Abstract
Bright/ARID3a has been implicated in mitogen- and growth factor-induced up-regulation of immunoglobulin heavy-chain (IgH) genes and in E2F1-dependent G1/S cell cycle progression. For IgH transactivation, Bright binds to nuclear matrix association regions upstream of certain variable region promoters and flanking the IgH intronic enhancer. While Bright protein was previously shown to reside within the nuclear matrix, we show here that a significant amount of Bright resides in the cytoplasm of normal and transformed B cells. Leptomycin B, chromosome region maintenance 1 (CRM1) overexpression, and heterokaryon experiments indicate that Bright actively shuttles between the nucleus and the cytoplasm in a CRM1-dependent manner. We mapped the functional nuclear localization signal to the N-terminal region of REKLES, a domain conserved within ARID3 paralogues. Residues within the C terminus of REKLES contain its nuclear export signal, whose regulation is primarily responsible for Bright shuttling. Growth factor depletion and cell synchronization experiments indicated that Bright shuttling during S phase of the cell cycle leads to an increase in its nuclear abundance. Finally, we show that shuttle-incompetent Bright point mutants, even if sequestered within the nucleus, are incapable of transactivating an IgH reporter gene. Therefore, regulation of Bright's cellular localization appears to be required for its function.
Confinement of biomolecules within compartments is an important feature of eukaryotic cells, because each compartment has its own specific functions (8, 20, 45). For all transcription factors, translocation to the nucleus is an essential process. Transport into or out of the nucleus takes place through large multiprotein structures, termed nuclear pores, that span the nuclear envelope. Small proteins (less than 40 to 60 kDa) can enter the nucleus by passive diffusion following a concentration gradient, although notable exceptions include histones (45). In most cases, molecules of more than 40 to 60 kDa are actively transported across nuclear pore complexes. Nuclear import of a protein is mediated by a nuclear localization signal (NLS), and the best known NLS is a stretch of basic amino acids, such as that found in the simian virus 40 (SV40) large T-antigen NLS (4). However, not all proteins that carry an NLS motif are nuclear (9), and conversely, noncanonical NLSs have been reported (17, 30, 36). Proteins exported from the nucleus to the cytoplasm require a nuclear export signal (NES). The most common type of NES is a leucine-rich hydrophobic amino acid stretch, such as LX1-3LX2-4LXL, which is recognized and bound by the export receptor, chromosome region maintenance 1 (CRM1)/exportin 1 (6, 34). Covalent binding of CRM1 by the antibiotic leptomycin B (LMB) inhibits CRM1-dependent nuclear export of NES-containing proteins (11), resulting in their nuclear accumulation. Therefore, LMB has been used extensively to examine the existence of CRM1-dependent NES activity.
Bright is a transcription factor discovered for its ability to bind certain immunoglobulin heavy-chain (IgH) promoters and the intronic enhancer following treatment with mitogens or growth factors (reviewed in reference 41). Stimulation of a mature B-cell line, BCL1, with interleukin-5 and antigen was shown to increase IgH gene transcription and to induce the formation of a nuclear matrix association region (MAR)-dependent DNA binding complex (38). A protein required for this complex was identified and named B-cell regulator of IgH gene transcription, Bright (16). As with other MAR binding proteins, Bright binds to ATC-rich sequences within MARs and, as such, became the founding member of the 15-membered (in humans and mice) ARID (AT-Rich Interacting Domain) family (reviewed in reference 42). However, while most ARID members bind AT-rich DNA relatively nonspecifically (28), Bright and its other ARID3 paralogue, Bright Dri-like protein (Bdp), show highly restricted specificity for an extended ATC-rich consensus found in promoter-associated MARs of some but not all variable region (VH) gene segments (13, 16) and within the MARs flanking the intronic enhancer (16). This specificity is engendered, at least in part, by the ability of ARID3 proteins to homo-oligomerize through a region conserved only in ARID3 termed the REKLES domain (reviewed in reference 41).
Mitogen- or growth factor-induced Bright-VH-associated MAR complexes were demonstrated to contain Bruton's tyrosine kinase (Btk) (40). Btk is required for efficient antigen receptor-mediated signaling during B lineage development and activation. Its loss or mutation leads to a lethal X-linked immunodeficiency condition in humans (25). MAR binding and transactivation by Bright are enhanced in the presence of Btk, raising the possibility that Bright might function in B-cell receptor-mediated signaling (31, 40). An evolutionarily conserved role for Bright in signal transduction is further supported by recent findings for Xenopus. The frog orthologue of Bright is essential for the signaling cascade activated by transforming growth factor β, which is required for mesodermal patterning and embryonic morphogenesis (7).
In addition to its participation in IgH transcriptional regulation, Bright and its human orthologue, Dril1, appear to function in cell cycle regulation. This was initially suggested by our finding (49) that a fraction of nuclear Bright, by virtue of an interaction through its REKLES domain with Sp100, fractionated into promyelocytic leukemia nuclear bodies (PML-NBs). Subsequently, it was found that when expressed ectopically in primary mouse embryonic fibroblasts (MEFs), Bright or Dril1 can rescue natural senescence or oncogenic RASV12-induced senescence in a p53-independent fashion (29). Furthermore, Dril1 can immortalize and transform MEFs through the regulation of cyclin E1 and, subsequently, E2F1 activity (29). While the mechanism underlying this G1/S acceleration remains unclear, a previous and unrelated report argued that Dril1 could physically interact with E2F1, leading to the alternative name, E2F1-binding protein 1 (35). Recently, it was reported that ectopic overexpression of Dril1 induced desumoylation of PML and the disintegration of PML-NBs, whereas reduction of Dril1 expression by small interfering RNA led to PML-NB accumulation and, consequently, PML-mediated premature senescence (12), an effect consistent with that observed previously in primary MEFs (29).
Based on its previous characterization as a MAR binding protein which accumulated in the nuclear matrix and in nuclear matrix-associated PML-NBs, we anticipated that Bright's transcriptional activity may require regulated nuclear matrix localization (41). In this paper, we investigated the cellular residency of Bright and determined the protein domains and motifs responsible. Unexpectedly, a significant amount of Bright was detected in the cytoplasm as well as in the nucleus. We found that Bright undergoes nucleocytoplasmic shuttling, and this appears to be regulated by a CRM1-dependent nuclear export mechanism. We show that shuttling occurs during S phase of the cell cycle and may be required for maintaining adequate Bright levels in the nucleus. However, this is insufficient for maximal transcription, as a nonshuttling, nucleus-only point mutant is a weaker transactivator than shuttle-competent wild-type Bright.
MATERIALS AND METHODS
Cell culture, transfections, and synchronization.
M12.4 is a BALB/c-derived B-cell lymphoma (24), and BCL1 is BALB/c-derived B-cell leukemia (14). Both are phenotypically the most similar to the mature B lymphocyte and have been shown to give a proliferative response and to be differentiation competent when treated with mitogens and growth factors, including lipopolysaccharide and interleukin-5 plus antigen (38). They were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS). The adherent cell lines, Cos-7, NIH 3T3, and HeLa, were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS under standard conditions. Mouse embryonic fibroblasts were isolated from day 13.5 BALB/c embryos using standard methods.
Non-B cells were transiently transfected using FuGene6 transfection reagent (Roche Diagnostics) following the manufacturer's directions. Briefly, 1.5 × 105 to 3 × 105 cells were cultured overnight in six-well plates containing 2 ml medium. The cells were transfected with 0.3 to 1 μg of plasmid DNA and cultured for 24 to 48 h. For cotransfection, we equalized the total DNA amounts by adding the appropriate amount of pCR3.1 empty circular vector. For LMB experiments, 10 ng/ml of LMB (provided by Minoru Yoshida, University of Tokyo, Japan) was added directly to the culture medium 12 h after transient transfection. Then, the cells were cultured for an additional 12 h and analyzed for Bright localization.
To synchronize the cell cycle at G1/S phase, BCL1 cells were grown in RPMI medium supplemented with 0.5% FCS for 18 h and then shifted to RPMI medium supplemented with 10% FCS and 1 mg/ml of aphidicolin (Sigma) for 16 h. The cells were washed with phosphate-buffered saline (PBS) to remove the cell cycle inhibitor and grown in RPMI medium supplemented 10% FCS.
Plasmids.
N-terminal green fluorescent protein (GFP) fusions of Bright and Bright truncation and point mutants were engineered as follows. First, we ligated the GFP open reading frame and the multicloning site-containing 0.8-kb BclI-NheI fragment of pEGFP-c1 (Clontech) to the 4.9-kb XbaI-NheI backbone fragment of pCR3.1 (Invitrogen). The vector (pCG) contains a cytomegalovirus promoter and a T7 promoter upstream of the GFP open reading frame and stop codons for each of the three reading frames at the end of the multicloning site. Bright wild type and deletion mutants were prepared by PCR and/or enzyme digestions and inserted into pCG. Bright point mutations were introduced by PCR-based site-directed mutagenesis. Bright wild type (1-601) and point mutants, K466A and G532A, were subcloned by PCR into pBabe, a murine leukemia virus-based puromycin-selectable retrovirus (provided by Daniel Peeper, Netherlands Cancer Research Institute) (10) for transduction of NIH 3T3 cells.
For the pGL3 5′ MAR construction, a fragment spanning the two MAR binding sites of Bright (previously termed Bf150 and Tx125) (39) that reside upstream of the S107 family member VH1 IgH promoter was PCR amplified using primers S107-5 (5′-GGATCCACATGTATGATTTTAATG-3′) and TxR5-3 (5′-AGATCTCAGCTATCAGTAACAATG-3′) from an S107-bearing construct used previously (21). The fragment contains the 5′ MARs but not the 125-bp core promoter of the VH1 gene, eliminating the octamer, ocaB, and TATA box binding sites (3). The 5′ MAR-containing fragment was inserted upstream of the minimal SV40 promoter of pGL3-promoter (Promega) and confirmed by DNA sequencing.
A neo mini construct, OSneo, was constructed by digesting OSDUPDEL with EcoRV and XhoI and by religating the 3.5-kb fragment. pcDNA hnRNP A1 (44) and pcDNA hnRNP C1 (44) were obtained from Karen J. Artzt (University of Texas at Austin). An expression plasmid encoding the human CRM1 full-length cDNA (hCRM1) was obtained from Tom Hope (University of Illinois at Chicago).
Retroviral transduction.
The Phoenix retroviral system was employed to establish stable transductants of Bright wild type and localization point mutants in NIH 3T3 cells according to published protocols (http://www.stanford.edu/group/nolan/retroviral_systems/phx.html). Briefly, 3 × 105 amphitrophic Phoenix 293 packaging cells were plated in 4 ml of DMEM supplemented with 10% fetal bovine serum (FBS) in 60-mm plates. After 12 h, the medium was replaced with fresh medium containing 25 μM chloroquine and cultured for 24 h. The Phoenix cells were transfected with pBabe, pBabe-Bright, or pBabe-Bright mutants with FuGene6. At 8 h posttransfection, the medium was replaced with medium lacking chloroquine. At 48 h after transfection, the viral supernatant was harvested, centrifuged, and filtered to remove live cells and debris. NIH 3T3 cells were plated 24 h before viral infection. Culture medium was replaced with the viral mixture (1 ml of viral supernatant, 4 μM of polybrene, and 2 ml of DMEM). After 3 h, 7 ml of DMEM medium supplemented with 10% FBS was added. Stable-expressing Bright cells were selected with 2 μg/ml of puromycin from day 2 posttransfection.
Protein fractionation.
We prepared the cytoplasmic and nuclear extract according to the procedure of Johnson et al. (19). Cells (1 × 106) were collected and washed twice with PBS. The cell pellet was suspended in 100 μl of sucrose buffer I-A (100 mM Tris-Cl [pH 8.0], 0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 1 mM dithiothreitol, and 0.5% NP-40) supplemented with protease inhibitors. Then, the nuclei were separated from the soluble cytoplasmic fraction by centrifugation at 2,000 rpm for 2 min (model 59-A; Fisher Scientific) at 4°C. The cytoplasmic fraction was recentrifuged at 13,000 rpm for 15 min at 4°C (5417R; Eppendorf). The supernatant was used as the cytoplasmic fraction. The nuclei were washed with 1 ml of sucrose buffer I-B (100 mM Tris-Cl [pH 8.0], 0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 1 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride) and spun down at 2,000 rpm for 2 min. The nuclei were suspended in 100 μl of urea buffer (8 M urea, 10 mM Tris-Cl [pH 8.0]), sonicated briefly at a low power to shear chromosomal DNA, and centrifuged at 13,000 rpm for 15 min at 4°C (5417R; Eppendorf). The supernatant was used for the nuclear extract. After determining the protein concentration using the Bradford assay (6a), 2 to 20 μg of each extract was loaded for Western blot analysis. The density of each band was measured using Scion Image 1.63 (Scion Corporation) after subtraction of the average backgrounds (10 random measurements).
Whole-cell lysates were prepared through protein extraction in 8 M urea buffer, brief sonication, and centrifugation at 13,000 rpm for 15 min.
Cytological studies.
Indirect immunofluorescence analysis was performed to examine the localization of Bright. B cells (1 × 106) were collected and washed twice with PBS. The cells were fixed with 500 μl of 4% paraformaldehyde (PFA) for 20 min at 25°C and permeabilized with 0.1% of Triton X-100 in PBS for 15 min at 25°C. After being washed with PBS three times, the cells were attached onto slides (Polytech) coated with 1% poly-l-lysine (Sigma), and the slides were immersed first in ice-cold methanol for 5 min and then in ice-cold acetone for 30 s. The cells were blocked with 20% FBS in PBS for 15 min at 25°C and incubated with rabbit anti-Bright antiserum (diluted 1:800) and/or goat anti-lamin B antibody (diluted 1:500; Santa Cruz) for 1 h at 25°C. After a washing, the cells were stained with donkey rhodamine-conjugated anti-goat antibody (diluted 1:500; Santa Cruz) and/or donkey fluorescein isothiocyanate-conjugated anti-rabbit antibody (diluted 1:500; Santa Cruz) for 1 h at 25°C in the dark. After being washed with PBS, the cells were stained with 1 ng/ml DAPI (4′,6′-diamidino-2-phenylindole) or Hoechst 33342 for 5 min at 25°C and then washed again with PBS. Slides were air dried, mounted with mounting medium (VECTASHIELD H-1000; Vector Laboratories, Inc.), covered with coverslips, and sealed with nail polish. The localization of Bright was observed via an Axioskop fluorescence microscope (Zeiss).
GFP-transfected Cos-7 cells were grown on coverslips for 24 h and fixed with 4% PFA for 20 min at 25°C. After being washed with PBS, coverslips were air dried, mounted with mounting medium supplemented with 1 ng/ml of DAPI, and covered with slide glasses. GFP or GFP-Bright localization was examined with Axioskop fluorescence microscopy (Zeiss). For each experiment, a minimum of 300 GFP-positive cells were grouped into three categories according to where the majority of the signal localized and scored as preferential nuclear localization (N), nuclear and cytoplasmic localization (W), or preferential cytoplasmic localization (C). The percentage of cells within each group was calculated for each transfection, and the average percentage was obtained from at least three independent transfections.
Heterokaryon fusions.
We performed heterokaryon experiments following the method of Blanc et al. (5). Briefly, we grew 3 × 105 HeLa cells/well in a six-well plate overnight. Then, we transfected the HeLa cells with 1 μg of myc-Bright, myc-Bright G532A, myc-hnRNP A1, or myc-hnRNP C1. On day 2, we replated 3 × 105 transfected cells onto sterilized cover glasses and cultured the cells overnight. On the following day, 3 × 105 NIH 3T3 cells were plated onto the HeLa cells and grown for 3 h with 10 μg/ml of cycloheximide. Cells were fused in 50% polyethylene glycol 3350 for 2 min, washed with 2 ml of PBS three times, and then grown with 10 μg/ml of cycloheximide for 3 h. After the cells were washed with PBS, they were immunostained with mouse anti-myc antibody (diluted 1:1,000) (BAbCO) as described above.
Cell cycle analysis.
B cells were fixed in ethanol, washed with PBS, incubated for 30 min with 10 μl of 50 μg/ml propidium iodide (Sigma) and 10 μl of 10 mg/ml RNase A, and then analyzed by flow cytometry for DNA content. Cos-7 cells transiently transfected with GFP-Bright were fixed in 4% PFA and then stained and analyzed as above.
Luciferase assays.
To establish a 5′ MAR luciferase-expressing NIH 3T3 stable cell line (NIH 3T3 5′ MAR-Luc), NIH 3T3 cells were cotransfected with OSneo, a neomycin-resistant gene-bearing mini vector, and the pGL3-5′ MAR construct. NeoR clones were selected with 600 μg/ml of G418 for 12 days, and then luciferase-positive clones were obtained. NIH 3T3 5′ MAR-Luc cells were transduced with pBabe-Bright wild type or K466A or G532A point mutant, and puromycin-resistant pools were used for further experiments. NIH 3T3 5′ MAR-Luc cells expressing Bright wild type or mutants were plated at 4 × 104 cells per well in 24-well plates. Twelve or 24 h later, the luciferase activity was measured within 10 μg of whole-cell lysate using the luciferase reporter assay system (Promega) according to the manufacturer's instructions. To evaluate the effect of Bright on luciferase reporter gene activity, we calculated the relative luciferase activities of Bright-expressing cells compared with that of NIH 3T3 5′ MAR-Luc. The relative activities were obtained as averages from four independent experiments.
Statistical analyses were performed using an unpaired Student t test (http://graphpad.com/quickcalcs/index.cfm). A P value of <0.05 was considered to be significant.
Northern blotting.
Total RNA was isolated using Trizol (Invitrogen), and 5 μg was separated on 0.8% agarose-formaldehyde gels. Probes for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (316-bp EcoRI-BamHI fragment of pTRI-GAPDH-human; Ambion) and for Bright (bases 1443 to 2067; GenBank U60335) were prepared using the Megaprime DNA labeling system (Amersham).
RESULTS
Bright accumulates in both the nucleus and the cytoplasm in various ratios in asynchronously growing cells.
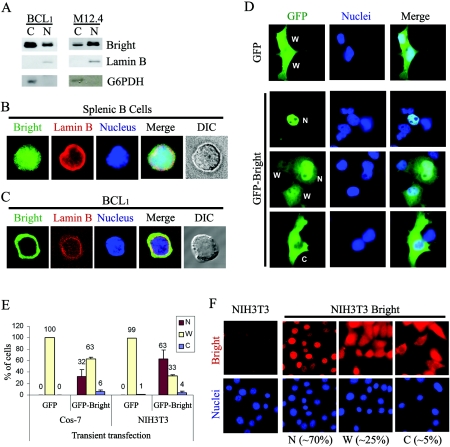
To investigate the subcellular localization of Bright, we performed biochemical fractionation of two mouse mature B-cell lines (M12.4 and BCL1) in which Bright-DNA complex induction by mitogens or growth factors had been previously observed (38). Cells were fractionated into nuclear and soluble cytoplasmic fractions, and Bright was detected in both (Fig. 1A). Unexpectedly, the majority of Bright existed within the cytoplasm of BCL1, whereas M12.4 expressed a significant amount of Bright in both the nucleus and the cytoplasm. The fractionation purity was confirmed by the lack of cross-contamination of cytoplasmic (glucose-6-phosphate dehydrogenase [G6PDH]) and nuclear (lamin B) controls. We observed identical fractionation profiles for M12.4 and BCL1 using an alternative protocol (32). Indeed, fractionation of any mouse or human B-cell line that expressed Bright (including 70Z, WEHI231, J558, Raji, and Daudi) as well as purified mouse splenic B cells revealed Bright accumulation to a various degree in both compartments (data not shown).
FIG. 1.
Nuclear and cytoplasmic localization of Bright. (A) Nuclear and cytoplasmic fractionation of Bright within B-cell lines BCL1 and M12.4. Five micrograms of nuclear or cytoplasmic proteins was analyzed by Western blotting with anti-Bright antiserum. The purity of the nuclear (lamin B) and cytoplasmic (G6PDH) fractions was confirmed by Western blotting of 20 μg of each extract with the appropriate antibodies. (B) Nucleocytoplasmic localization of Bright within mouse splenic B cells. Mouse CD43− splenic B cells were isolated from spleens using anti-CD43 antibody-conjugated magnetic beads. Costaining of the cells with anti-Bright (green, fluorescein isothiocyanate) and anti-lamin B (red, rhodamine) allowed discrimination between the nucleus and the relatively small volume of cytoplasm. DIC, differential interference contrast. (C) Cytoplasmic accumulation of Bright in a mature B-cell line, BCL1. Immunostaining with anti-Bright revealed various localization patterns, including a small portion of cells expressing Bright exclusively within their cytoplasm. (D) Cellular localization of GFP-Bright expressed ectopically in Cos-7 cells. The localization of GFP-Bright was determined at 24 h posttransfection of Cos-7 cells. W, dispersed across the whole cell; N, preferential nuclear localization; and C, preferential cytoplasmic localization. (E) Quantitative analysis of GFP-Bright and GFP-only localization in Cos-7 and NIH 3T3 cells after transient transfection. (F) Cellular localization of Bright ectopically and stably expressed in NIH 3T3 cells. Retroviral transduced, Bright-expressing NIH 3T3 cells or NIH 3T3 cells alone were immunostained with anti-Bright antiserum (red, rhodamine) and DAPI (blue). Population sizes are shown with the representative localization patterns. The data in panels E and F were quantified as described in Materials and Methods.
To confirm the fractionation results, we analyzed the localization of Bright at a single cell level using an immunostaining/fluorescence microscopic approach. While nuclear in the majority of splenic B cells, Bright could clearly be found in the cytoplasm of some cells (nucleocytoplasmic form in Fig. 1B). In BCL1 and M12.4, we consistently observed three patterns: preferentially nuclear, simultaneously in the nucleus and cytoplasm, or preferentially in the cytoplasm (cytoplasmic form in Fig. 1C).
Even though Bright is preferentially expressed in B lymphocytes in the adult mouse, it is expressed quite broadly in the developing embryo (41). Also, B cells pose strong technical limitations (e.g., extremely small cytoplasmic volume relative to nuclear volume and low transfection efficiency) for detailed structure-function analyses. Therefore, it was necessary to determine whether ectopic expression of Bright in more suitable non-B-cell lines or primary cells would mimic the localization phenotype seen in B cells. So, we expressed full-length Bright (residues 1 to 601) as an N-terminal GFP fusion protein (GFP-Bright) in the monkey kidney cell line, Cos-7. At 24 h posttransfection, the cellular localization of Bright was observed by fluorescence microscopy (Fig. 1D and E). As with B cells, three phenotypes were observed: accumulation in both the nucleus and the cytoplasm, preferentially within the nucleus, or preferentially within the cytoplasm. As expected, the GFP-only control was evenly distributed across the entire cell without any preferential localization. To address potential construct artifacts, we fused GFP to the C terminus of Bright (Bright-GFP) and observed a similar localization pattern (data not shown). Bright-GFP localized preferentially (∼65% of transfected cells) within the nucleus, with the other two phenotypes represented as smaller percentages. These phenotypes were observed regardless of the recipient transfected cell (e.g., NIH 3T3 in Fig. 1E and HeLa, data not shown), with preferential nuclear expression dominating.
To address potential artifacts of GFP fusion and cytomegalovirus promoter-mediated overexpression, we produced stably expressing Bright cell lines in NIH 3T3 cells (NIH 3T3-Bright) by retroviral infection of the full-length, untagged wild-type protein. Localization of Bright was detected by immunostaining using anti-Bright antiserum (Fig. 1F). About 70% of the cells expressed Bright in the nucleus, while Bright was distributed across the entire cell or restricted to the cytoplasm in ∼30% of cells.
Because the cell lines used for ectopic expression were either immortal (NIH 3T3) or transformed (HeLa and Cos-7), we could not exclude their malignant features (e.g., aneuploidy and deregulated growth) as important for the asymmetric nucleocytoplasmic localization of Bright. Therefore, we transiently transfected E13.5 MEFs with GFP-Bright or GFP only and examined their localization (data not shown). While GFP diffused across the whole cell, GFP-Bright localized in the nucleus and/or in the cytoplasm.
We conclude that regardless of whether measurements are performed on the endogenous or ectopically introduced protein, whether GFP tagged or wild type, and whether in primary or transformed cells, Bright localization is observed in both the nucleus and the cytoplasm in various ratios in asynchronously growing cells. This indicates that the factors required for asymmetric nucleocytoplasmic distribution of Bright are ubiquitous and that a transformed non-B-cell line such as Cos-7 provides an adequate model for further study.
Bright is exported from the nucleus to the cytoplasm under CRM1 control.
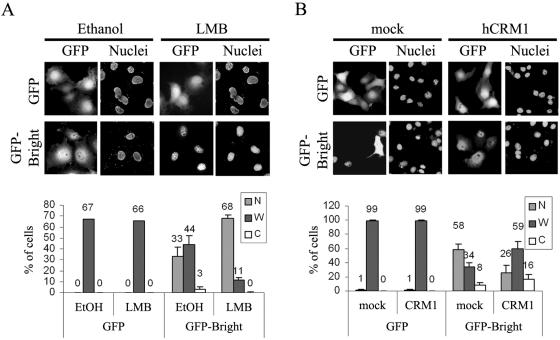
Because Bright localizations appeared to differ in individual cells in an asynchronous culture and a few cells expressed Bright exclusively in the cytoplasm, we reasoned that Bright might be translocated during cell growth by an active export system. We tested whether LMB, a specific and potent inhibitor of CRM1/exportin 1, blocks the nuclear export of GFP-Bright. The addition of LMB at concentrations (10 ng/ml) not toxic to cell growth (data not shown) resulted in the nuclear accumulation of GFP-Bright in Cos-7 cells while ethanol (solvent in which LMB is dissolved) had no effect (Fig. 2A). Ethanol or LMB treatment had no effect on GFP-only localization, indicating that the cytoplasmic export of Bright requires a functional CRM1. Cos-7 cells transiently cotransfected with CRM1 and GFP-Bright (Fig. 2B) significantly increased their cytoplasmic localization of GFP-Bright, while neither GFP alone nor mock transfections showed such effects. Therefore, Bright is actively exported from the nucleus to the cytoplasm by CRM1.
FIG. 2.
Bright is exported from the nucleus to the cytoplasm by CRM1. (A) LMB treatment produces enhanced nuclear accumulation of GFP-Bright. (Upper panel) Cos-7 cells were transiently transfected with GFP-Bright and after 12 h were treated with 10 ng/ml of LMB and incubated for another 12 h. The solvent used to dissolve LMB, ethanol (EtOH), was used for mock treatment. (Lower panel) Quantitation of increased nuclear localization of Bright after LMB treatment. (B) CRM1 enhanced the nuclear export of GFP-Bright. (Upper panel) Cos-7 cells were transiently cotransfected with GFP-Bright and CRM1, and GFP-Bright localization was observed at 24 h posttransfection. Cotransfection of Bright with pCR3.1 empty vector served as mock treatment. (Lower panel) Quantitation of increased cytoplasmic localization of Bright produced by CRM1 overexpression.
An NLS resides in the REKLESα subdomain of Bright.
As a first approach toward identification of the NLS for Bright, we constructed C-terminally truncated GFP-Bright mutants and analyzed their localization into Cos-7 cells at 24 h posttransfection (Fig. 3A and D). A short C-terminal truncation (residues 6 to 562) had no effect on the cellular localization of Bright (data not shown). Further truncation of the C terminus (residues 1 to 541) led to Bright accumulation within the nucleus in a significantly higher percentage of cells (∼70%) than that of wild-type GFP-Bright (∼32%), indicating that residues 542 to 562 are critical for cytoplasmic localization. Mutant 1-493, in which the C-terminal half of the REKLES domain was deleted, still accumulated in the nucleus of ∼80% of transfected cells. However, the localization of mutant 1-381, in which the entire REKLES domain was deleted, was evenly distributed across the nucleus and cytoplasm of ∼98% of transfected cells, i.e., the same pattern as that seen for GFP alone. This indicated that the region between residues 381 and 493 is required for NLS activity. As expected from these results, the cellular localization of mutant 1-269 was also evenly distributed within all cells (data not shown). Because the 1-381 and 1-269 mutants had no preferential localization within either the nucleus or the cytoplasm, we suspect that their appearance in both compartments reflects unregulated diffusion through nuclear pores.
FIG.3.
NLS and NES activities of Bright reside within different regions of the REKLES domain. (A) Schematic of Bright domains (boxes) and truncation mutants. REKLES is divided into N-terminal (α) and C-terminal (β) subdomains. The cellular localization phenotypes (W, N, and C; described in previous legend and in Materials and Methods) of each mutant analyzed with or without (mock) the addition of 10 ng/ml of LMB are shown to the right of each construct tested. The first character represents the most frequently observed pattern. Boxes at the bottom represent the minimal regions mapped for NLS and NES activities. (B, upper panel) The REKLESα subdomain and residues that are required for the nuclear import of Bright. Bold enlarged letters denote residues whose mutation abolished nuclear import. Mutation of the K (small bold) within the REKLES motif (underlined) had no effect on nuclear import. (Lower panel) Schematic of point mutation constructs and phenotypes. The vertical lines denote the positions of mutations within the REKLESα subdomain. (C) Residues within the REKLESβ subdomain required for the nuclear export of Bright. Point mutations, their positions, and localization phenotypes are represented as in panel B. (D) Exemplary images of the cellular localization of GFP-Bright and selected mutants collected 24 h posttransfection of Cos-7 cells.
A series of GFP-fused, N-terminal truncation mutants were examined as described above (Fig. 3A and D). Mutant 117-589 exhibited all three localization phenotypes (N, W, and C) in a manner indistinguishable from that of wild-type Bright (data not shown). However, mutant 238-601, which lacks a highly acidic region (residues 128 to 164 in Fig. 3A) of unknown function, accumulated preferentially in the cytoplasm in ∼65% of the transfected cells, but no preferentially nuclearly accumulating cells were observed. Surprisingly, the deletion of another 157 residues (mutant 395-601) restored the localization pattern of this mutant to that of wild-type Bright. Further deletion (residues 450 to 601) increased the preferential nuclear localization of Bright within ∼70% of transfected cells. A shorter mutant (mutant 500-601) accumulated in the cytoplasm in ∼40% of transfected cells. Even though ∼60% of mutant 500-601-transfected cells expressed the protein in both the nucleus and the cytoplasm, we were unable to detect any cells in which the 500-601 mutant accumulated exclusively in the nucleus. This indicated that the deletion within mutant 500-601 removed a functional NLS. In addition, the concomitant increase in cytoplasmic accumulation of mutant 500-601 indicated that it might be actively exported out of the nucleus. Because mutants 450-601 and 1-493 localized preferentially within the nucleus, we concluded that residues 450 to 493, the N-terminal half of the REKLES domain, are necessary for the regulated nuclear localization of Bright.
Next, we examined whether mutant 450-493 is both necessary and sufficient for NLS activity (Fig. 3A and D). Contrary to our expectation, mutant 450-493 was evenly distributed in virtually every transfected Cos-7 cell. One possible explanation for this is that residues 450 to 493, when removed from their wild-type context within the conserved REKLES domain, are insufficient to adopt an appropriate secondary conformation. To test this, we examined the localization of the entire REKLES domain in mutant 450-541, in which the C-terminal endpoint of the 450-to-493 truncation is extended by 48 amino acids. As hypothesized, mutant 450-541 accumulated preferentially in the nucleus in ∼87% of the transfected cells. However, extension of residues 450 to 493 in the other (N-terminal) direction (mutant 395-493) resulted in a whole-cell distribution indistinguishable from that of mutant 450-493 (data not shown).
We introduced internal deletions (Fig. 3A and D) to more precisely define the region within REKLES that is important for the nuclear localization of Bright. Deletion of the N-terminal region of the REKLES domain (mutant d453-500) led to cytoplasmic accumulation of the mutant in ∼85% of the transfected cells. Treatment with LMB did not induce the nuclear retention of this mutant, indicating that residues 453 to 500 are required for the nuclear entry of Bright.
The REKLES domain was named for an amino acid motif (Fig. 3B) which is relatively conserved only among ARID3 paralogues and orthologues (42). The domain has been operationally divided into N-terminal (REKLESα) and C-terminal (REKLESβ) subdomains (Fig. 3A) (23). REKLESα, which was implicated directly by the results described above, contains no classical NLS-like sequences (Fig. 3B). Nevertheless, site-directed mutagenesis showed that two lysines within REKLESα were required for nuclear entry of Bright (Fig. 3B and D). Point mutation K466A or K467A abolished the nuclear localization of Bright and resulted in cytoplasmic accumulation of the protein in ∼86% of transfected cells. Importantly, LMB treatment could not retain K466A in the nucleus (data not shown). This strongly supported the contention that the cytoplasmic accumulation of this point mutant was caused by the abolishment of its NLS, as opposed to enhancement of its export activity. However, K457A, which alters the K within the REKLES motif itself, had no effect on the localization pattern of Bright. Mutation of the nearby P463 to A, which is also conserved in Bright homologues, abolished the nuclear import of Bright and further emphasized the importance of REKLESα for the nuclear localization of Bright.
We conclude that Bright has a functional NLS between residues 450 and 493 of its REKLESα that requires at least two noncanonical (with respect to SV40-type motifs) lysines for nuclear entry. In addition, our data raise the possibility that the remaining C terminus, the acidic domain, and even the ARID itself contribute to Bright's complex cellular localization.
Nuclear export of Bright requires the REKLESβ subdomain and the 19 amino acids downstream.
Because the loss of the REKLESβ domain in mutant 1-493 resulted in nuclear accumulation and because the C-terminal region of Bright (mutant 500-601) was sufficient for cytoplasmic accumulation, we suspected that the nuclear export signal(s) might reside in the C-terminal region of Bright. To test this, we transiently transfected Cos-7 cells with truncation mutants that localize to the cytoplasm (Fig. 3A and D). At 12 h posttransfection, we treated the cells with 10 ng/ml LMB for 12 h and then observed the localization of the mutant proteins (Fig. 3A). GFP localized across the entire cell irrespective of LMB treatment. While LMB had no effect on mutant 1-381, the nuclear accumulation of mutant 395-601 was significantly increased. LMB also enhanced the nuclear appearance of mutant 500-601, while this small, NLS-deficient protein was preferentially detected in the cytoplasm with ethanol treatment. Likewise, a similar phenotype for the even shorter 500-to-562 truncation can be rationalized in the same manner, implying that the amino acids from residues 500 to 562 were sufficient for NES.
The 63 residues comprising the REKLESβ subdomain (residues 500 to 562) do not encode a canonical leucine repeat-type nuclear export signal (Fig. 3C and data not shown). So, we constructed a series of internal deletions (Fig. 3A and D). The deletion from 521 to 541 (mutant d521-541) accumulated in the nucleus in ∼86% of transfected cells, while mutant d500-521 localized indistinguishably from wild-type Bright in the cytoplasm and/or in the nucleus. Because mutant d521-541 retains functional NLS activity, its nuclear accumulation in most transfected cells would most likely result from the loss of its NES activity. Within this region, all Bright homologues have conserved a cluster of residues: G532, Y535, G537, and L539. A single point mutation of any of these residues to alanine abolished nuclear export activity, i.e., resulted in the nuclear accumulation of the mutants (Fig. 3C and D). However, the conservative substitution Y535F had no effect on Bright localization, indicating that the planar conformation—not the charge—of this residue is crucial for the nuclear export activity of Bright.
We conclude that Bright is actively exported through CRM1-dependent interactions mediated by the REKLESβ subdomain. We further suggest that the variable nuclear and cytoplasmic phenotypes observed for the endogenous protein or the ectopic protein observed early (24 h) following transient transfection are a consequence of alteration of the relative efficiencies of Bright's nuclear import and export activities.
Bright shuttles between the nucleus and the cytoplasm.
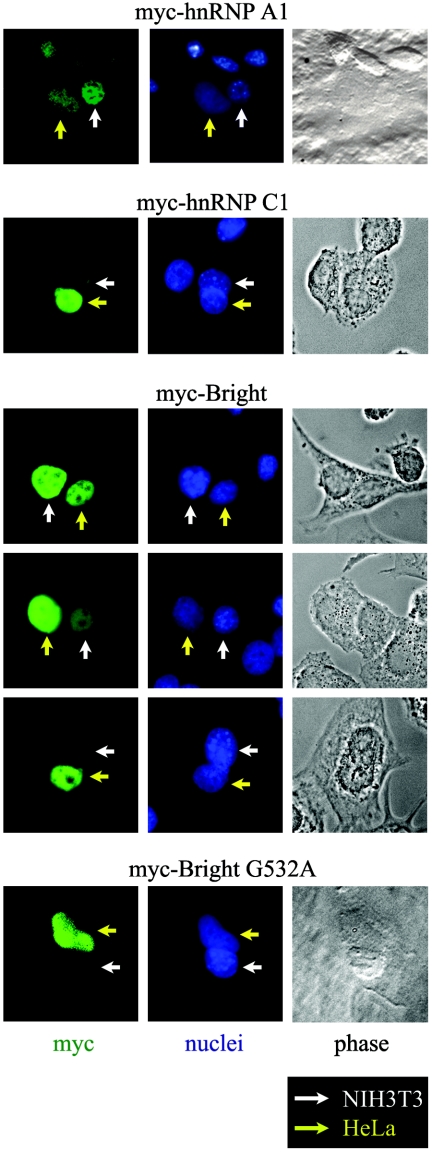
Shuttling proteins require NLS and NES activities, but the presence of an NLS and NES does not necessarily establish shuttling ability. Therefore, we investigated whether Bright shuttles between the nucleus and the cytoplasm by performing heterokaryon fusions between murine and human derived cell lines that allow discrimination between the nuclei by Hoechst staining. We transiently transfected human HeLa cells with an N-terminal myc-tagged full-length Bright (myc-Bright) and fused the transfectants to untransfected mouse-derived NIH 3T3 cells. As controls, we employed HeLa transfections of previously characterized shuttling (myc-hnRNP A1) or nonshuttling (myc-hnRNP C1) myc-tagged constructs (44). As expected, myc-hnRNP A1 was transferred into the NIH 3T3 nuclei of the HeLa NIH 3T3 heterokaryons, while myc-hnRNP C1 was detected only in the HeLa nuclei (Fig. 4). Although the intensity of the Bright signals varied among individual heterokaryons, myc-Bright was clearly detected in NIH 3T3 nuclei. As further confirmation, we observed that the shuttling activity of the NES-incompetent G532A mutant was severely reduced (Fig. 4). These data provide formal evidence that Bright shuttles between the nucleus and the cytoplasm.
FIG. 4.
Bright shuttles between the nucleus and cytoplasm. HeLa cells were transiently transfected with myc-Bright wild type or the NES-deficient G532A mutant and then fused with NIH 3T3 cells. Bright proteins were detected by immunostaining the resulting heterokaryons with anti-myc monoclonal antibody. myc-hnRNP A1 was previously shown to be a shuttling protein, whereas myc-hnRNP C1 served as a nucleus-only control (44).
Nuclear accumulation of Bright results from reduced nuclear export activity.
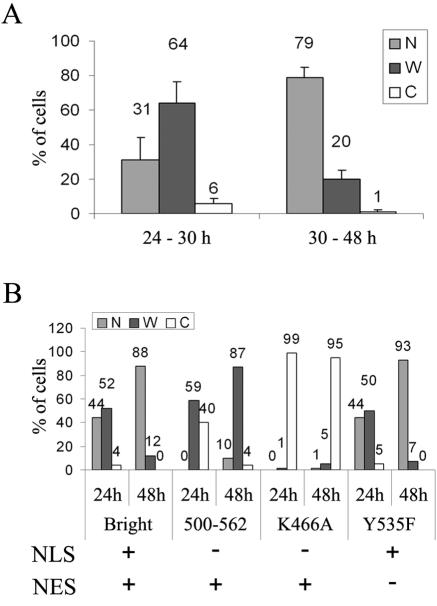
When endogenously or ectopically expressed in any asynchronously growing cells, Bright localizes in variable patterns. We found that the time following initiation of Bright ectopic expression strongly influenced its localization. Up to 30 h posttransfection, Bright localized within both the nucleus and the cytoplasm in a significant proportion of transfected Cos-7 cells. However, after 30 h, most cells contained Bright in their nuclei (Fig. 5A). GFP-only localization was unaffected by incubation times (data not shown), indicating that this shift was specific for Bright. We found no evidence that this post-30 h nuclear accumulation was caused by differential protein expression, growth factor starvation, or contact inhibition during prolonged cultures (i.e., carried out for 3 to 4 days following serum refreshment and splitting of the cells) (data not shown).
FIG. 5.
Reduction in NES activity can lead to enhanced nuclear accumulation of ectopically expressed Bright following prolonged culture. (A) Summary of localization phenotypes for wild-type GFP-Bright analyzed early (24 to 30 h) or late (30 to 48 h) posttransfection of Cos-7 cells. (B) Comparison of localization phenotypes in short and prolonged cultures of the wild type and localization mutants in transiently transfected Cos-7 cells. Functions retained (+) or lost (−) in each protein are indicated below the corresponding data.
To investigate the mechanism contributing to the increased nuclear/cytoplasmic ratios of Bright described above, we monitored the cellular localization of Bright wild type and selected localization mutants at 24 or 48 h posttransfection of Cos-7 cells (Fig. 5B; Table 1). As expected, we observed enhanced nuclear appearance at 48 h for wild-type Bright and the Y535F mutant, which are both NLS and NES competent. However, the NLS-deficient K466A mutant continued to accumulate in the cytoplasm at 48 h, indicating that the nuclear accumulation of Bright at 48 h requires NLS activity. The nuclear localization of mutant 500-562, which lacks an NLS but contains full NES activity, is also enhanced at 48 h. But, in spite of the presence of an NES, if increased nuclear Bright results only from enhanced NLS activity, then this mutant should not be imported into the nucleus but should remain in the cytoplasm at 48 h posttransfection.
TABLE 1.
Nuclear accumulation of Bright depends on reduced NES activitya
| Wild type or mutant | Increased cytoplasmic localization with CRM1 | Increased nuclear localization with LMB | Increased nuclear localization at 48 h |
|---|---|---|---|
| GFP | − | − | − |
| Bright | + | + | + |
| 395-601 mutant | ND | + | + |
| 238-601 mutant | ND | + | + |
| 500-562 mutant | − | + | + |
| 450-541 mutant | +/− | ND | ND |
| K466A mutant | ND | − | − |
| Y535F mutant | ND | + | + |
+, change of localization pattern in >20% of cells; +/−, change of localization pattern in >10% of cells; −, no change of localization pattern or change in <10% of cells; and ND, not determined.
These results indicate that reduced NES activity is responsible for the increased nuclear localization of Bright at 48 h. However, we cannot exclude the possibility that, at least in the NLS-containing constructs listed in Table 1, an enhanced NLS activity functioned in concert with reduced export activity to increase nuclear Bright levels at the later time point.
Nuclear export and import of Bright can be modulated in serum-depleted B-cell cultures.
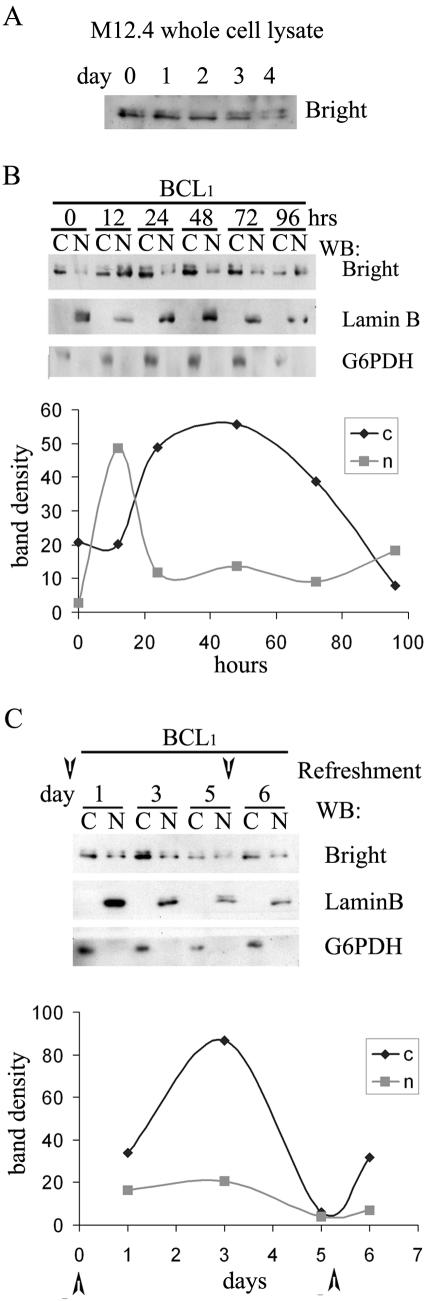
Mitogen and growth factor stimulation have been shown to increase Bright DNA binding in normal B cells and in B-cell lines, such as M12.4 and BCL1, that are responsive to these inductive stimuli in culture (41). However, we found that this effect results from increased overall expression of Bright and not from enhancement of its nuclear/cytoplasmic ratio (data not shown). Therefore, we investigated the reverse effect of growth factor withdrawal on Bright localization. Traditional serum starvation of the B-cell lines that we employed was toxic to their growth; for example, with M12.4 cells, aberrant chromosomal nondisjunction was observed (data not shown). So, we adopted a less severe deprivation protocol in which B cells were cultured under normal conditions for 5 days without serum replenishment. Aliquots were removed from the culture periodically for subcellular fractionation and Western analysis. We observed that total Bright expression in BCL1 and M12.4 cells gradually decreased for 4 days after replenishment (Fig. 6A and data not shown for BCL1). However, Bright levels within both the nuclear and cytoplasmic fractions of BCL1 fluctuated as a function of deprivation time (Fig. 6B). Following an initial burst of nuclear import within the first 12 h, nuclear accumulation rapidly decreased to a level that was still elevated relative to that at time zero (serum addition). That this decline resulted from nuclear export is suggested by the reciprocal rise in cytoplasmic accumulation over the same (10 to 24 h) time interval. Over the next 3 days of fasting, nuclear Bright levels were maintained while cytoplasmic levels gradually declined. We observed equivalent localization behavior in fasted M12.4 B cells despite the gradual decrease of total Bright levels after replenishment (data not shown). Replenishing cells at day 5 rescued cytoplasmic expression but not at the expense of nuclear depletion (Fig. 6C). These results indicate that Bright localization is regulated in B cells under conditions in which growth factors are limiting.
FIG. 6.
Fluctuation in the nucleocytoplasmic ratio of Bright in BCL1 B cells is induced by serum deprivation. (A) Overall Bright expression level is gradually reduced following replenishment. M12.4 cells were grown for 4 days without replenishment, and whole-cell lysates were prepared at the indicated time points. Five micrograms of lysates was analyzed for Bright expression. Equivalent protein loading among lanes was confirmed by ink staining (not shown). (B) Alteration of nuclear/cytoplasmic ratio of Bright in BCL1 B cells. (Upper panel) Western analysis of subcellular fractionation of Bright and nuclear (lamin B) or cytoplasmic (G6PDH) controls. Samples were prepared at 12-h intervals after replenishment (time zero). (Lower panel) Quantitation (Scion Image) of the data indicates an early (12 to 24 h) export-import phase, followed by a prolonged reduction in cytoplasmic Bright over 96 h. (C) Recovery of cytoplasmic expression of Bright following replenishment of cells. BCL1 cultures were manipulated as in panel B and then at day 5 were replenished with medium. Fractionations (at the indicated time points) and quantitation were performed as described above. Arrowheads represent the time points of replenishment.
Cellular localization of Bright is altered during S phase.
We rationalized that the change in Bright localization during S phase might correspond to active nucleocytoplasmic shuttling at a relative NES/NLS rate required to maintain minimal Bright nuclear function under the stress of growth factor depletion. To address this, Cos-7 cells were transiently transfected with GFP-Bright, and their cell cycle was analyzed by propidium iodide staining and fluorescence-activated cell sorter analysis (Fig. 7A). At 12 h posttransfection (Fig. 7A, left panel), most GFP-Bright-expressing cells (solid curve) were in S phase. By 24 h (Fig. 7A, right panel), the majority of the GFP-Bright population had progressed into G2/M. Because a similar cell cycle pattern was observed in GFP-only-expressing cells (data not shown), the enrichment in S-phase cells at 12 h is not a consequence of Bright expression. Meanwhile, during this 12- to 24-h interval, LMB treatment retained Bright (but not the GFP control) within the nucleus (Fig. 2A). These results indicate that Bright is actively imported into and exported out of the nucleus (shuttling) during S phase.
FIG. 7.
Cellular localization of Bright is altered during S phase. (A) GFP-Bright-expressing Cos-7 cells are in S phase between 12 to 24 h posttransfection. DNA content of transfected Cos-7 cells was analyzed at the indicated time points through propidium iodide staining and fluorescence-activated cell sorter analysis. (B) Nuclear/cytoplasmic ratio of Bright increases during S phase in BCL1 cells. (Upper panel) Cell cycle analysis after synchronization of BCL1 cells at G1/S with serum reduction and aphidicolin treatment. Four hours after release of the cell cycle block, a significant number of the cells are in S phase. (Middle panel) Bright fractionation at the indicated times after release of the cell cycle block. Five micrograms of nuclear or cytoplasmic proteins was analyzed by Western blotting. (Lower panel) Quantitation (Scion Image) of the data indicates an increase in the nuclear/cytoplasmic ratio of Bright at 4 h after removing aphidicolin, coinciding with the majority of cells being in early S phase.
Next, we examined Bright localization during S phase following synchronization of BCL1 B cells at G1/S. At 4 h after release of the cell cycle block, a significant number of the cells were in S phase (Fig. 7B, upper panel). Biochemical fractionation followed by Western analysis demonstrated an increase in the nuclear/cytoplasmic ratio of Bright at 4 h after removing aphidicolin, coinciding with the majority of cells being in early S phase (Fig. 7B, middle and lower panels). The Bright expression level and the nuclear/cytoplasmic ratio decreased at 6 h after release of the cell cycle block. These data indicate that endogenous Bright localization is actively regulated during S phase in B cells.
MAR-mediated transactivation by Bright is regulated by its cellular localization.
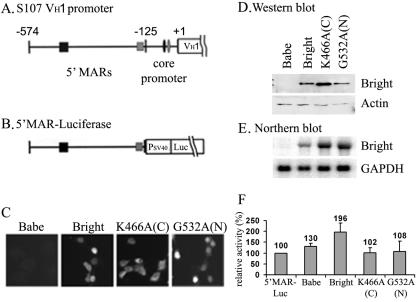
Bright binds and (presumably) transactivates via IgH-associated promoter and enhancer MARs as a homo-oligomer (16). So, in order to test the impact of Bright shuttling on its transcriptional activity, we required a system in which endogenous Bright contribution was eliminated. Otherwise, heteromeric REKLES-mediated interaction of localization mutants and endogenous wild-type Bright would obscure results. Furthermore, we found previously that maximal MAR-mediated activity in B cells required chromosomally integrated reporters (21). Therefore, we appended the region spanning the two VH1 promoter-associated MAR binding sites (Fig. 8A) to the minimal SV40 promoter driving pGL3 firefly luciferase (5′MAR-Luc) (Fig. 8B) and cotransfected the construct and a neomycin mini vector, OSneo, stably into NIH 3T3 cells. Neomycin-resistant, luciferase-expressing cell lines were selected and then retrovirally transduced with either wild-type Bright, an NLS point mutant (K466A) whose expression is restricted to the cytoplasm, an NES point mutant (G532A) that accumulates exclusively in the nucleus, or the murine leukemia virus-based pBabe vector alone (Fig. 3 and 8C).
FIG. 8.
Shuttling activity is required for Bright to transactivate an integrated IgH promoter-associated MAR reporter in NIH 3T3 cells. (A and B) Schematic of (A) the S107 VH1 5′ upstream region and (B) the 5′ MAR luciferase reporter construct. Squares represent MARs previously shown (21) to contain specific Bright binding sites (centered at −574 and −125 with respect to transcriptional start of VH1) required for promoter activation by Bright in B cells. Small black and gray ovals denote octB and octamer binding sites. (C) Cellular localization of Bright wild type and mutants stably expressed in NIH 3T3 cells bearing integrated 5′ MAR luciferase reporters. Cytoplasmic-restricted expression (C) of NLS point mutant K466A, nuclearly restricted expression (N) of NES point mutant G532A, and variable expression of the wild type were confirmed by anti-Bright immunostaining. (D and E) Wild-type and mutant Bright expression levels in 5′ MAR luciferase/NIH 3T3. For the Western blot shown in panel D, 5 μg of each whole-cell lysate was analyzed with anti-Bright, and 20 μg of each lysate was used for the antiactin loading control. For the Northern blot shown in panel E, total RNA was hybridized with Bright or GAPDH-specific probes. (F) Relative Bright transactivation in each 5′ MAR luciferase/NIH 3T3 cell line. Luciferase activities were measured from 10 μg of whole-cell extract prepared from Bright wild-type or mutant transductants. Babe denotes mock infection with the pBabe retroviral backbone (10). K466A is the cytoplasmically restricted (C) mutant, and G532A is the nuclearly restricted (N) mutant. Each column and vertical bar represent the mean and standard deviation from four independent cultures of retrovirally transduced cell lines, respectively. Bright, P < 0.05; others, P > 0.05.
Because puromycin-resistant cells were bulk selected, total expression levels of Bright or Bright mutants were analyzed by Western and Northern blotting (Fig. 8D and E). Luciferase activities were then measured and normalized. Although the magnitude of transactivation was about half of that observed in B cells (21), we obtained reproducible and statistically significant results (Fig. 8F). Wild-type Bright consistently transactivated the integrated 5′ MAR reporter about twofold more than the level observed in the parent cell line (P < 0.05). In spite of the higher whole-cell expression level of cytoplasmically restricted Bright (K466A) protein and mRNA (Fig. 8D and E), this mutant had no effect on reporter gene expression (Fig. 8F). But, unexpectedly, neither did the exclusively nucleus-only G532A mutant, whose overall expression is equivalent to the wild type (Fig. 8D and E).
Because both mutant proteins could self-associate and bind to the MARs (data not shown), these results indicate that the regulation of nucleocytoplasmic shuttling of Bright contributes to the control of IgH gene transcription. However, nuclear localization per se was insufficient for its transcriptional activation. It implies that Bright may have to shuttle to efficiently activate immunoglobulin promoters or that G532 may participate in unknown functions required for the transactivation activity of Bright.
DISCUSSION
Bright was previously characterized as an IgH transactivator and MAR binding protein which functions in the nucleus (reviewed in reference 41). Mouse Bright and its human orthologue, Dril1, interact with LYSp100, Sp100, and PML, components of PML-NBs nuclear dot structures (12, 49). Therefore, we were surprised to discover that a vast amount of Bright partitioned into the cytoplasm in every murine and human B-cell line that expresses the protein as well as in purified splenic B cells. Bright expressed ectopically, either transiently or stably in primary or transformed cells, partitioned within both compartments at variable ratios. However, the highly similar Bright paralogue, Bdp (26), localized exclusively within the nucleus (data not shown). Therefore, the cytoplasmic localization of Bright seems to be a characteristic of the Bright protein itself—not the ARID family or the cell in which Bright is expressed.
Our experiments with LMB treatment and CRM1 overexpression indicated that Bright is actively exported from the nucleus to the cytoplasm, resulting in its cytoplasmic appearance. In addition, heterokaryon experiments indicated that the fate of at least some Bright molecules that exit the nucleus is to shuttle back again, formally establishing a potential for regulated nucleocytoplasmic shuttling. This suggests that Bright has evolved an active nuclear export mechanism to execute a cytoplasmic function, as opposed to merely a means to down-regulate unfavorably high nuclear levels. One obvious rationale is to facilitate Bright's interaction with the cytoplasmic tyrosine kinase, Btk. Btk is essential for several phases of antigen receptor-mediated signal transduction (reviewed in reference 41), and its association with Bright enhances DNA binding and transactivation (31). Perhaps by passing through the cytoplasm, Bright might also serve as a transporter of Btk to essential nuclear phosphorylation targets, such as TFII-I (41).
There are 15 ARID family genes in humans and mice, but the REKLES domain is conserved only among the 3 ARID3 members (42). REKLES was shown to be required for the self-association of Bright and, consequently, for its in vitro MAR binding activity (16). REKLES is also required for Bright interactions with coactivators and repressors (49). Here, we show that REKLES is essential for both nuclear import and export of Bright, further extending the multifunctional nature of this domain. Even though the REKLES domain contains no classical NLS motifs, several residues (P463, K466, and K467) within the N-terminal or REKLESα subdomain were required for nuclear import. These noncanonical lysines, nonetheless, might interact directly with importins in a manner analogous to that of prototypic NLS basic residues (reviewed in reference 18).
We suspect that other domains within Bright contribute to its REKLESα-mediated NLS activity (Fig. 9A). The deletion of the acidic domain (e.g., mutant 395-601 in Fig. 2 and mutant 290-541, data not shown) abolished the nuclear accumulation of these mutant proteins and increased the cytoplasmic accumulation despite the presence of the NLS-bearing REKLESα domain. This seemed to result from abolished (or reduced) NLS activity, because LMB did not induce the nuclear accumulation of these mutant proteins. Moreover, a further deletion in mutant 395-601 restored the nuclear import activity of the mutant proteins, implying that mutant 290-394 interfered with the NLS activity of REKLESα. However, the presence of the acidic domain appeared to suppress the blocking effect exerted on the NLS by the C-terminal region of the ARID (residues 290 to 395) (data not shown). While we cannot rule out artificial effects of these deletions, the data systematically indicate that the C-terminal region of the ARID and the acidic domain contribute to the regulation of the nuclear import of Bright and, consequently, the cellular localization of Bright (Fig. 9A).
FIG. 9.
Functional domains and hypothesized regulation of Bright nucleocytoplasmic shuttling. (A) Domains contributing to Bright localization. In addition to motifs within the REKLESα (NLS-required) and REKLESβ (NES-required) subdomains, data from mutants shown in Fig. 3 indicate that regions (lower boxes) partially overlapping the ARID and the acidic domain (upper boxes) affect cellular localization of Bright through inter- or intramolecular interactions (arrows). Protein Y represents an unknown factor(s) required to mediate nuclear export in lieu of a conventional NES motif within REKLESβ. (B) Hypothesized regulation of Bright localization during B-cell growth under limiting growth factor conditions (fasting). At the top is a reproduction of Bright subcellular fractionation in serum-deprived BCL1 cells. This fluctuation pattern may be controlled by two distinct mechanisms. First, nuclear export may be regulated. After replenishing cells with growth factors, overall Bright expression (denoted by the number of circles) increases, but the nuclear export activity remains low, resulting in a unidirectional (small arrows at 12 h) nuclear accumulation of Bright. Shortly thereafter, robust nuclear export activity (large arrows) results in a rapid (day 1, 2) accumulation of Bright in the cytoplasm. However, our data indicate that a pool of Bright may remain behind, by virtue of interaction with an unknown nuclear factor(s) (X) that mediates a second control mechanism, nuclear retention. This ensures that a minimal level of Bright must be retained in the nucleus irrespective of its overall expression level. The remaining Bright pool undergoes nucleocytoplasmic shuttling (bidirectional arrows), which persists throughout the course of the fasting/growth factor-depleting culture.
The NES activity of Bright was mapped to the more C-terminal REKLESβ subdomain and the adjacent C-terminal 19 amino acids. However, CRM1 overexpression can force cytoplasmic export of a mutant (mutant 450-541) (data not shown) which lacks the downstream 19 residues. Similarly, the nucleus-only ARID3 paralogue, Bdp, shares no conservation of the downstream region (26); yet, it too can be exported by CRM1 overexpression, albeit to a significantly lower extent (data not shown). These results indicate that the REKLESβ domain is sufficient for minimal NES activity, whereas the C-terminal 19 residues contribute to maximal efficiency. REKLESβ contains no prototypic leucine-rich NES motif, although several residues (Y535, G532, G537, and L539) conserved between Bright and Bdp were required for Bright nuclear export. We speculate that these residues constitute part of an interaction surface for adapters or other types of proteins that provide NES motifs in trans (protein Y in Fig. 9A).
Heterogeneous localization patterns of Bright were detected after prolonged and/or growth factor-deficient cell culture of B cells and transfected Cos-7 cells (Fig. 5 and 6). Analysis of NLS and NES mutants indicated that loss of nuclear export and not gain of nuclear import activity was crucial for this effect in Cos-7 cells. Our model to explain the various localization patterns of Bright in B cells is shown in Fig. 9B. The initial nuclear surge, followed by a cytoplasmic surge, might result from regulated nuclear export activity. We suggest that the subsequent phase of prolonged nuclear maintenance and the cytoplasmic reduction of Bright could be accomplished by the existence of a pool of nuclear Bright shielded from the shuttling machinery. A putative factor (X in Fig. 9B) might facilitate nuclear retention such that when bound to Bright, this complex remains sequestered in the nucleus. Such a retention function has been proposed previously for nuclear matrix-associated proteins p54nrb and matrin in sequestering early polyoma RNA transcripts in the nucleus (46). Even though the total expression level of Bright may be reduced in the fasted cells, the nuclear import, export, and retention activities are balanced so as to maintain a minimal required level of nuclear Bright for 5 days.
Factors involved in cell cycle control, such as Cdc25B (43), Cdc25C (33, 37), cyclin B (15, 37), and cyclin D1 (1, 2), were reported to be regulated by cellular localization and to shuttle constantly between the nucleus and cytoplasm. Their localization is thought to be controlled by a balance of nuclear import and export activities. Bright localization shares some of these features. As shown in our LMB (Fig. 2A) and cell cycle (Fig. 7A) experiments, Bright appears to be shuttling actively within transfected Cos-7 cells during S phase. Furthermore, S-phase BCL1 B cells displayed an elevated nuclear/cytoplasmic ratio of Bright, which decreased as the majority of the cell population progressed into G2/M (Fig. 7B). These observations contribute to the growing evidence of Bright involvement in cell cycle regulation (12, 29, 35). Senescence rescue appears to require activation of E2F1 through a cyclin E1-dependent mechanism (29). E2F1 is almost exclusively nuclearly localized. Perhaps the cytoplasmic localization of Bright could be critical for the timing of its proposed interaction with E2F1 (35). That is, a cytoplasmic localization for Bright in G1 would be an ideal location if Bright's job were to sequester hypophosphorylated pRb from potential E2F/pRb repressive complexes in the nucleus.
If nuclear localization is the only mechanism by which Bright regulates its target genes, then a form constitutively localized to the nucleus should be an equivalent or possibly an even more efficient transactivator than the shuttling, wild-type form of Bright. To address this, we employed an NIH 3T3 cell system with stable integration of a reporter (luciferase) driven by the S107 VH1-associated 5′ MARs and stable transduction with Bright wild-type or mutant retroviruses. We found that nuclear localization of Bright in itself is insufficient for transactivation activity. The NES-deficient G532A mutant, which accumulated in the nucleus at levels equivalent to that of the wild type, failed to transactivate the integrated 5′ MAR target. The G532A mutant is fully competent to oligomerize and to bind DNA (data not shown). Therefore, either Bright must shuttle through the cytoplasm to transactivate or this residue within the REKLESβ is essential for a yet-to-be-determined function (e.g., a heterologous protein-protein interaction) required for the transactivation activity.
A number of signaling pathways regulate transcription factors by controlling their localization through nucleocytoplasmic shuttling (reviewed in reference 48). This has become increasingly evident with inducible factors, such as NFAT, NF-κB, and Pho4. What are their fates if rendered constitutively nuclear? Nucleus-only NFATs are strongly transactivating (27). However, translocation of NF-κB or Pho4 into the nucleus in itself is insufficient to drive target genes (22, 47). The common control feature of these and most other shuttling proteins is phosphorylation. The phosphorylation events that modify each of the examples cited above have unique and separable roles in regulating export, import, and ability to activate transcription in the nucleus (48). Although Bright interacts with the cytoplasmic tyrosine kinase Btk (40), it has not been shown to be phosphorylated by Btk (40) or by any other tyrosine or serine/threonine kinases, as judged by conventional immunoprecipitation/Western and in vitro assays (C. Webb, personal communication; P. W. Tucker, unpublished results). At our current level of resolution, the nuclear and cytoplasmic forms of Bright are indistinguishable. Thus, another mechanism, distinct from phosphorylation, is likely to regulate Bright localization and is intimately linked to its transcriptional control as well.
Acknowledgments
We thank Chhaya Das and Maya Ghosh for superb technical assistance and members of our laboratory for critical comments on the manuscript.
The research was supported by the NIH (CA31534 and AI64886) and the Mary Betzner Morrow Centennial Endowment in Molecular Genetics to P.W.T.
REFERENCES
- 1.Alt, J. R., J. L. Cleveland, M. Hannink, and J. A. Diehl. 2000. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14:3102-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. [DOI] [PubMed] [Google Scholar]
- 3.Avitahl, N., and K. A. Calame. 1996. 125 bp region of the Ig VH1 promoter is sufficient to confer lymphocyte-specific expression in transgenic mice. Int. Immunol. 8:1359-1366. [DOI] [PubMed] [Google Scholar]
- 4.Benditt, J. O., C. Meyer, H. Fasold, F. C. Barnard, and N. Riedel. 1989. Interaction of a nuclear location signal with isolated nuclear envelopes and identification of signal-binding proteins by photoaffinity labeling. Proc. Natl. Acad. Sci. USA 86:9327-9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc, V., S. Kennedy, and N. O. Davidson. 2003. A novel nuclear localization signal in the auxiliary domain of apobec-1 complementation factor regulates nucleocytoplasmic import and shuttling. J. Biol. Chem. 278:41198-41204. [DOI] [PubMed] [Google Scholar]
- 6.Bogerd, H. P., R. A. Fridell, R. E. Benson, J. Hua, and B. R. Cullen. 1996. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell. Biol. 16:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Callery, E. M., J. C. Smith, and G. H. Thomsen. 2005. The ARID domain protein dril1 is necessary for TGFβ signaling in Xenopus embryos. Dev. Biol. 278:542-559. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright, P., and K. Helin. 2000. Nucleocytoplasmic shuttling of transcription factors. Cell. Mol. Life Sci. 57:1193-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christophe-Hobertus, C., V. Duquesne, B. Pichon, P. P. Roger, and D. Christophe. 1999. Critical residues of the homeodomain involved in contacting DNA bases also specify the nuclear accumulation of thyroid transcription factor-1. Eur. J. Biochem. 265:491-497. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, R. L., P. Broeker, and C. R. Ashman. 1988. DNA base sequence changes and sequence specificity of bromodeoxyuridine-induced mutations in mammalian cells. Proc. Natl. Acad. Sci. USA 85:4406-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida. 1997. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308-311. [DOI] [PubMed] [Google Scholar]
- 12.Fukuyo, Y., K. Mogi, Y. Tsunematsu, and T. Nakajima. 2004. E2FBP1/hDril1 modulates cell growth through downregulation of promyelocytic leukemia bodies. Cell Death Differ. 11:747-759. [DOI] [PubMed] [Google Scholar]
- 13.Goebel, P., A. Montalbano, N. Ayers, E. Kompfner, L. Dickinson, C. F. Webb, and A. J. Feeney. 2002. High frequency of matrix attachment regions and cut-like protein x/CCAAT-displacement protein and B cell regulator of IgH transcription binding sites flanking Ig V region genes. J. Immunol. 169:2477-2487. [DOI] [PubMed] [Google Scholar]
- 14.Gronowicz, E. S., C. A. Doss, F. D. Howard, D. C. Morrison, and S. Strober. 1980. An in vitro line of the B cell tumor BCL1 can be activated by LPS to secrete IgM1. J. Immunol. 125:976-980. [PubMed] [Google Scholar]
- 15.Hagting, A., C. Karlsson, P. Clute, M. Jackman, and J. Pines. 1998. MPF localization is controlled by nuclear export. EMBO J. 17:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrscher, R. F., M. H. Kaplan, D. L. Lelsz, C. Das, R. Scheuermann, and P. W. Tucker. 1995. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 9:3067-3082. [DOI] [PubMed] [Google Scholar]
- 17.Ishidate, T., S. Yoshihara, Y. Kawasaki, B. C. Roy, K. Toyoshima, and T. Akiyama. 1997. Identification of a novel nuclear localization signal in Sam68. FEBS Lett. 409:237-241. [DOI] [PubMed] [Google Scholar]
- 18.Jans, D. A., C. Y. Xiao, and M. H. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22:532-544. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. R., S. Levanat, and A. E. Bale. 1995. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. BioTechniques 19:192-195. [PubMed] [Google Scholar]
- 20.Kaffman, A., and E. K. O'Shea. 1999. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15:291-339. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M. H., R. T. Zong, R. F. Herrscher, R. H. Scheuermann, and P. W. Tucker. 2001. Transcriptional activation by a matrix associating region-binding protein. Contextual requirements for the function of Bright. J. Biol. Chem. 276:21325-21330. [DOI] [PubMed] [Google Scholar]
- 22.Komeili, A., and E. K. O'Shea. 1999. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284:977-980. [DOI] [PubMed] [Google Scholar]
- 23.Kortschak, R. D., P. W. Tucker, and R. Saint. 2000. ARID proteins come in from the desert. Trends Biochem. Sci. 25:294-299. [DOI] [PubMed] [Google Scholar]
- 24.Laskov, R., J. K. Kim, V. L. Woods, P. E. McKeever, and R. Asofsky. 1981. Membrane immunoglobulins of spontaneous B-lymphomas of aged BALB/c mice. Eur. J. Immunol. 11:462-471. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson, P. T., M. Vihinen, and C. I. Smith. 1996. X-linked agammaglobulinemia (XLA): a genetic tyrosine kinase (Btk) disease. Bioessays 18:825-834. [DOI] [PubMed] [Google Scholar]
- 26.Numata, S., P. P. Claudio, C. Dean, A. Giordano, and C. M. Croce. 1999. Bdp, a new member of a family of DNA-binding proteins, associates with the retinoblastoma gene product. Cancer Res. 59:3741-3747. [PubMed] [Google Scholar]
- 27.Okamura, H., J. Aramburu, C. Garcia-Rodriguez, J. P. Viola, A. Raghavan, M. Tahiliani, X. Zhang, J. Qin, P. G. Hogan, and A. Rao. 2000. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell 6:539-550. [DOI] [PubMed] [Google Scholar]
- 28.Patsialou, A., D. Wilsker, and E. Moran. 2005. DNA-binding properties of ARID family proteins. Nucleic Acids Res. 33:66-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeper, D. S., A. Shvarts, T. Brummelkamp, S. Douma, E. Y. Koh, G. Q. Daley, and R. Bernards. 2002. A functional screen identifies hDRIL1 as an oncogene that rescues RAS-induced senescence. Nat. Cell Biol. 4:148-153. [DOI] [PubMed] [Google Scholar]
- 30.Pollard, V. W., W. M. Michael, S. Nakielny, M. C. Siomi, F. Wang, and G. Dreyfuss. 1996. A novel receptor-mediated nuclear protein import pathway. Cell 86:985-994. [DOI] [PubMed] [Google Scholar]
- 31.Rajaiya, J., M. Hatfield, J. C. Nixon, D. J. Rawlings, and C. F. Webb. 2005. Bruton's tyrosine kinase regulates immunoglobulin promoter activation in association with the transcription factor Bright. Mol. Cell. Biol. 25:2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes, J. C., C. Muchardt, and M. Yaniv. 1997. Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J. Cell Biol. 137:263-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwindling, S. L., A. Noll, M. Montenarh, and C. Gotz. 2004. Mutation of a CK2 phosphorylation site in cdc25C impairs importin α/β binding and results in cytoplasmic retention. Oncogene 23:4155-4165. [DOI] [PubMed] [Google Scholar]
- 34.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, M., S. Okuyama, S. Okamoto, K. Shirasuna, T. Nakajima, T. Hachiya, H. Nojima, S. Sekiya, and K. Oda. 1998. A novel E2F binding protein with Myc-type HLH motif stimulates E2F-dependent transcription by forming a heterodimer. Oncogene 17:853-865. [DOI] [PubMed] [Google Scholar]
- 36.Takei, Y., K. Yamamoto, and G. Tsujimoto. 1999. Identification of the sequence responsible for the nuclear localization of human Cdc6. FEBS Lett. 447:292-296. [DOI] [PubMed] [Google Scholar]
- 37.Takizawa, C. G., and D. O. Morgan. 2000. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12:658-665. [DOI] [PubMed] [Google Scholar]
- 38.Webb, C. F., C. Das, S. Eaton, K. Calame, and P. W. Tucker. 1991. Novel protein-DNA interactions associated with increased immunoglobulin transcription in response to antigen plus interleukin-5. Mol. Cell. Biol. 11:5197-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb, C. F., C. Das, K. L. Eneff, and P. W. Tucker. 1991. Identification of a matrix-associated region 5′ of an immunoglobulin heavy chain variable region gene. Mol. Cell. Biol. 11:5206-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb, C. F., Y. Yamashita, N. Ayers, S. Evetts, Y. Paulin, M. E. Conley, and E. A. Smith. 2000. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J. Immunol. 165:6956-6965. [DOI] [PubMed] [Google Scholar]
- 41.Webb, C. F., R. T. Zong, D. Lin, Z. Wang, M. Kaplan, Y. Paulin, E. Smith, L. Probst, J. Bryant, A. Goldstein, R. Scheuermann, and P. Tucker. 1999. Differential regulation of immunoglobulin gene transcription via nuclear matrix-associated regions. Cold Spring Harbor Symp. Quant. Biol. 64:109-118. [DOI] [PubMed] [Google Scholar]
- 42.Wilsker, D., L. Probst, H. M. Wain, L. Maltais, P. W. Tucker, and E. Moran. 2005. Nomenclature of the ARID family of DNA-binding proteins. Genomics 86:247-251. [DOI] [PubMed] [Google Scholar]
- 43.Woo, E. S., R. L. Rice, and J. S. Lazo. 1999. Cell cycle dependent subcellular distribution of Cdc25B subtypes. Oncogene 18:2770-2776. [DOI] [PubMed] [Google Scholar]
- 44.Wu, J., L. Zhou, K. Tonissen, R. Tee, and K. Artzt. 1999. The quaking I-5 protein (QKI-5) has a novel nuclear localization signal and shuttles between the nucleus and the cytoplasm. J. Biol. Chem. 274:29202-29210. [DOI] [PubMed] [Google Scholar]
- 45.Yoneda, Y. 2000. Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells 5:777-787. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Z., and G. G. Carmichael. 2001. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell 106:465-475. [DOI] [PubMed] [Google Scholar]
- 47.Zhong, H., M. J. May, E. Jimi, and S. Ghosh. 2002. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Mol. Cell 9:625-636. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler, E. C., and S. Ghosh. 2005. Regulating inducible transcription through controlled localization. Sci. STKE 284:re6. [DOI] [PubMed] [Google Scholar]
- 49.Zong, R. T., C. Das, and P. W. Tucker. 2000. Regulation of matrix attachment region-dependent, lymphocyte-restricted transcription through differential localization within promyelocytic leukemia nuclear bodies. EMBO J. 19:4123-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]