Abstract
The p38 mitogen-activated protein kinase (MAPK) signaling pathway can be activated by a variety of stress stimuli such as UV radiation and osmotic stress. The regulation and role of this pathway in death receptor-induced apoptosis remain unclear and may depend on the specific death receptor and cell type. Here we show that binding of Fas ligand to Fas activates p38 MAPK in CD8+ T cells and that activation of this pathway is required for Fas-mediated CD8+ T-cell death. Active p38 MAPK phosphorylates Bcl-xL and Bcl-2 and prevents the accumulation of these antiapoptotic molecules within the mitochondria. Consequently, a loss of mitochondrial membrane potential and the release of cytochrome c lead to the activation of caspase 9 and, subsequently, caspase 3. Therefore, the activation of p38 MAPK is a critical link between Fas and the mitochondrial death pathway and is required for the Fas-induced apoptosis of CD8+ T cells.
The members of the p38 mitogen-activated protein kinase (MAPK) family are widely distributed among different tissues and have been implicated in differentiation, cell death, proliferation, and DNA repair (34). Four different p38 MAPK family members (α, β, γ, and δ) have been identified. There is some specificity among members of this group toward different substrates, but their specific functions are not well understood. p38 MAPKs are regulated by phosphorylation at Tyr and Thr residues by dual-specificity MAPK kinase 3(MKK3), MKK4, and MKK6 (34, 38). These MKKs can be phosphorylated and activated by a large group of less-specific MKK kinases, including MEKKs 1 to 4, MLK2/3, and ASK1. More recently, it has also been proposed that p38 MAPK can be activated via an alternative, MKK-independent pathway (44).
The p38 MAPK signaling pathway is activated by different stimuli associated with stress (such as UV radiation or inflammatory cytokines), as well as some nonstress stimuli (i.e., insulin, transforming growth factor β, and T-cell receptor ligation) (34). Despite a large number of in vitro studies regarding the function of p38 MAPK, the role of p38 in cell death remains controversial. Several studies have shown that p38 MAPK mediates apoptosis in different cell types, including neurons (15, 59), fibroblasts (46), cardiac muscle cells (29, 55), and endothelial cells (63). Other studies have described the antiapoptotic effects of this pathway. Activation of p38 MAPK has a protective effect on cardiac myocytes (64). Anthrax lethal toxin induces macrophage cell death by inhibiting p38 MAPK (36). Antiapoptotic effects of the p38 MAPK pathway have also been observed during neuronal differentiation (48). Relatively few studies have been done on the function of p38 MAPK in vivo. Inhibition of p38 MAPK in cardiac myocytes in vivo promotes hypertrophic cardiomyopathy (2), whereas activation of p38 MAPK induces heart failure (26). We have previously shown that activation of p38 MAPK in vivo causes death of CD8+ T cells (31). Thus, the final outcome of p38 MAPK activation may be determined not only by cell type, but also by other factors such as the specific stimuli and/or the signaling context present when this pathway is activated.
In addition to stress stimuli, p38 MAPK is also activated by death receptors, such as Fas and the tumor necrosis factor alpha (TNF-α) receptors (reviewed in reference 53). However, the relative contribution of p38 MAPK to death receptor-induced apoptosis and its integration with other death/survival signaling pathways triggered by these receptors are less understood. Activation of p38 MAPK by TNF-α has been shown to mediate apoptosis in endothelial cells (11) but survival in neurons (35). Although several studies have reported the activation of p38 MAPK in response to Fas ligation, most of them indicate that p38 MAPK activation by Fas is secondary and dependent on caspase activation (3, 18, 43, 51). A more recent study proposes that p38 MAPK can interfere with the recruitment of Fas death-inducing signaling complex (DISC) components in several tumor cell types (49). p38 MAPK can also contribute to Fas mediated-death by upregulating FasL or downregulating Fas expression (16, 17).
Fas plays a key role in maintaining peripheral T-cell numbers and in activation-induced cell death (33). Apoptosis through Fas is initiated by the activation of caspase 8, following recruitment to the membrane signaling complex, and the subsequent cleavage and activation of caspase 3 (52). Mitochondrial damage and activation of caspase 9 can also contribute to Fas-mediated activation of caspase 3, but the mechanism is less clear (25). Although activation of p38 MAPK has been shown to induce death in CD8+ T cells in vivo (31), no studies have demonstrated a role for p38 MAPK in Fas-mediated cell death in primary resting T cells. Here, we show that activation of p38 MAPK is critical for efficient induction of apoptosis in unstimulated CD8+ T cells through Fas. Activation of p38 MAPK leads to phosphorylation and translocation of Bcl-2 and Bcl-xL out of the mitochondria, inducing the release of cytochrome c and activation of caspase 3 through caspase 9. Thus, p38 MAPK is a critical component of Fas signaling that mediates the activation of caspase 3 via the mitochondrial death pathway.
MATERIALS AND METHODS
Transgenic mice.
The dominant-negative p38 (dnp38) and MKK6(Glu) transgenic mice (B10.BR background) have been previously described (41). Expression of the transgene in each of these mouse lines is under control of the distal lck promoter. Wild-type B10.BR strain mice were obtained from Jackson Laboratories. Procedures that involved mice were approved by institutional guidelines for animal care.
Cell preparation and culture conditions.
CD8+ T cells were isolated from spleen and lymph nodes by negative selection, as previously described (41). Reagents used during cell culture include zVAD-fmk (Enzyme Systems Products) and recombinant FLAG-CD95L (FasL) (Alexis Biochemicals). FasL treatment was performed by preincubating CD8+ T cells in medium at 37°C in 5% CO2 for 30 min prior to the addition of 200 ng/ml of FasL (unless otherwise specified) combined with 4 μg/ml anti-Flag M2 mouse monoclonal antibody (Sigma) for the indicated times.
Cell death analysis.
For the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay used for cell death analysis, cells treated with medium, FasL, dexamethasone, or TNF-α for the indicated periods of time were washed in phosphate-buffered saline (PBS), fixed in 1% paraformaldehyde, and permeabilized with 70% ethanol as recommended by the manufacturer (Pharmingen). Apoptotic cells were detected using terminal deoxynucleotidyltransferase-mediated fluorescein isothiocyanate-dUTP incorporation according to the manufacturer's protocol (Pharmingen). Annexin V staining was performed using the Annexin V-PE kit as recommended by the manufacturer (Molecular Probes, Eugene, OR).
Cell lysates and Western blotting.
Whole-cell lysates were prepared in Triton lysis buffer (20 mM Tris, pH 7.4, 250 mM NaCl, 0.2 mM EDTA, 2 mM Na4P2O7, 1% Triton X-100, 10% glycerol, 0.5 mM dithiothreitol, 25 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 5 μg/ml pepstatin) as previously described (37). Mitochondrial lysates were prepared using a mitochondrial fractionation kit (Active Motif Incorporated) according to the manufacturer's protocol. Whole-cell and mitochondrial lysates (10 to 40 μg each) were then separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes as described previously (31). Primary antibodies for Western blotting include anti-p38, anti-phospho-p38, anti-phospho-JNK1/2, anti-JNK1/2, and anti-total or -cleaved caspase 3 from Cell Signaling Technologies; anti-actin and anti-STAT1 from Santa Cruz Biotechnology; anti-caspase 3 (a kind gift of Y. Lazebnik, Cold Spring Harbor Laboratories); anti-caspase 8 from Apotech Corporation; and anti-caspase 9 from Stressgen Biotechnologies. Anti Bcl-2, anti-Bcl-xL, and anti-Bax were from the Bcl-2 Related Sampler kit (BD Transduction Laboratories), and anti-cytochrome c was from BD PharMingen.
Kinase activity assays.
Total p38 MAPK was immunoprecipitated from whole-cell lysates with agarose-conjugated anti-p38 antibody (Santa Cruz Biotechnology), repeatedly washed, and resuspended in kinase buffer (25 mM HEPES, pH 7.4, 25 mM β-glycerophosphate, 25 mM MgCl2). Reactions were performed by incubating immunoprecipitates, 10 mM ATP, 5 μCi [γ-32P]ATP (Perkin-Elmer, Inc.), and 2 μg of either recombinant Bcl-2 substrate or recombinant glutathione S-transferase (GST)-Bcl-xL substrate (Protein X Labs). For the generation of Bcl-xL mutants, cDNA coding sequence for truncated Bcl-xL (positions 1 to 212, deletion of C-terminal 20 amino acids) was subcloned into pTopoTA vector (InVitrogen), further subcloned into expression vector pGEX.4T.1 (Amersham), and used for PCR-based site-directed mutagenesis (QuikChange kit; Stratagene) to generate Thr115Ala single- and Thr47Ala Thr115Ala double-amino-acid substitution mutants. GST-Bcl-xL wild-type and mutant fusion proteins were expressed in Escherichia coli BL21(DE3) under IPTG (isopropyl-β-d-thiogalactopyranoside) induction. Cells were harvested 3 h later and lysed, and the GST fusion proteins were captured onto glutathione Sepharose (Amersham Biosciences) as described previously (9). Bound proteins were eluted from the matrix with 50 mM Tris, pH. 8.0, plus 50 mM reduced glutathione and concentrated, and the buffer was exchanged with 50 mM Tris, pH. 8.0, using Microcon Centrifugal Filter Devices (Amicon). Protein concentrations were determined by the Bradford method (Bio-Rad), and 2 μg of the purified proteins was used for in vitro kinase assays as described above. For in vitro kinase reactions, some mixtures contained 2.5 μM SB203580 (Calbiochem), as indicated. Reactions were terminated after 20 min at 30°C by the addition of Laemmli sample buffer. Total reaction volumes were separated by SDS-PAGE and transferred to nitrocellulose prior to visualization and quantitation using a Bio-Rad phosphorimager.
JC-1 staining.
CD8+ T cells were preincubated in medium at 37°C in 5% CO2 for 60 min prior to adding 0.1 mg/ml of JC-1 dye (Molecular Probes) for 10 min, and washing in phosphate-buffered saline (PBS), pH 7.4. In healthy cells with high mitochondrial membrane potential, JC-1 molecules accumulate within the mitochondria and form aggregates that emit at ∼590 nm (red). In cells with mitochondrial damage, JC-1 fails to aggregate within the mitochondria and therefore emits at ∼527 nm (green). Thus, a shift in fluorescence from 590 to 527 nm indicates a loss of membrane potential (40). The percentage of cells emitting JC-1 green fluorescence was quantitated by flow cytometry (LSRII; BD Biosciences).
Caspase activity assays.
CD8+ T cells (5 × 104) in 100 μl of medium were added to an equal volume of the appropriate Caspase-Glo reagent (Promega) at room temperature. Cleaved-substrate luminescence was measured in relative light units with a TD 20/20 Luminometer (Turner Designs).
RNase protection assay.
Total RNA was isolated from cells using Ultraspec RNA isolation reagent (Biotecx Laboratories) according to the manufacturer's protocol. An RNase protection assay (RPA) was performed using the mAPO-2 template kit (BD PharMingen) as recommended by the manufacturer. Briefly, 3 μg of total RNA was hybridized overnight with [32P]UTP-radiolabeled in vitro-transcribed RNA probes. Overlapping single-stranded RNA (ssRNA) on hybridized double-stranded RNAs (dsRNAs) was digested with RNases A and T1, and the protected dsRNA duplexes were purified and resolved on urea-denaturing gels. Gels were then dried and analyzed by autoradiography.
Confocal microscopy.
CD8+ T cells were cytospun onto slides, fixed in 3.7% formaldehyde, permeabilized in 0.1% Triton X-100, blocked in 1% bovine serum albumin in PBS, and incubated with primary antibodies against Bcl-2 and Bcl-xL (BD PharMingen) or cleaved caspase 3 followed by incubation with Alexa 568-conjugated goat anti-rabbit or goat anti-mouse antibodies (Molecular Probes) as previously described (7). Visualization of nuclei and mitochondria was accomplished by staining with YOYO-1-iodide and MitoTracker Deep Red 633 (Molecular Probes), respectively, in the presence of 50 μg/ml RNase. Images were collected using a Bio-Rad MRC 1024 confocal workstation with Olympus BX50 fluorescence microscope and LaserSharp scanning software.
RESULTS
Caspase-independent activation of p38 MAPK by Fas ligation in CD8+ T cells.
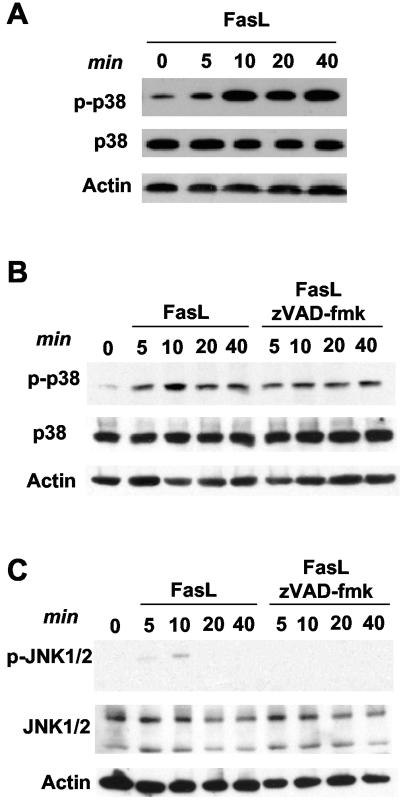
Although p38 MAPK has been shown to be activated by Fas ligation in several cell types (3, 18, 43, 51), no previous studies have addressed the regulation of p38 MAPK by Fas in primary nonactivated T cells. Since p38 MAPK can induce death of CD8+ T cells in vivo (31), we examined whether p38 MAPK was activated by Fas ligand (FasL) in these cells. CD8+ T cells were isolated from wild-type mice and treated with FasL for different periods of time. Whole-cell lysates were examined for phosphorylated p38 MAPK by Western blot analysis. p38 MAPK was rapidly activated in CD8+ T cells in response to treatment with FasL (Fig. 1A).
FIG. 1.
Activation of p38 MAPK in response to FasL in CD8+ T cells. (A) Wild-type CD8+ T cells were cultured for 30 min in medium alone, prior to the addition of FasL (200 ng/ml) for the times indicated. Cells were then lysed, and a Western blot was performed for activated p38 MAPK (phospho-p38 [p-p38]), total p38 MAPK, and actin (as a loading control). (B) Wild-type CD8+ T cells were cultured in the presence or absence of zVAD-fmk (50 μM) for 30 min, prior to the addition of FasL (200 ng/ml) for the times indicated. Phospho-p38 and total p38 were examined by Western blot analysis as described for panel A. (C) Wild-type CD8+ T cells were cultured as described for panel B. Phospho-JNK1 and -JNK2 (p-JNK1/2) and total JNK were examined by Western blot analysis.
JNK can also be activated by Fas, but its activation appears to be indirect, caspase mediated, and not required for death (4, 23, 28). To rule out the possibility that p38 MAPK activation by Fas was also dependent on caspase activity, we pretreated CD8+ T cells with zVAD (a pan-caspase inhibitor) prior to and throughout treatment with FasL. FasL could still activate p38 MAPK in the presence of zVAD, indicating that p38 MAPK activation is not dependent upon caspase activity (Fig. 1B). To further confirm that JNK is not the primary kinase that initiates the apoptotic response of CD8+ T cells to FasL treatment, we examined the activation of JNK1 and JNK2 by Western blot analysis using an anti-phospho JNK1/2 antibody. We have previously shown that the mRNA and protein levels of JNK1 and JNK2 are very low in primary unstimulated CD4+ and CD8+ T cells, in contrast to the levels of these kinases in T-cell clones, T-cell lines, and activated primary T cells (6, 56). In correlation with those studies, low levels of JNK1 and slightly higher levels of JNK2 were detected in CD8+ T cells (Fig. 1C). Consequently, no phosphorylated JNK1 and low levels of phosphorylated JNK2 could be detected in CD8+ T cells in response to FasL treatment (Fig. 1C). Furthermore, the weak phosphorylation of JNK2 by FasL was abrogated by zVAD (Fig. 1C). Together, these results indicate that p38 MAPK is likely the primary MAPK pathway to be involved in Fas-induced death in these cells.
Activation of p38 MAPK is required for Fas-mediated cell death in CD8+ T cells.
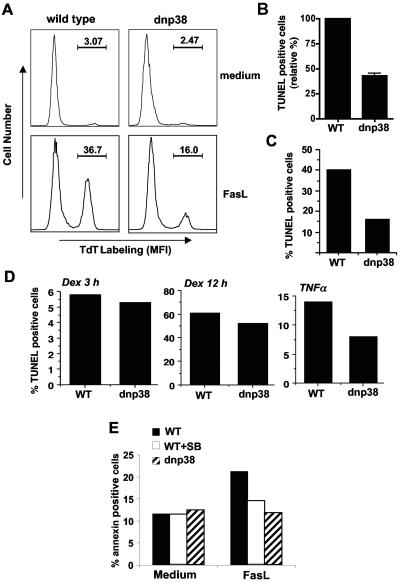
To investigate whether the activation of p38 MAPK was required for Fas-induced cell death, we used the previously described transgenic mice expressing a dominant-negative mutant of p38 MAPK (dnp38) (41). Expression of the dominant-negative p38 mutant in T cells partially inhibits (40 to 60%) endogenous p38 MAPK activity (41). CD8+ T cells from wild-type and dnp38 transgenic mice were treated with FasL, and apoptosis was measured by TUNEL assay after 4 to 5 h. While a large percentage of wild-type CD8+ T cells were apoptotic following treatment with FasL, a substantially lower percentage of apoptotic cells (twofold reduction) were observed in FasL-treated dnp38 cells (Fig. 2A and 2B). Similar resistance to FasL-induced apoptosis was observed even when a higher concentration of FasL was used (Fig. 2C). Kinetics analysis indicated that the protection against FasL-mediated apoptosis was already observed after short periods of treatment (2 h) with FasL (see Fig. S1 in the supplemental material). Less effect was observed when death was examined after 12 h of FasL treatment (see Fig. S1 in the supplemental material), probably because p38 MAPK did not protect from a Fas-independent late death triggered by dead cell debris.
FIG. 2.
Activation of p38 MAPK is necessary for FasL-induced death in CD8+ T cells. (A) Freshly isolated CD8+ T cells from wild-type mice or dnp38 transgenic mice were cultured in medium alone or treated with FasL (200 ng/ml) for 4 h prior to detection of apoptosis via TUNEL and flow cytometry. The percentage of TdT-labeled cells is indicated. MFI, mean fluorescence intensity. This is a representative experiment, and panel B shows the mean percentage of TUNEL-positive (TdT labeled) cells (in response to FasL) relative to wild-type (WT) cells in four independent experiments (n = 4). Error bars represent standard error. (C) Wild-type and dnp38 CD8+ T cells were treated with a higher dose of FasL (400 ng/ml) for 4 h. Apoptosis was determined by TUNEL assay. (D) CD8+ T cells were treated with dexamethasone (Dex; 1 μM) for 3 or 12 h or with TNF-α (50 ng/ml) for 12 h. Apoptosis was determined by TUNEL assay. (E) CD8+ T cells from wild-type and dnp38 transgenic mice were treated with FasL (200 ng/ml). Wild-type CD8+ T cells were also treated with FasL (200 ng/ml) in the presence of the p38 MAPK inhibitor SB203580 (5 μM) (WT+SB). Apoptosis was examined after 2 h by Annexin V staining and flow cytometry.
We therefore tested whether p38 MAP kinase inhibition will affect apoptosis mediated by other death stimuli. CD8+ T cells from wild-type and dnp38 transgenic mice were treated with dexamethasone, and apoptosis was measured by TUNEL assay after different periods of time. Unlike FasL, dexamethasone requires longer periods of time to induce a substantial amount of death in peripheral T cells. Nevertheless, no difference in the induction of apoptosis between wild-type and dnp38 CD8+ T cells was detected after 3 or 12 h of exposure to dexamethasone (Fig. 2D). Similar to Fas, p38 MAPK is also activated by other death receptors, such as TNF-αR (39). We examined TNF-α-induced death in wild-type and dnp38 CD8+ T cells by treating them with TNF-α for 12 h since T cells are relatively resistant to TNF-α-induced death and longer periods of treatment are needed to detect some apoptosis. CD8+ T cells from dnp38 mice were also more resistant to TNF-α-mediated apoptosis than wild-type CD8+ T cells (Fig. 2D). Thus, activation of p38 MAPK is required for apoptosis induced by Fas and, probably, other death receptors in CD8+ T cells.
To further confirm the role of p38 MAPK in Fas-induced death in CD8+ T cells, we examined the effect of the pharmacological inhibitor of p38 MAPK, SB203580. CD8+ T cells from wild-type mice were treated with FasL in the absence or presence of SB203580. In parallel, we also examined FasL-induced death in dnp38 CD8+ T cells. We used Annexin V staining as an alternative method to measure apoptosis. The presence of SB203580 also impaired FasL-induced apoptosis (Fig. 2E). Similar results were obtained using TUNEL to measure apoptosis (see Fig. S2 in the supplemental material). Together, the data show that Fas ligation activates p38 MAPK in CD8+ T cells and that activation of this pathway is required for Fas-induced apoptosis.
p38 MAPK is essential for activation of caspase 3 in response to FasL in vitro.
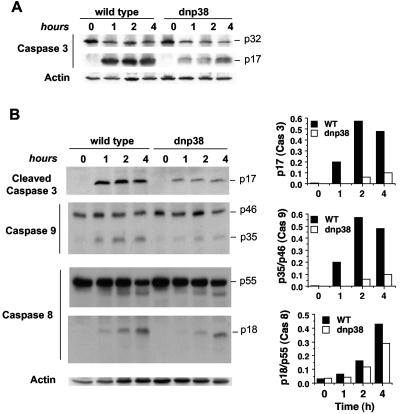
Interactions of Fas with its cognate FasL trigger the activation of upstream caspases leading to the downstream activation of caspase 3 (25, 27, 52). To determine whether caspase 3 activation by Fas is dependent upon the p38 MAPK pathway, we treated CD8+ T cells from wild-type or dnp38 mice with FasL for the indicated times. Whole-cell lysates were analyzed by Western blotting for the presence of the active cleaved fragment of caspase 3 using an antibody that recognizes both the procaspase (p32) and cleaved (p17) forms of caspase 3. The ability of dnp38 CD8+ T cells to activate caspase 3 in response to FasL was remarkably diminished compared with that of wild-type cells (Fig. 3A).
FIG. 3.
Inhibition of p38 MAPK in CD8+ T cells prevents activation of caspase 3 and caspase 9 in response to FasL. (A) CD8+ T cells from wild-type or dnp38 transgenic mice were treated with FasL for the indicated time periods. Cells were then lysed, and a Western blot was performed using an antibody that recognizes both the full-length (p32) and cleaved (p17) caspase 3. (B) CD8+ T cells from wild-type (WT) or dnp38 transgenic mice were treated with FasL for the indicated time periods and lysed, and whole extracts were used for Western blots using antibodies against cleaved caspase 3 (p17), full-length (p46) and cleaved (p35) caspase 9, or full-length (p55) and cleaved (p18) caspase 8. Actin was examined as a loading control. Densitometry analysis was performed, and relative values for caspase 3 and for the cleaved/full-length-form ratio of caspase 8 and caspase 9 are shown. Results shown are representative of four independent experiments.
Cleavage and activation of caspase 3 can be triggered by either caspase 9, in response to mitochondrial damage, or caspase 8, as a result of death receptor signaling (25, 27, 52). In order to determine how p38 MAPK activates caspase 3 in response to FasL, we examined activation of caspases 9 and 8 in wild-type and dnp38 CD8+ T cells treated with FasL. Caspase 9 activation, as indicated by the presence of the cleaved (p35) fragment, was almost abolished in FasL-treated dnp38 CD8+ T cells compared with wild-type cells (Fig. 3B). In contrast, only a slight reduction in cleaved caspase 8 (p18) was detected between dnp38 and wild-type CD8+ T cells (Fig. 3B). The reduction in caspase 3 activation was further confirmed using an antibody that only recognizes the cleaved (p17) form (Fig. 3B). These data indicate that the activation of caspase 3 by p38 MAPK in response to FasL is most likely mediated by caspase 9 and not caspase 8.
Activation of p38 MAPK in vivo is sufficient to activate caspase 9 and caspase 3.
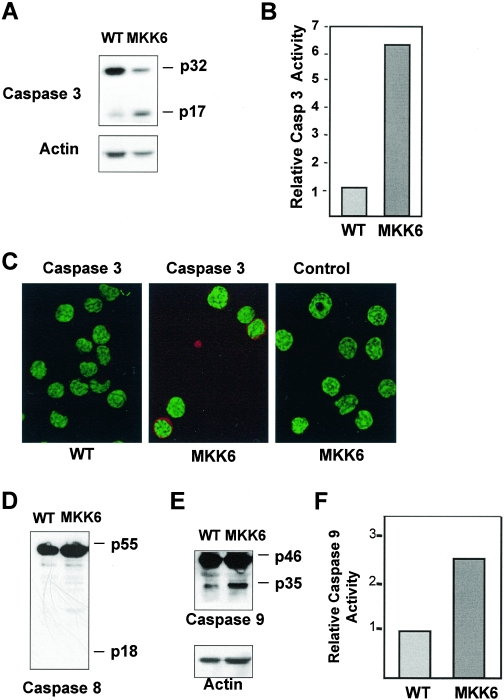
The above results indicate that p38 MAPK is essential for efficient caspase 9 and caspase 3 activation in response to FasL in CD8+ T cells. To determine whether the activation of p38 MAPK alone was sufficient to induce caspase activation in these cells in vivo, we used the previously described MKK6(Glu) transgenic mice. These mice express a constitutively active mutant of MKK6 [MKK6(Glu)], a specific upstream activator of p38 MAPK, in T cells (41). Thus, p38 MAPK is already active in T cells from these mice in the absence of any stimulation. Whole-cell lysates from freshly isolated wild-type or MKK6(Glu) CD8+ T cells were analyzed by Western blotting for caspase 3. CD8+ T cells from MKK6(Glu) transgenic mice had an increased quantity of cleaved (p17) caspase 3 compared to wild-type cells (Fig. 4A). To confirm that caspase 3 was active in CD8+ T cells from MKK6(Glu) mice, we measured the specific activity of caspase 3 in freshly isolated cells using substrate-specific cleavage-induced luminescence. In correlation with the presence of cleaved caspase 3, MKK6(Glu) CD8+ T cells contained high levels of caspase 3 activity (Fig. 4B). We also examined the presence of active caspase 3 in freshly isolated MKK6(Glu) CD8+ T cells by immunostaining and confocal microscopy using the antibody that only recognizes the cleaved (p17) caspase 3 form. No cells with active caspase 3 were detected within wild-type CD8+ T cells, but a significant number (20 to 30%) of the MKK6(Glu) CD8+ T cells contain a high level of active caspase 3 (Fig. 4C). Thus, activation of p38 MAPK by itself causes the activation of caspase 3 in CD8+ T cells in vivo.
FIG. 4.
Activation of p38 MAPK alone is sufficient to induce activation of caspase 3 and caspase 9, but not caspase 8, in CD8+ T cells. (A) Whole-cell lysates of freshly isolated CD8+ T cells from wild-type (WT) or MKK6(Glu) transgenic mice were analyzed by Western blotting for full-length (p32) and cleaved (p17) caspase 3. Actin was examined as a loading control. (B) Relative caspase 3 activity of CD8+ T cells from wild-type or MKK6(Glu) transgenic mice as measured by cleavage of a luminescent caspase 3 substrate. (C) Freshly isolated CD8+ T cells from wild-type and MMK6(Glu) transgenic mice were used to examined active caspase 3 by immunostaining and confocal microscopy analysis using an anti-cleaved caspase 3 (p17) antibody and a secondary antibody (red). YOYO (green) was used as a nuclear marker. Staining with the secondary antibody (Control) in MKK6(Glu) CD8+ T cells is also shown. (D and E) Whole-cell lysates of CD8+ T cells from wild-type or MKK6(Glu) transgenic mice were analyzed by Western blotting for full-length (p55) and cleaved (p18) caspase 8 (D) or full-length (p46) and cleaved (p35) caspase 9 (E). Actin was examined as a loading control. (F) Relative caspase 9 activity of CD8+ T cells from wild-type or MKK6(Glu) transgenic mice as measured by cleavage of a luminescent caspase 9 substrate. Results are representative of three independent experiments.
To observe the effects of p38 MAPK activation on upstream caspases, whole-cell lysates from wild-type and MKK6(Glu) transgenic mice were examined for the presence of cleaved caspases 8 and 9 by Western blot analysis. In correlation with the minimal effect of p38 MAPK inhibition on Fas-mediated caspase 8 activation, no cleaved caspase 8 (p18) was observed in MKK6(Glu) CD8+ cells (Fig. 4D). Interestingly, MKK6(Glu) CD8+ T cells contained increased levels of cleaved caspase 9 (p35) (Fig. 4E). To confirm that caspase 9 was active in MKK6(Glu) CD8+ T cells, we measured caspase 9 activity using a caspase 9 substrate with cleavage-induced luminescence. MKK6(Glu) CD8+ T cells contained increased levels of caspase 9 activity compared to wild-type cells (Fig. 4F). In addition, MKK6(Glu) CD8+ T cells exhibited no detectable activation of caspases 2 and 7, which can also cleave caspase 3 (data not shown). Thus, p38 MAPK activates caspase 9, but not caspase 8, 2, or 7, indicating that caspase 3 cleavage and activation by this pathway are likely mediated by caspase 9.
Activation of p38 MAPK in vivo increases mitochondrial permeability and induces the release of cytochrome c in CD8+ T cells.
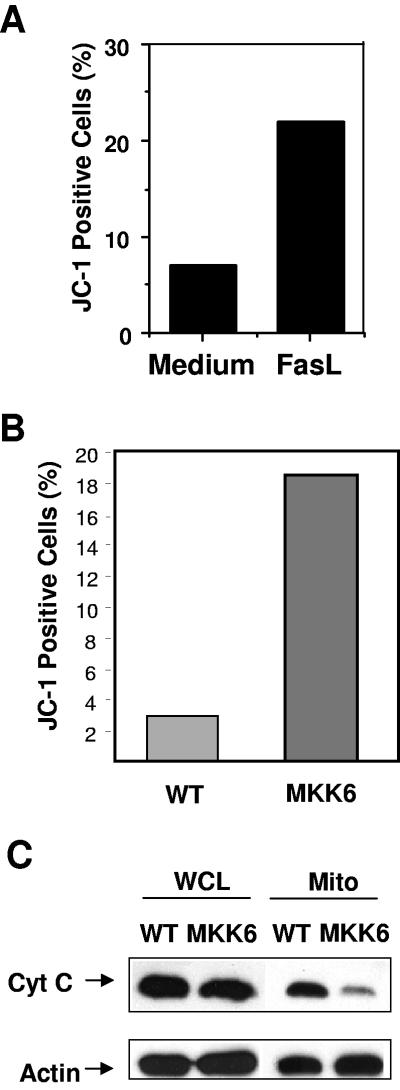
Activation of caspase 9 is triggered by the leakage of cytochrome c from the mitochondria into the cytosol, followed by formation of the Apaf/cytochrome c/procaspase 9 apoptosome complex (25). Using the JC-1 dye and flow cytometry, we confirmed that treatment with FasL also increases mitochondrial membrane potential in wild-type CD8+ T cells (Fig. 5A). To determine if activation of p38 MAPK was sufficient to cause changes in mitochondrial permeability, we performed JC-1 staining in CD8+ T cells freshly isolated from wild-type and MKK6(Glu) transgenic mice. While very few wild-type CD8+ T cells stained positive for JC-1, a markedly increased percentage of MKK6(Glu) CD8+ T cells were JC-1 positive (Fig. 5B). These results indicate that activation of p38 MAPK increases mitochondrial permeability resulting in a loss of membrane potential.
FIG. 5.
CD8+ T cells from MKK6(Glu) transgenic mice have decreased mitochondrial membrane potential and decreased mitochondrial cytochrome c. (A) Wild-type CD8+ T cells were incubated with medium alone or FasL (200 ng/ml). After 4 h, cells were stained with JC-1 dye and analyzed by flow cytometry. The percentages of cells containing JC-1 green fluorescence (indicating a loss of mitochondrial membrane potential) are shown. (B) Freshly isolated CD8+ T cells from wild-type (WT) or MKK6(Glu) transgenic mice were stained with JC-1 dye and analyzed by flow cytometry. (C) Whole-cell lysates (WCL) or mitochondrial lysates (Mito) from wild-type or MKK6(Glu) CD8+ T cells were analyzed by Western blotting for cytochrome c (Cyt C) or actin (as a loading control). The results shown are representative of three independent experiments.
To confirm the increased mitochondrial membrane permeability in MKK6(Glu) CD8+ T cells, we prepared mitochondrial extracts of freshly isolated wild-type and MKK6(Glu) CD8+ T cells and examined cytochrome c levels by Western blot analysis. The amount of cytochrome c within the mitochondria was highly reduced in MKK6(Glu) CD8+ T cells relative to wild-type CD8+ T cells (Fig. 5C). Activation of p38 MAPK, therefore, leads to increased mitochondrial membrane permeability and cytochrome c release, which in turn activates the caspase 9-mediated death pathway.
p38 MAPK prevents the mitochondrial accumulation of Bcl-2 and Bcl-xL in CD8+ T cells.
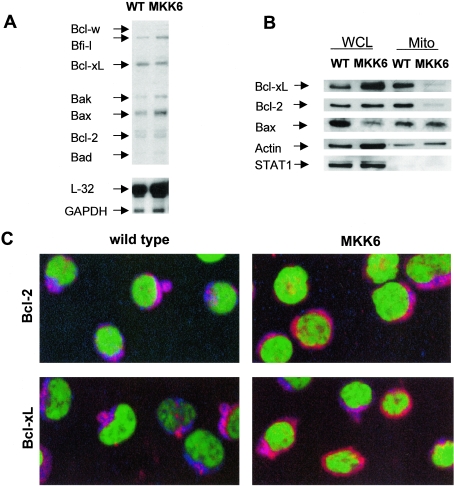
The Bcl-2 family is a group of proteins that coordinately control apoptotic cell death by regulating the release of cytochrome c from the mitochondria (47). This family includes both proapoptotic (Bax, Bad, Bid, Bim, and Bak) and antiapoptotic (Bcl-2, Bcl-xL, and Bcl-w) members. We performed an RPA to determine whether the increased cytochrome c release induced by p38 MAPK could be due to changes in expression of Bcl-2 family proteins. No significant differences were observed in the mRNA levels of Bcl-2 family members between MKK6(Glu) and wild-type CD8+ T cells (Fig. 6A). Although Bax mRNA levels appeared to be slightly increased in MKK6(Glu) CD8+ T cells compared to those in wild-type CD8+ T cells, we observed no increase in Bax protein levels (see below).
FIG. 6.
Activation of p38 MAPK in CD8+ T cells prevents mitochondrial accumulation of Bcl-2 and Bcl-xL. (A) Total RNA from freshly purified wild-type (WT) or MKK6(Glu) CD8+ T cells was analyzed for expression of various Bcl-2 family members by RPA using the mAPO-2 template kit (BD PharMingen). L-32 and GAPDH were examined as controls for loading. (B) Whole-cell lysates (WCL) or mitochondria lysates (Mito) from wild-type or MKK6(Glu) CD8+ T cells were analyzed by Western blotting for Bcl-xL, Bcl-2, and Bax. We also examined the levels of actin (as a loading control) and STAT1 (as a marker of cytoplasmic contamination). (C) Cytospun CD8+ T cells from wild-type or MKK6(Glu) transgenic mice were stained with antibodies against either Bcl-2 or Bcl-XL (red), YOYO nuclear dye (green), and MitoTracker mitochondrial dye (blue). Colocalization of Bcl-2 or Bcl-xL with MitoTracker is displayed as a pink/purple color, while cytosolic Bcl-2 and Bcl-xL appear red. Cells were visualized by confocal microscopy at a ×60 magnification. The results shown are representative of at least two independent experiments.
In order to confirm that the levels of expression of Bcl-2 family members were similar in MKK6(Glu) CD8+ T cells compared to wild-type CD8+ T cells, protein levels were examined by Western blot analysis. No substantial differences were observed in the amount of Bcl-2 or Bcl-xL in whole-cell lysates between wild-type and MKK6(Glu) CD8+ T cells (Fig. 6B). In contrast to the mRNA levels, the quantity of Bax protein was not increased in MKK6(Glu) CD8+ T cells, but instead appeared to be slightly lower or unaffected (Fig. 6B). Neither Bad nor Bid was detected in whole-cell lysates from wild-type or MKK6(Glu) CD8+ T cells (data not shown).
Since the presence of antiapoptotic Bcl-2 family proteins within the mitochondria is thought to be crucial in the maintenance of mitochondrial membrane potential and the prevention of mitochondrial damage (14), we tested whether p38 MAPK may affect the mitochondrial accumulation of Bcl-2 family members. We examined the levels of Bcl-2, Bcl-xL, and Bax in mitochondrial fractions of wild-type and MKK6(Glu) CD8+ T cells by Western blot analysis. The levels of Bcl-2 and Bcl-xL proteins in the mitochondria of MKK6(Glu) CD8+ T cells were profoundly reduced compared to those in mitochondria from wild-type cells (Fig. 6B). To rule out any possible cytoplasmic contamination of the mitochondrial extracts, we analyzed the presence of the transcription factor STAT1, which is normally confined to the cytoplasm. STAT1 was undetectable in mitochondrial extracts from either wild-type or MKK6(Glu) CD8+ T cells (Fig. 6B).
To further demonstrate the effect of p38 MAPK activation on the mitochondrial accumulation of Bcl-2 and Bcl-xL, we examined the intracellular localization of these proteins by confocal microscopy, using MitoTracker as a mitochondrial marker. Freshly isolated CD8+ T cells from wild-type or MKK6(Glu) transgenic mice were cytospun and stained with MitoTracker (mitochondria), YOYO (nucleus), and antibodies against either Bcl-2 or Bcl-xL. While Bcl-2 and Bcl-xL colocalized with MitoTracker in wild-type CD8+ T cells, both Bcl-2 and Bcl-xL appeared to be distributed within the cytosol in most MKK6(Glu) CD8+ T cells (Fig. 6C), indicating that these cells had less mitochondrion-associated Bcl-2 and Bcl-xL. Together, these data show that in vivo activation of p38 MAPK prevents the accumulation of the antiapoptotic proteins Bcl-2 and Bcl-xL within the mitochondria of CD8+ T cells.
Phosphorylation of Bcl-2 and Bcl-xL by p38 MAPK in response to Fas ligation.
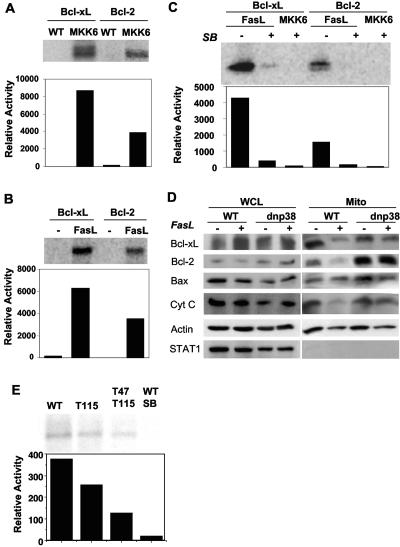
Previous studies have shown that phosphorylation of Bcl-2 and Bcl-xL by JNK is associated with the inactivation of their antiapoptotic function, although the mechanism remains unclear (20, 30, 60). To investigate the possibility that p38 MAPK is regulating the mitochondrial accumulation of Bcl-2 and Bcl-xL via phosphorylation, we performed in vitro kinase assays using recombinant Bcl-2 and Bcl-xL as substrates. Total p38 MAPK was immunoprecipitated from whole-cell lysates of wild-type or MKK6(Glu) CD8+ T cells and incubated with either recombinant Bcl-xL or Bcl-2 in vitro. As expected, no phosphorylation of Bcl-2 or Bcl-xL was observed in the presence of p38 MAPK from wild-type CD8+ T cells, since p38 MAPK is normally inactive (Fig. 7A). In contrast, strong phosphorylation of both Bcl-2 and Bcl-xL was detected using p38 MAPK from MKK6(Glu) CD8+ T cells (Fig. 7A). Thus, active p38 MAPK phosphorylates both Bcl-2 and Bcl-xL.
FIG. 7.
p38 MAPK from MKK6(Glu) transgenic or FasL-treated wild-type, CD8+ T cells can phosphorylate Bcl-2 and Bcl-xL. (A) Total p38 MAPK was immunoprecipitated from whole-cell lysates of wild-type (WT) or MKK6(Glu) CD8+ T cells and incubated with recombinant Bcl-xL or Bcl-2 substrates in vitro in the presence of [γ-32P]ATP. Substrate phosphorylation following SDS-PAGE was detected by autoradiography (upper panel) and quantitated by PhosphorImager (lower panel). (B) Wild-type CD8+ T cells were cultured in the presence (FasL) or absence (−) of FasL for 2 h prior to lysis and immunoprecipitation of total p38 MAPK. The ability of p38 MAPK to phosphorylate Bcl-xL and Bcl-2 was analyzed as described for panel A. (C) Total p38 MAPK was immunoprecipitated from wild-type CD8+ T cells treated for 2 h with FasL (FasL) or freshly isolated MKK6(Glu) CD8+ T cells (MKK6). The phosphorylation of Bcl-xL and Bcl-2 in vitro was analyzed as described for panel A, in the presence (+) or absence (−) of the p38 MAPK inhibitor SB203580 (2.5 μM). (D) CD8+ T cells from wild-type or dnp38 transgenic mice were treated with FasL (+) or medium alone (−) for 2 h. Whole-cell lysates (WCL) or mitochondrial lysates (Mito) were prepared and examined for the presence of Bcl-xL, Bcl-2, Bax, and cytochrome c (Cyt C) by Western blot analysis. We also analyzed the levels of actin (as a loading control) and STAT1 (as a marker of cytoplasmic contamination). (E) Total p38 MAPK was immunoprecipitated from freshly isolated MKK6(Glu) CD8+ T cells (MKK6). The phosphorylation of wild-type Bcl-xL, the Thr115Ala Bcl-xL mutant (Thr115) and the Thr115Ala Thr47Ala double Bcl-xL mutant (T47T115) by p38 MAPK in vitro was analyzed as described for panel A. Phosphorylation of wild-type Bcl-xL in the presence of SB203580 (WT/SB) is also shown. The results shown are representative of at least two independent experiments.
Our data above (Fig. 1A and 1B) indicate that FasL treatment activates p38 MAPK in wild-type CD8+ T cells. We therefore examined whether p38 MAPK activated in response to FasL could also phosphorylate Bcl-2 and Bcl-xL. Wild-type CD8+ T cells were cultured in the presence or absence of FasL and lysed, and total p38 MAPK was immunoprecipitated for in vitro kinase assays with either Bcl-2 or Bcl-xL. As expected, phosphorylation of Bcl-2 and Bcl-xL was undetectable when using p38 MAPK from wild-type CD8+ T cells cultured in medium alone. However, p38 MAPK from FasL-treated wild-type cells was able to strongly phosphorylate both Bcl-2 and Bcl-xL (Fig. 7B).
To demonstrate that the phosphorylation of Bcl-2 and Bcl-xL in vitro was directly mediated by p38 MAPK, we used SB203580, a specific pharmacological p38 MAPK inhibitor. Total p38 MAPK was immunoprecipitated from whole-cell lysates of wild-type CD8+ T cells treated with FasL, and in vitro kinase assays were performed using Bcl-2 or Bcl-xL substrates in the presence or absence of SB203580. Both Bcl-2 and Bcl-xL were strongly phosphorylated by p38 MAPK in the absence of SB203580, but phosphorylation was abolished when SB203580 was present in the reaction (Fig. 7C). SB203580 also abolished the phosphorylation of Bcl-2 and Bcl-xL by p38 MAPK immunoprecipitated from MKK6(Glu) CD8+ T cells (Fig. 7C). Thus, p38 MAPK activated by Fas ligation directly phosphorylates both Bcl-2 and Bcl-xL.
Our data show that activation of p38 MAPK in CD8+ T cells prevents the mitochondrial accumulation of Bcl-2 or Bcl-xL (Fig. 6B and C) and that Fas ligation activates p38 MAPK, which phosphorylates Bcl-2 and Bcl-xL (Fig. 7B and C). We therefore examined whether FasL treatment would also reduce mitochondrial levels of these proteins. Wild-type CD8+ T cells were cultured in medium alone, or in the presence of FasL, and whole-cell lysates or mitochondrial lysates were prepared for Western blot analysis. No significant difference in the total protein levels of Bcl-2, Bcl-xL, or Bax was detected between CD8+ T cells in medium alone and FasL-treated cells (Fig. 7D). However, FasL treatment profoundly reduced the amount of Bcl-2 and Bcl-xL within the mitochondria of wild-type CD8+ T cells relative to medium-treated control cells (Fig. 7D). In contrast, we observed no change in the amount of mitochondrial Bax due to FasL treatment. Therefore, stimulation of CD8+ T cells by FasL results in a selective reduction in mitochondrion-associated Bcl-2 and Bcl-xL levels, but not mitochondrial Bax levels.
Our data above show that active p38 MAPK also causes the release of cytochrome c from the mitochondria (Fig. 5C). Accordingly, we asked whether treatment with FasL affected mitochondrial cytochrome c levels in CD8+ T cells. As with Bcl-2 and Bcl-xL, we observed decreased levels of mitochondrial cytochrome c in FasL-treated CD8+ T cells compared to those in cells cultured in medium alone (Fig. 7D). Together, these data indicate that Fas ligation in wild-type CD8+ T cells results in less mitochondrion-associated Bcl-2 and Bcl-xL, subsequently inducing the release of cytochrome c.
To ascertain whether the decrease in mitochondrial Bcl-2 and Bcl-xL levels in FasL-treated cells were the result of p38 MAPK activation, we performed parallel experiments using CD8+ T cells from dnp38 transgenic mice. CD8+ T cells from dnp38 mice were cultured in the presence or absence of FasL, and whole-cell lysates or mitochondrial lysates were prepared for Western blot analysis. Similar to wild-type CD8+ T cells, no difference in total protein levels of Bcl-2, Bcl-xL, or Bax was observed between FasL-treated and medium-treated dnp38 CD8+ T cells (Fig. 7D). Likewise, no differences in overall protein expression were detected between wild-type and dnp38 CD8+ T cells (Fig. 7D). However, while FasL diminished mitochondrial levels of Bcl-2 and Bcl-xL in wild-type CD8+ T cells, no significant reduction of mitochondrial Bcl-2 or Bcl-xL levels was observed in FasL-treated dnp38 CD8+ T cells (Fig. 7D). Thus, translocation of Bcl-2 and Bcl-xL out of the mitochondria in response to Fas ligation is dependent upon activation of p38 MAPK in CD8+ T cells. In correlation with the presence of Bcl-2 and Bcl-xL in the mitochondria, dnp38 CD8+ T cells exhibited almost normal mitochondrial cytochrome c levels upon treatment with FasL (Fig. 7D). No STAT1 was detected in the mitochondrial extracts (Fig. 7D). Together, our data indicate that the loss of mitochondrial Bcl-2 and Bcl-xL in FasL-treated CD8+ T cells is mediated by p38 MAPK, likely due to phosphorylation.
JNK has been shown to phosphorylate Bcl-xL on Thr-47 and Thr-115 (20). Since the phosphorylation target motifs (Ser Pro) for p38 MAPK and JNK are highly conserved (57), it was highly possible that p38 MAPK uses the same residues described for JNK. We therefore generated recombinant wild-type Bcl-xL and mutant Bcl-xL proteins where the Thr115 and/or Thr47 was replaced by Ala. These proteins were used as substrates for in vitro kinase using p38 MAPK immunoprecipitated from MKK6(Glu) CD8+ T cells. The Thr115Ala mutation caused a reduction in the phosphorylation levels of Bcl-xL, but the effect was more profound in the Thr47Ala Thr115Ala double Bcl-xL mutant (Fig. 7E), indicating that both residues were phosphorylated by p38 MAPK. The residual phosphorylation detected in the double mutant could be due to the presence of an additional SP site generated by the fusion of GST and the truncated Bcl-xL, since it was blocked by SB203580. Thus, as expected, p38 MAPK shares with JNK the phosphorylation motifs in Bcl-xL. It is also likely that the Bcl2 residues (Ser70, Ser87, and Thr69) shown to be phosphorylated by JNK (60) are the targets for p38 MAPK.
DISCUSSION
p38 MAPK was initially identified as a kinase activated in response to stress stimuli and associated with apoptotic signaling (13, 59). Subsequently, the activation of p38 MAPK by death receptors has been described by several groups (34). However, the involvement of p38 MAPK in receptor-induced cell death has not been well investigated, and little is known about the participation of p38 MAPK in Fas signaling in CD4+ and CD8+ T cells. Studies with activated T cells and Jurkat cell lines indicate that Fas can activate p38 MAPK, but inhibition of this pathway does not prevent Fas-induced cell death (3, 43). Here, we demonstrate that p38 MAPK is activated by Fas in primary unstimulated CD8+ T cells and that such activation is required for mitochondrial caspase activation and cell death induced by Fas ligation.
The contribution of p38 MAPK to Fas-induced cell death has often been overlooked due to the ability of the Fas signaling complex to trigger alternative downstream pathways. The best-characterized pathway is the activation of caspase 8, which then cleaves and activates caspase 3 (19, 52). Fas also leads to the activation of JNK, but JNK activation seems to be secondary to caspase activation and JNK-deficient mouse embryonic fibroblasts remain sensitive to Fas ligation (4, 23, 28, 50). Unlike JNK or caspase 8, activation of NF-κB by Fas is associated with the expression of antiapoptotic proteins such as inhibitor of apoptosis proteins (IAPs) and Bcl-2, which can protect cells from apoptosis (54). The overall balance of these multiple downstream signals likely determines the life-or-death decision for each cell. Our data indicate that activation of the p38 MAPK pathway is a crucial signaling component for the induction of apoptosis through Fas in CD8+ T cells.
Although it is clear that caspases are involved in Fas-mediated apoptosis, it is also clear that the specific pathways used by this receptor may depend on the specific type of cell and the situation. Studies using caspase 3-deficient mice have shown that caspase 3 is required for Fas-mediated cell death in activated peripheral T cells and hepatocytes but not in thymocytes (22, 58, 65, 66). The requirement of caspase 3 for Fas-mediated cell death in primary unstimulated CD4+ and CD8+ T cells has not been described. Here, we show that inhibition of caspase 3 cleavage by expression of the dnp38 MAPK increases resistance to Fas-mediated cell death in freshly isolated CD8+ T cells, suggesting that caspase 3 may contribute to Fas-mediated death in these cells. We have previously shown that activation of p38 MAPK in vivo causes death of CD8+ T cells, but not CD4+ T cells (31). While activation of p38 MAPK is sufficient to activate caspase 3 in CD8+ T cells (Fig. 4), it is not sufficient to activate caspase 3 in CD4+ T cells (see Fig. S3 in the supplemental material). In correlation, we found that CD4+ T cells are more resistant to Fas-induced death than CD8+ T cells (see Fig. S3 in the supplemental material; also unpublished observations) and that inhibition of p38 MAPK did not significantly affect Fas-induced death in CD4+ T cells (see Fig. S3 in the supplemental material). Thus, it is possible CD4+ T cells have a mechanism (e.g., accumulation of antiapoptotic molecules) that increased death resistance compared with CD8+ T cells.
Caspase 8 plays a necessary and nonredundant role in Fas-mediated cell death (42, 52). The involvement of p38 MAPK in Fas-dependent caspase 8 activation remains unclear. It has been proposed that p38α, but not p38β, facilitates Fas-mediated activation of caspase 8 by inhibiting the phosphorylation and presence of c-FLIPS in the DISC in Jurkat tumor cells (49). In contrast, in neutrophils p38 MAPK directly phosphorylates and inhibits the activities of caspase 8 and caspase 3 (1). Here, we show that p38 MAPK inhibition has little effect on Fas-induced caspase 8 cleavage. Thus, p38 MAPK does not seem to be required for caspase 8 activation by Fas in CD8+ T cells.
Although it has been shown that mitochondrion-mediated caspase 9 activation also contributes to Fas-mediated cell death by cleaving and activating caspase 3, this pathway appears to be more redundant and its relative contribution to Fas-mediated death depends on the cell type. Thymocytes deficient for caspase 9 remain sensitive to anti-Fas antibody-induced cell death (21). Hepatocytes and activated T cells are also equally sensitive to Fas death signals in the absence of caspase 9 (12, 65). It has been proposed that type I cells exclusively use the caspase 8-mediated caspase 3 activation pathway, while type II cells also require mitochondrial damage to mediate cell death through Fas (45). Although the specific contribution of caspase 9 to Fas-mediated cell death in primary nonstimulated CD8+ T cells has not been described, here we show that inhibition of p38 MAPK in these cells impairs Fas-triggered caspase 3 activation and cell death by inhibiting caspase 9. Thus, these results suggest that caspase 9 is likely to play a role in the Fas ligation-induced cell death of CD8+ T cells.
While the activation of the caspase 8 pathway by Fas is well established, the activation of mitochondrial damage and caspase 9 is not as clear. It is now believed that upon Fas ligation, Bid is cleaved by active caspase 8 and translocates into the mitochondria, thus facilitating cytochrome c release and activating caspase 9 (24, 61). However, Bid expression is imperceptible in resting CD4+ T lymphocytes (32). Similarly, we were unable to detect the Bid protein in CD8+ T cells (data not shown), suggesting that Bid is unlikely to play a role in the mitochondrion-mediated Fas death of nonactivated T lymphocytes. In this study, we propose an alternative mechanism for the induction of mitochondrial damage and activation of caspase 9 in response to Fas ligation. We propose that Fas-induced cytochrome c release from the mitochondria is the result of decreased mitochondrial Bcl-2 and Bcl-xL levels and that this effect is mediated by p38 MAPK.
Several mechanisms have been proposed for p38 MAPK to induce cell death by regulating Bcl-2 family members, depending on the stimuli or the cell type. p38 MAPK has been reported to play a role in regulating the cleavage of Bid in response to oxygen species, but the mechanism for this process has not been investigated (67). As mentioned above, no Bid protein was detected in resting CD8+ T cells. p38 MAPK has also been shown to phosphorylate Bax in neurons and thymocytes, affecting its translocation to the mitochondria in response to nitric oxide (10) and glucocorticoids (62), respectively. We did not observe any significant differences in mitochondrial Bax accumulation in CD8+ T cells as a result of FasL treatment. In correlation, activation of p38 MAPK itself did not increase the levels of mitochondrial Bax (Fig. 5B and 6D). Instead, we observed a profound decrease in the mitochondrial levels of Bcl-2 and Bcl-xL in CD8+ T cells caused by p38 MAPK activation. Moreover, we demonstrate that FasL decreases the mitochondrial levels of Bcl-2 and Bcl-xL in CD8+ T cells and that this effect is mediated by p38 MAPK. We therefore propose that p38 MAPK leads to mitochondrial damage in response to Fas ligation by preventing mitochondrial accumulation of these two antiapoptotic molecules in CD8+ T cells.
It has been shown that JNK phosphorylates Bcl-2 on Ser70, Ser87, and Thr69 (60) and Bcl-xL on Thr47 and Thr115 (20). In this study, we show for the first time that p38 MAPK activated upon Fas ligation also phosphorylates Bcl-2 and Bcl-xL (Fig. 7). In addition, we show that p38 MAPK shares the JNK phosphorylation sites in Bcl-xL and it is highly possible that it shares the JNK phosphorylation sites in Bcl-2. Thus, both pathways can regulate cell death by phosphorylation of antiapoptotic Bcl-2 family members and the relative contribution could depend on the cell type or the environment where activation takes place. We have shown that unstimulated CD8+ T cells contain very low levels of JNK1 and JNK2 (6) and that activation in response to FasL treatment is very low, transient, and dependent upon caspase activation (Fig. 1). Thus, we propose that p38 MAPK is likely the kinase to initiate the phosphorylation of the Bcl-2 family proteins, activation of caspases, and induction of death in response to Fas ligation in unstimulated CD8+ T cells.
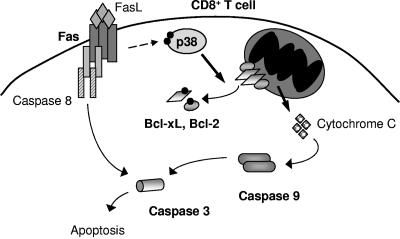
Our studies suggest a novel mechanism by which p38 MAPK can mediate apoptosis via the phosphorylation of two newly identified p38 MAPK substrates, Bcl-2 and Bcl-xL (Fig. 8). Our results also establish p38 MAPK activation as a critical link between Fas signaling and the mitochondrial death pathway in unstimulated CD8+ T cells. Furthermore, the p38 MAPK pathway appears to be the critical pathway in CD8+ T cells for eliciting cell death in response to FasL. The role of Fas in death of T cells prior to activation remains poorly understood. Since Fas-deficient mice accumulate CD8+ and CD4+ T cells in addition to the CD4− CD8− T-cell receptor α/β-positive T cells (5), it is clear that Fas has a critical role in regulating T-cell homeostasis in vivo. In this regard, a recent study has shown that in the absence of Fas, T cells accumulate to significantly higher levels after transfer into lymphopenic recipients (8), indicating that Fas-mediated death could be a principal regulator of homeostasis for unstimulated CD8+ T cells. We propose that the p38 MAPK may play a role in this event.
FIG. 8.
Role for Fas-induced p38 MAPK activity in apoptosis of CD8+ T cells.
Supplementary Material
Acknowledgments
We thank Colette Charland for technical help with flow cytometry analysis; Ralph Budd, Karen Fortner, and Miroslav Koulnis for critical reading of the manuscript; and the personnel in the Microscopy Imaging Facility and the DNA sequencing facility.
This research was supported in part by the Arthritis Foundation Research and grant R01 AI051454 (M.R.). N.F. was supported by the Cancer Biology Training Grant from the National Cancer Institute (T32 CA09286) and the COBRE Program of the National Center for Research Resources (RR15557; M.R.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alvarado-Kristensson, M., F. Melander, K. Leandersson, L. Ronnstrand, C. Wernstedt, and T. Andersson. 2004. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med. 199:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braz, J. C., O. F. Bueno, Q. Liang, B. J. Wilkins, Y.-S. Dai, S. Parsons, J. Braunwart, B. J. Glascock, R. Klevitsky, T. F. Kimball, T. E. Hewett, and J. D. Molkentin. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 111:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner, B., U. Koppenhoefer, C. Weinstock, O. Linderkamp, F. Lang, and E. Gulbins. 1997. Fas- or ceramide-induced apoptosis is mediated by a Rac1-regulated activation of Jun N-terminal kinase/p38 kinases and GADD153. J. Biol. Chem. 272:22173-22181. [DOI] [PubMed] [Google Scholar]
- 4.Cahill, M. A., M. E. Peter, F. C. Kischkel, A. M. Chinnaiyan, V. M. Dixit, P. H. Krammer, and A. Nordheim. 1996. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene 13:2087-2096. [PubMed] [Google Scholar]
- 5.Cohen, P. L., and R. A. Eisenberg. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243-269. [DOI] [PubMed] [Google Scholar]
- 6.Conze, D., T. Krahl, N. Kennedy, L. Weiss, J. Lumsden, P. Hess, R. A. Flavell, G. Le Gros, R. J. Davis, and M. Rincon. 2002. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8+ T cell activation. J. Exp. Med. 195:811-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl, S., C.-W. Chow, L. Weiss, A. Palmetshofer, T. Twardzik, L. Rounds, E. Serfling, R. J. Davis, J. Anguita, and M. Rincon. 2002. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J. Exp. Med. 196:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner, K. A., and R. C. Budd. 2005. The death receptor Fas (CD95/APO-1) mediates the deletion of T lymphocytes undergoing homeostatic proliferation. J. Immunol. 175:4374-4382. [DOI] [PubMed] [Google Scholar]
- 9.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 10.Ghatan, S., S. Larner, Y. Kinoshita, M. Hetman, L. Patel, Z. Xia, R. J. Youle, and R. S. Morrison. 2000. p38 MAP kinase mediates Bax translocation in nitric oxide-induced apoptosis in neurons. J. Cell Biol. 150:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grethe, S., M. P. Ares, T. Andersson, and M. I. Porn-Ares. 2004. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp. Cell Res. 298:632-642. [DOI] [PubMed] [Google Scholar]
- 12.Hakem, R., A. Hakem, G. S. Duncan, J. T. Henderson, M. Woo, M. S. Soengas, A. Elia, J. L. de la Pompa, D. Kagi, W. Khoo, J. Potter, R. Yoshida, S. A. Kaufman, S. W. Lowe, J. M. Penninger, and T. W. Mak. 1998. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94:339-352. [DOI] [PubMed] [Google Scholar]
- 13.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 14.Harris, M. H., and C. B. Thompson. 2000. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 7:1182-1191. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich, K. A., and J. L. Kummer. 1996. Inhibition of p38 mitogen-activated protein kinase by insulin in cultured fetal neurons. J. Biol. Chem. 271:9891-9894. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, S.-C., M. A. Gavrilin, M.-H. Tsai, J. Han, and M.-Z. Lai. 1999. p38 mitogen-activated protein kinase is involved in Fas ligand expression. J. Biol. Chem. 274:25769-25776. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov, V. N., and Z. Ronai. 2000. p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene 19:3003-3012. [DOI] [PubMed] [Google Scholar]
- 18.Juo, P., C. J. Kuo, S. E. Reynolds, R. F. Konz, J. Raingeaud, R. J. Davis, H.-P. Biemann, and J. Blenis. 1997. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Mol. Cell. Biol. 17:24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 20.Kharbanda, S., S. Saxena, K. Yoshida, P. Pandey, M. Kaneki, Q. Wang, K. Cheng, Y.-N. Chen, A. Campbell, T. Sudha, Z.-M. Yuan, J. Narula, R. Weichselbaum, C. Nalin, and D. Kufe. 2000. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA damage. J. Biol. Chem. 275:322-327. [DOI] [PubMed] [Google Scholar]
- 21.Kuida, K., T. F. Haydar, C. Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M. S. Su, P. Rakic, and R. A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325-337. [DOI] [PubMed] [Google Scholar]
- 22.Kuida, K., T. S. Zheng, S. Na, C.-Y. Kuan, D. Yang, H. Karasuyama, P. Rakic, and R. A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. 384:368-372. [DOI] [PubMed]
- 23.Lenczowski, J. M., L. Dominguez, A. M. Eder, L. B. King, C. M. Zacharchuk, and J. D. Ashwell. 1997. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol. Cell. Biol. 17:170-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 25.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 26.Liao, P., D. Georgakopoulos, A. Kovacs, M. Zheng, D. Lerner, H. Pu, J. Saffitz, K. Chien, R.-P. Xiao, D. A. Kass, and Y. Wang. 2001. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. USA 98:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Los, M., M. Van de Craen, L. C. Penning, H. Schenk, M. Westendorp, P. A. Baeuerle, W. Droge, P. H. Krammer, W. Fiers, and K. Schulze-Osthoff. 1995. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature 375:81-83. [DOI] [PubMed] [Google Scholar]
- 28.Low, W., A. Smith, A. Ashworth, and M. Collins. 1999. JNK activation is not required for Fas-mediated apoptosis. Oncogene 18:3737-3741. [DOI] [PubMed] [Google Scholar]
- 29.Mackay, K., and D. Mochly-Rosen. 1999. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J. Biol. Chem. 274:6272-6279. [DOI] [PubMed] [Google Scholar]
- 30.Maundrell, K., B. Antonsson, E. Magnenat, M. Camps, M. Muda, C. Chabert, C. Gillieron, U. Boschert, E. Vial-Knecht, J.-C. Martinou, and S. Arkinstall. 1997. Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J. Biol. Chem. 272:25238-25242. [DOI] [PubMed] [Google Scholar]
- 31.Merritt, C., H. Enslen, N. Diehl, D. Conze, R. J. Davis, and M. Rincon. 2000. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8+ but not CD4+ T cells. Mol. Cell. Biol. 20:936-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra, R. S., D. M. Jelley-Gibbs, J. Q. Russell, G. Huston, S. L. Swain, and R. C. Budd. 2005. Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates. J. Immunol. 174:3999-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagata, S., and P. Golstein. 1995. The Fas death factor. Science 267:1449-1456. [DOI] [PubMed] [Google Scholar]
- 34.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 35.Park, J. G., Y. Yuk, H. Rhim, S. Y. Yi, and Y. S. Yoo. 2002. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J. Biochem. Mol. Biol. 35:267-272. [DOI] [PubMed] [Google Scholar]
- 36.Park, J. M., F. R. Greten, Z.-W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 37.Pedraza-Alva, G., S. Sawasdikosol, Y. C. Liu, L. B. Merida, M. E. Cruz-Munoz, F. Oceguera-Yanez, S. J. Burakoff, and Y. Rosenstein. 2001. Regulation of Cbl molecular interactions by the co-receptor molecule CD43 in human T cells. J. Biol. Chem. 276:729-737. [DOI] [PubMed] [Google Scholar]
- 38.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 39.Raingeaud, J., A. J. Whitmarsh, T. Barrett, B. Dérijard, and R. J. Davis. 1996. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reers, M., T. W. Smith, and L. B. Chen. 1991. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30:4480-4486. [DOI] [PubMed] [Google Scholar]
- 41.Rincon, M., H. Enslen, J. Raingeaud, M. Recht, T. Zapton, M. S.-S. Su, L. A. Penix, R. J. Davis, and R. A. Flavell. 1998. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 17:2817-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmena, L., B. Lemmers, A. Hakem, E. Matysiak-Zablocki, K. Murakami, P. Y. B. Au, D. M. Berry, L. Tamblyn, A. Shehabeldin, E. Migon, A. Wakeham, D. Bouchard, W. C. Yeh, J. C. McGlade, P. S. Ohashi, and R. Hakem. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmon, R., I. Foltz, P. Young, and J. Schrader. 1997. The p38 mitogen-activated protein kinase is activated by ligation of the T or B lymphocyte antigen receptors, Fas or CD40, but suppression of kinase activity does not inhibit apoptosis induced by antigen receptors. J. Immunol. 159:5309-5317. [PubMed] [Google Scholar]
- 44.Salvador, J. M., P. R. Mittelstadt, T. Guszczynski, T. D. Copeland, H. Yamaguchi, E. Appella, A. J. Fornace, and J. D. Ashwell. 2005. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat. Immunol. 6:390-395. [DOI] [PubMed] [Google Scholar]
- 45.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K.-M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwenger, P., P. Bellosta, I. Vietor, C. Basilico, and E. Y. Skolnik. 1997. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 94:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu, S., M. Narita, Y. Tsujimoto, and Y. Tsujimoto. 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399:483-487. [DOI] [PubMed] [Google Scholar]
- 48.Takeda, K., T. Hatai, T. S. Hamazaki, H. Nishitoh, M. Saitoh, and H. Ichijo. 2000. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275:9805-9813. [DOI] [PubMed] [Google Scholar]
- 49.Tourian, L., Jr., H. Zhao, and C. B. Srikant. 2004. p38α, but not p38β, inhibits the phosphorylation and presence of c-FLIPs in DISC to potentiate Fas-mediated caspase-8 activation and type I apoptotic signaling. J. Cell Sci. 117:6459-6471. [DOI] [PubMed] [Google Scholar]
- 50.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 51.Toyoshima, F., T. Moriguchi, and E. Nishida. 1997. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J. Cell Biol. 139:1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 53.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 54.Wang, C.-Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 55.Wang, Y., S. Huang, V. P. Sah, J. Ross, Jr., J. H. Brown, J. Han, and K. R. Chien. 1998. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273:2161-2168. [DOI] [PubMed] [Google Scholar]
- 56.Weiss, L., A. J. Whitmarsh, D. D. Yang, M. Rincon, R. J. Davis, and R. A. Flavell. 2000. Regulation of c-Jun NH2-terminal kinase (Jnk) gene expression during T cell activation. J. Exp. Med. 191:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitmarsh, A. J., and R. J. Davis. 2001. Analyzing JNK and p38 mitogen-activated protein kinase activity. Methods Enzymol. 332:319-336. [DOI] [PubMed] [Google Scholar]
- 58.Woo, M., R. Hakem, M. S. Soengas, G. S. Duncan, A. Shahinian, D. Kagi, A. Hakem, M. McCurrach, W. Khoo, S. A. Kaufman, G. Senaldi, T. Howard, S. W. Lowe, and T. W. Mak. 1998. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto, K., H. Ichijo, and S. J. Korsmeyer. 1999. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol. Cell. Biol. 19:8469-8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 62.Yoshino, T., H. Kishi, T. Nagata, K. Tsukada, S. Saito, and A. Muraguchi. 2001. Differential involvement of p38 MAP kinase pathway and Bax translocation in the mitochondria-mediated cell death in TCR- and dexamethasone-stimulated thymocytes. Eur. J. Immunol. 31:2702-2708. [DOI] [PubMed] [Google Scholar]
- 63.Yue, T.-L., J. Ni, A. M. Romanic, J.-L. Gu, P. Keller, C. Wang, S. Kumar, G.-L. Yu, T. K. Hart, X. Wang, Z. Xia, W. E. DeWolf, Jr., and G. Z. Feuerstein. 1999. TL1, a novel tumor necrosis factor-like cytokine, induces apoptosis in endothelial cells. Involvement of activation of stress protein kinases (stress-activated protein kinase and p38 mitogen-activated protein kinase) and caspase-3-like protease. J. Biol. Chem. 274:1479-1486. [DOI] [PubMed] [Google Scholar]
- 64.Zechner, D., R. Craig, D. S. Hanford, P. M. McDonough, R. A. Sabbadini, and C. C. Glembotski. 1998. MKK6 activates myocardial cell NF-kappa B and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 273:8232-8239. [DOI] [PubMed] [Google Scholar]
- 65.Zheng, T. S., S. Hunot, K. Kuida, T. Momoi, A. Srinivasan, D. W. Nicholson, Y. Lazebnik, and R. A. Flavell. 2000. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat. Med. 6:1241-1247. [DOI] [PubMed] [Google Scholar]
- 66.Zheng, T. S., S. F. Schlosser, T. Dao, R. Hingorani, I. N. Crispe, J. L. Boyer, and R. A. Flavell. 1998. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc. Natl. Acad. Sci. USA 95:13618-13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang, S., J. T. Demirs, and I. E. Kochevar. 2000. p38 mitogen-activated protein kinase mediates Bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 275:25939-25948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.