:39-47. [DOI] [PubMed] [Google Scholar]
- 6.Cauwels, A., B. Janssen, A. Waeytens, C. Cuvelier, and P. Brouckaert. 2003. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat. Immunol. 4:387-393. [DOI] [PubMed] [Google Scholar]
- 7.Chipuk, J. E., T. Kuwana, L. Bouchier-Hayes, N. M. Droin, D. D. Newmeyer, M. Schuler, and D. R. Green. 2004. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010-1014. [DOI] [PubMed] [Google Scholar]
- 8.Deng, Y., X. Ren, L. Yang, Y. Lin, and X. Wu. 2003. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115:61-70. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., Y. Lin, and Z. G. Liu. 2003. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 4:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekert, P. G., J. Silke, and D. L. Vaux. 1999. Caspase inhibitors. Cell Death Differ. 6:1081-1086. [DOI] [PubMed] [Google Scholar]
- 11.Fiore, C., V. Trezeguet, A. Le Saux, P. Roux, C. Schwimmer, A. C. Dianoux, F. Noel, G. J. Lauquin, G. Brandolin, and P. V. Vignais. 1998. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie 80:137-150. [DOI] [PubMed] [Google Scholar]
- 12.Flierl, A., Y. Chen, P. E. Coskun, R. J. Samulski, and D. C. Wallace. 2005. Adeno-associated virus-mediated gene transfer of the heart/muscle adenine nucleotide translocator (ANT) in mouse. Gene Ther. 12:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, D. R., and G. Kroemer. 2004. The pathophysiology of mitochondrial cell death. Science 305:626-629. [DOI] [PubMed] [Google Scholar]
- 14.Halestrap, A. P., K. Y. Woodfield, and C. P. Connern. 1997. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 272:3346-3354. [DOI] [PubMed] [Google Scholar]
- 15.Hatanaka, T., Y. Kihira, Y. Shinohara, E. Majima, and H. Terada. 2001. Characterization of loops of the yeast mitochondrial ADP/ATP carrier facing the cytosol by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 286:936-942. [DOI] [PubMed] [Google Scholar]
- 16.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 17.Jaattela, M., and J. Tschopp. 2003. Caspase-independent cell death in T lymphocytes. Nat. Immunol. 4:416-423. [DOI] [PubMed] [Google Scholar]
- 18.Kamata, H., S. Honda, S. Maeda, L. Chang, H. Hirata, and M. Karin. 2005. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649-661. [DOI] [PubMed] [Google Scholar]
- 19.Kelliher, M. A., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 20.Kokoszka, J. E., K. G. Waymire, S. E. Levy, J. E. Sligh, J. Cai, D. P. Jones, G. R. MacGregor, and D. C. Wallace. 2004. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427:461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreuz, S., D. Siegmund, J. J. Rumpf, D. Samel, M. Leverkus, O. Janssen, G. Hacker, O. Dittrich-Breiholz, M. Kracht, P. Scheurich, and H. Wajant. 2004. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J. Cell Biol. 166:369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassus, P., X. Opitz-Araya, and Y. Lazebnik. 2002. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297:1352-1354. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, J., A. Devin, A. Miller, Y. Lin, Y. Rodriguez, L. Neckers, and Z. G. Liu. 2000. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 275:10519-10526. [DOI] [PubMed] [Google Scholar]
- 24.Lin, D. T., and J. D. Lechleiter. 2002. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J. Biol. Chem. 277:31134-31141. [DOI] [PubMed] [Google Scholar]
- 25.Lin, Y., S. Choksi, H. M. Shen, Q. F. Yang, G. M. Hur, Y. S. Kim, J. H. Tran, S. A. Nedospasov, and Z. G. Liu. 2004. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 279:10822-10828. [DOI] [PubMed] [Google Scholar]
- 26.Liu, H., Y. Ma, L. J. Pagliari, H. Perlman, C. Yu, A. Lin, and R. M. Pope. 2004. TNF-alpha-induced apoptosis of macrophages following inhibition of NF-kappaB: a central role for disruption of mitochondria. J. Immunol. 172:1907-1915. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., H. Perlman, L. J. Pagliari, and R. M. Pope. 2001. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J. Exp. Med. 194:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, H., and R. M. Pope. 2004. Phagocytes: mechanisms of inflammation and tissue destruction. Rheum. Dis. Clin. N. Am. 30:19-39. [DOI] [PubMed] [Google Scholar]
- 29.Ma, Y., H. Liu, H. Tu-Rapp, H. J. Thiesen, S. M. Ibrahim, S. M. Cole, and R. M. Pope. 2004. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat. Immunol. 5:380-387. [DOI] [PubMed] [Google Scholar]
- 30.Machida, K., Y. Hayashi, and H. Osada. 2002. A novel adenine nucleotide translocase inhibitor, MT-21, induces cytochrome c release by a mitochondrial permeability transition-independent mechanism. J. Biol. Chem. 277:31243-31248. [DOI] [PubMed] [Google Scholar]
- 31.Maianski, N. A., D. Roos, and T. W. Kuijpers. 2003. Tumor necrosis factor alpha induces a caspase-independent death pathway in human neutrophils. Blood 101:1987-1995. [DOI] [PubMed] [Google Scholar]
- 32.Majewski, N., V. Nogueira, P. Bhaskar, P. E. Coy, J. E. Skeen, K. Gottlob, N. S. Chandel, C. B. Thompson, R. B. Robey, and N. Hay. 2004. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16:819-830. [DOI] [PubMed] [Google Scholar]
- 33.Majima, E., H. Koike, Y. M. Hong, Y. Shinohara, and H. Terada. 1993. Characterization of cysteine residues of mitochondrial ADP/ATP carrier with the SH-reagents eosin 5-maleimide and N-ethylmaleimide. J. Biol. Chem. 268:22181-22187. [PubMed] [Google Scholar]
- 34.Nakagawa, T., S. Shimizu, T. Watanabe, O. Yamaguchi, K. Otsu, H. Yamagata, H. Inohara, T. Kubo, and Y. Tsujimoto. 2005. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652-658. [DOI] [PubMed] [Google Scholar]
- 35.Newmeyer, D. D., and S. Ferguson-Miller. 2003. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481-490. [DOI] [PubMed] [Google Scholar]
- 36.Perlman, H., L. J. Pagliari, C. Georganas, T. Mano, K. Walsh, and R. M. Pope. 1999. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp. Med. 190:1679-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham, C. G., C. Bubici, F. Zazzeroni, S. Papa, J. Jones, K. Alvarez, S. Jayawardena, E. De Smaele, R. Cong, C. Beaumont, F. M. Torti, S. V. Torti, and G. Franzoso. 2004. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119:529-542. [DOI] [PubMed] [Google Scholar]
- 38.Proskuryakov, S. Y., A. G. Konoplyannikov, and V. L. Gabai. 2003. Necrosis: a specific form of programmed cell death? Exp. Cell Res. 283:1-16. [DOI] [PubMed] [Google Scholar]
- 39.Saelens, X., N. Festjens, E. Parthoens, I. Vanoverberghe, M. Kalai, F. van Kuppeveld, and P. Vandenabeele. 2005. Protein synthesis persists during necrotic cell death. J. Cell Biol. 168:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert, A., and S. Grimm. 2004. Cyclophilin D, a component of the permeability transition-pore, is an apoptosis repressor. Cancer Res. 64:85-93. [DOI] [PubMed] [Google Scholar]
- 41.Schulze, K., B. Witzenbichler, C. Christmann, and H. P. Schultheiss. 1999. Disturbance of myocardial energy metabolism in experimental virus myocarditis by antibodies against the adenine nucleotide translocator. Cardiovasc. Res. 44:91-100. [DOI] [PubMed] [Google Scholar]
- 42.Su, H., N. Bidere, L. Zheng, A. Cubre, K. Sakai, J. Dale, L. Salmena, R. Hakem, S. Straus, and M. Lenardo. 2005. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 307:1465-1468. [DOI] [PubMed] [Google Scholar]
- 43.Vanden Berghe, T., M. Kalai, G. van Loo, W. Declercq, and P. Vandenabeele. 2003. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J. Biol. Chem. 278:5622-5629. [DOI] [PubMed] [Google Scholar]
- 44.Vanden Berghe, T., G. van Loo, X. Saelens, M. Van Gurp, G. Brouckaert, M. Kalai, W. Declercq, and P. Vandenabeele. 2004. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J. Biol. Chem. 279:7925-7933. [DOI] [PubMed] [Google Scholar]
- 45.Vander Heiden, M. G., N. S. Chandel, P. T. Schumacker, and C. B. Thompson. 1999. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3:159-167. [DOI] [PubMed] [Google Scholar]
- 46.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verrier, F., A. Deniaud, M. Lebras, D. Metivier, G. Kroemer, B. Mignotte, G. Jan, and C. Brenner. 2004. Dynamic evolution of the adenine nucleotide translocase interactome during chemotherapy-induced apoptosis. Oncogene 23:8049-8064. [DOI] [PubMed] [Google Scholar]
- 48.Vivarelli, M. S., D. McDonald, M. Miller, N. Cusson, M. Kelliher, and R. S. Geha. 2004. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp. Med. 200:399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weaver, J. G., A. Tarze, T. C. Moffat, M. Lebras, A. Deniaud, C. Brenner, G. D. Bren, M. Y. Morin, B. N. Phenix, L. Dong, S. X. Jiang, V. L. Sim, B. Zurakowski, J. Lallier, H. Hardin, P. Wettstein, R. P. van Heeswijk, A. Douen, R. T. Kroemer, S. T. Hou, S. A. Bennett, D. H. Lynch, G. Kroemer, and A. D. Badley. 2005. Inhibition of adenine nucleotide translocator pore function and protection against apoptosis in vivo by an HIV protease inhibitor. J. Clin. Investig. 115:1828-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, L., A. Alva, H. Su, P. Dutt, E. Freundt, S. Welsh, E. H. Baehrecke, and M. J. Lenardo. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304:1500-1502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.