Abstract
Apoptotic cell death of photoreceptors is the final event leading to blindness in the heterogeneous group of inherited retinal degenerations. GDNF (glial cell-line-derived neurotrophic factor) was found to rescue photoreceptor function and survival very effectively in an animal model of retinal degeneration (M. Frasson, S. Picaud, T. Leveillard, M. Simonutti, S. Mohand-Said, H. Dreyfus, D. Hicks, and J. Sahel, Investig. Ophthalmol. Vis. Sci. 40:2724-2734, 1999). However, the cellular mechanism of GDNF action remained unresolved. We show here that in porcine retina, GDNF receptors GFRα-1 and RET are expressed on retinal Mueller glial cells (RMG) but not on photoreceptors. Additionally, RMG express the receptors for the GDNF family members artemin and neurturin (GFRα-2 and GFRα-3). We further investigated GDNF-, artemin-, and neurturin-induced signaling in isolated primary RMG and demonstrate three intracellular cascades, which are activated in vitro: MEK/ERK, stress-activated protein kinase (SAPK), and PKB/AKT pathways with different kinetics in dependence on stimulating GFL. We correlate the findings to intact porcine retina, where GDNF induces phosphorylation of ERK in the perinuclear region of RMG located in the inner nuclear layer. GDNF signaling resulted in transcriptional upregulation of FGF-2, which in turn was found to support photoreceptor survival in an in vitro assay. We provide here a detailed model of GDNF-induced signaling in mammalian retina and propose that the GDNF-induced rescue effect on mutated photoreceptors is an indirect effect mediated by retinal Mueller glial cells.
A major cause of blindness in the Western world is the degeneration of photoreceptors (PR) as a result of point mutations in genes coding for either phototransduction-related proteins or other proteins important for retinal function (reviewed in reference 25). Irrespective of the diversity of mutated genes and proteins involved in this heterogeneous group of progressive retinal dystrophies with homologous phenotypes, the final event leading to blindness is apoptosis of PR. This has prompted investigation of the effects of neuroprotective agents on PR survival in animal models of retinitis pigmentosa. One of the major effective molecules discovered to rescue retinal PR was glial cell line-derived neurotrophic factor (GDNF), initially purified from a rat glioma cell line supernatant as a trophic factor for embryonic midbrain dopamine neurons (35) and later found to have pronounced effects on other neuronal subpopulations (reviewed in reference 2). GDNF is a distant member of the transforming growth factor β (TGF-β) superfamily and a founder protein of the GDNF family ligand (GFL), which includes neurturin (NRTN), artemin (ARTN), and persephin (PSPN). GFLs bind specific GFRα1-4 coreceptors that are either linked to the plasma membrane by a glycosyl phosphatidylinositol (GPI) anchor or, by cleavage through an unknown protease, provided as a soluble coreceptor. All four different GFLs (GDNF, NRTN, ARTN, and PSPN) signal via the activation of the RET receptor tyrosine kinase, a single-pass transmembrane protein containing four cadherin-like repeats in the extracellular domain and a typical intracellular tyrosine kinase domain (reviewed in reference 2). GDNF is widely distributed in the central and peripheral nervous systems and is also expressed in the inner ear, olfactory epithelium, carotid body, kidney, and gastrointestinal tract (38). In the eye, GDNF is primarily expressed in the retina, and several investigators have shown its potential therapeutic value by providing neuroprotection in the context of retinal degeneration. GDNF has been shown to rescue retinal ganglion cells after axotomy (29, 55) and has been proven very effective in retarding retinal PR degeneration in the rd1 mouse (19). Subretinal application of GDNF has led to decreased loss of PR, as well as a significant functional rescue, shown by recordable ERG on PN22 compared to untreated animals.
Recent evidence has proposed that neurotrophic rescue of PR may be indirect, mediated by interaction of neurotrophic factors with retinal Mueller glial cells (RMG), which in turn release or present secondary factors acting directly on PR (20, 21, 53; reviewed in reference 7). Upon subretinal injection of GDNF in the rd1 mouse, RMG react by upregulation of glial fibrillary acidic protein (19), indicating that GDNF-induced regulation in RMG gene expression and subsequent effects on PR may be mediated through an indirect pathway.
To elucidate the role of RMG in GDNF-mediated protection of PR, we analyzed expression and activity of transducer elements involved in GDNF signaling in porcine retina and further analyzed candidate target genes of GDNF signaling.
MATERIALS AND METHODS
Adult porcine eyes were provided by a local slaughterhouse. They were removed from the animals within 5 min after death and kept on ice in CO2-independent medium (GIBCO) until further use. Within 1 h after the death of the animals, either the eyes were fixed for immunolabeling or retinae were dissected for preparation of RMG or PR.
Immunolabeling.
Porcine eyes were fixed in 10% formalin. For adequate and fast fixation, both cornea and lens were removed and the aqueous humor was replaced by fixative. After dehydration, the eyes were embedded in paraffin.
Tissue sections (5 μm) were mounted on coated slides (Super Frost Plus), deparaffinized, and rehydrated. High-temperature antigen retrieval was performed using 1 mM EDTA buffer, pH 8.0, as described previously (37). Slides were rinsed in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBS-T; pH 7.3), and nonspecific binding was blocked with 3% bovine serum albumin (BSA) in PBS-T. Sections were then incubated with primary antibodies from R&D (anti-RET, anti-GFRα-1, anti-GFRα-2, and anti-GFRα-3 at 1:200 in 3% BSA-PBS-T) overnight at 4°C in a humid chamber, followed by incubation with Alexa 546 donkey anti-goat immunoglobulin G (IgG) conjugate (1:200; Molecular Probes) and DAPI (4′,6′-diamidino-2-phenylindole; 200 nM; Sigma) for 2 h at room temperature. After washing, sections were incubated for another 8 h at 4°C with primary antibodies anti-glutamine-synthetase (anti-GS) (1:500; Transduction Laboratories) or anti-GLAST (1:200; Alpha Diagnostic), followed by incubation for 2 h with Alexa 488 anti-mouse or anti-rabbit IgG, respectively, and, after final washing, mounted in FluorSave (Calbiochem). Images were obtained with a Zeiss APO-TOME.
Immunolabeling on GDNF-stimulated retina sections.
Retinae were dissected from porcine eyes as described previously (18), cut into equal pieces, and placed into 12-well dishes containing Dulbecco's modified Eagle's medium combined with F-12 medium (DMEM/F-12; GIBCO) at one piece per well. After an initial incubation phase (10 min), stimulation was performed with 100 ng/ml GDNF (PeproTech) in DMEM/F-12 or medium alone. After the indicated time points, the medium was replaced by ice-cold paraformaldehyde (4% in PBS, 5% sucrose) and the tissue was fixed for 1 h at 4°C. Cryopreservation was performed by incubation for 1 h each in 5% and 10% sucrose in PBS, followed by incubation overnight in 20% sucrose in PBS, all at 4°C. The tissue samples were frozen in TissueTek on liquid nitrogen and stored at −80°C. Tissue pieces were sectioned at 10 μm in a cryostat, collected on gelatin-coated Super Frost slides, and stored at −20°C.
For immunolabeling, sections were rinsed in PBS and incubated for 10 min in 0.1% Triton 100 in PBS, followed by blocking for 30 min in 3% BSA in PBS-T. Incubation with primary antibodies (anti-pERK, 1:250 [Cell Signaling Technology]; or anti-GS at 1:500 [BD Transduction Laboratories], diluted in 3% BSA-PBS-T) was done overnight at 4°C and then followed by incubation with Alexa 488/568-coupled secondary antibodies (1:200; Molecular Probes) for 2 h at room temperature. After a final washing, sections were mounted in FluorSave (Calbiochem) and images were obtained with a Zeiss Axioskop II. pERK-positive cells within the inner nuclear layer (INL) were counted on two visual fields (×100 magnification) per section, there were 10 different sections per experiment, and the experiments were repeated twice.
NCAM immunolabeling.
Paraformaldehyde-fixed retinae were cryopreserved, sectioned at 10 μm, and stored at −80°C. Immunolabeling was performed as described above, with anti-neural cell adhesion molecule (NCAM) antibody (MAB2120Z, 1:200; Chemicon), followed by anti-mouse Alexa 488-coupled secondary antibody.
Statistical significance.
Statistical significance was calculated using the Peritz F parametric test (22).
Isolation and in vitro culturing of primary RMG.
RMG were isolated from porcine eyes employing a panning method as described previously (23) and cultured in DMEM/HEPES (GIBCO) with 10% fetal calf serum (FCS) to confluence (2 to 3 weeks). The purity of these cultures is above 97% (22).
Stimulation of RMG and preparation of cell lysates.
One day prior to stimulation, RMG were washed, transferred into serum-free medium (DMEM/HEPES; GIBCO), and stimulated with either GDNF (100 ng/ml; PeproTech), NRTN (100 ng/ml; PeproTech), or ARTN (100 ng/ml; PeproTech) for the indicated time points. Inhibitors were applied 20 min both prior to and during stimulation at the following concentrations: U0126 (Promega), 10 μM; and LY294002 (Promega), 50 μM. For phosphotyrosine detection, stimulation was performed in the presence of phosphatase inhibitor cocktail 2 (Sigma), composed of sodium orthovanadate, sodium molybdate, sodium tartrate, and imidazole. Stimulation was stopped by addition of liquid nitrogen, and lysates were then prepared by addition (per 10-cm dish) of 300 μl lysis buffer (50 mM Tris, pH 7.4, 250 mM NaCl, 25 mM EDTA, 1% NP-40, 10% glycerol, and Complete protease inhibitor cocktail; Roche), including phosphatase inhibitor cocktail 2 (Sigma). Protein content of the lysates was determined by Bradford assay.
Photoreceptor preparation and survival assay.
Porcine PR were prepared as described elsewhere (18), with the following slight modifications: enzymatic digestion of retinal pieces was stopped by addition of DMEM/F-12 medium (GIBCO) supplemented with 2% FCS. PR were collected from supernatant and plated at 1.3 × 105 cells per well onto precoated Falcon 96-well plates that were first coated with p-lysine (2 μg/cm2, 3 h; Sigma) followed by laminin (1 μg/cm2, overnight; BD Bioscience). Different concentrations of FGF-2 (20 ng/ml, 100 ng/ml, 500 ng/ml, or 1,000 ng/ml; purified FGF-2 from bovine brain; R&D) or respective GFLs (GDNF, NRTN, or ARTN, all 100 ng/ml and 500 ng/ml; PeproTech) in DMEM/F-12 medium or medium alone were applied on attached PR 20 h after preparation. PR survival was monitored by performing an esterase calcein-fluorophore assay (Molecular Probes), whereby living cells fluoresce bright green. Fluorescence was measured daily on a different row of the plate using a fluorescence reader (BioTek; Synergy HT). All values were compared to initial fluorescence at the beginning of the survival assays. Relative fluorescence was calculated as a percentage of initial fluorescence, whereby every value is a mean of two to four wells per day. Every experiment was performed at least in triplicate.
Western blots.
Protein lysates (10 μg) of RMG were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted semidry onto polyvinylidene difluoride membranes. Unspecific binding was blocked for 1 h with 5% BSA in TBS-T. Blots were incubated with primary antibodies in 5% BSA-TBS-T overnight at 4°C [anti-pTyr100, 1:1,000; anti-pMEK1/2, 1:1,000; anti-pERK, 1:5,000; anti-pSAPK (stress-activated protein kinase), 1:2,000; or anti-pAKT (Ser473), 1:1,000] (Cell Signaling Technology), washed, and incubated in horseradish peroxidase-coupled secondary antibodies (1:15,000; Jackson Laboratories). Signal was developed with the ECL+ enhanced chemiluminescence kit (Amersham) according to the manufacturer's instructions and detected on Hyperfilm ECL (Amersham). Blots were stripped and reprobed with actin (1:1,000; Oncogene) or vimentin (clone V9, 1:1,000; Sigma) antibodies to verify equivalent protein loading.
RT-PCR.
Total RNA was prepared from retina, RMG (either directly after the panning procedure or from confluent cultures), or PR with an RNeasy kit (QIAGEN) according to the manufacturer's instructions. Reverse transcription (RT) was performed with the RT kit (QIAGEN), and 70 ng cDNA was set for PCR (annealing at 65°C, 35 cycles) with the following primers: RET for (CTGGACTCCATGGAGAACC) and RET rev (TCCAGGTCTTTGCTGATGTC), product size, 929 bp; GFRα-1 for (TAAAGGAAAACTACGCTGACTGCC) and GFRα-1 rev (TGCCCGACACATTGGATTTC), product size, 353 bp; GFRα-2 for (TTTGTCGTGAGCTCTGTGAAGC) and GFRα-2 rev (GCATGATTGGGTCCGAGATAAC), product size, 388 bp; GFRα-3 for (AGGAGCCTTCAGTCCCCAAG) and GFRα-3 rev (TGAGGCAGCGATCCCAATC), product size, 200 bp; GDNF for (CAGTGACTCAAATATGCCAGAGGA) and GDNF rev (AGATACATCCACACCTTTTAGCGG), product size, 491 bp; vimentin for (GTTTCCAAGCCTGACCTCAC) and vimentin rev (AATCTCATCCTGCAGGCG), product size, 335 bp; rhodopsin for (GGCTTCCCCATCAACTTCCTC) and rhodopsin rev (GTGGCTGACTCCTGCTGCTG), product size, 534 bp; GS for (GACTGCGCTGCAAGACCCG) and GS rev (ATGTGGTACTGGTGCCGCTTG), product size, 699 bp; GLAST for (ATTGTTGAGATGGAAGACATGGGAGTGATTG) and GLAST rev (TGGCTGTGATGCTGATGGTGATAATCTGTC), product size, 400 bp; and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) for (CGGGAAGCTTGTCATCAATGG) and GAPDH rev (GGCAGTGATGGCATGGACTG), product size, 358 bp. Products were of the expected sizes and were sequenced for identity verification.
Northern blot analysis.
One day prior to stimulation, RMG were washed, transferred into serum-free medium (DMEM/HEPES), and stimulated for 24 h with GDNF (100 ng/ml; PeproTech). Northern blotting was performed as described elsewhere (42). Briefly, total RNA was prepared with the RNeasy kit (QIAGEN), and examination of integrity and quantification were performed with an RNA-nano chip on a 2100 Bioanalyzer (Agilent). Equal amounts (5 μg) were loaded onto formaldehyde gels, resolved, and blotted. Detection of FGF-2 transcript was performed by generation of an FGF-2-specific digoxigenin (DIG)-labeled RNA with a DIG labeling kit (Roche) using an RT-PCR product generated from RMG with the following primers: FGF-2 for (CAAGCGGCTGTACTGCAAAAAC) and FGF-2 T7 promoter rev (TAATACGACTCACTATAGGATGTGGCCATTAAAATCAGCTCTT). The detected transcript was approximately 6 kbp and thus similar in size to that of the full-length human transcript (6.774 kbp, ENST00000264498; http://www.ensembl.org/).
FGF-2 ELISA.
RMG were seeded onto 96-well culture plates at 2 × 104 cells per well, incubated for 24 h in DMEM/25 mM HEPES in the presence of 10% FCS, and then, prior to stimulation, incubated for an additional 24 h without FCS. RMG were then left either unstimulated or stimulated with GDNF, NRTN, or ARTN (all 100 ng/ml; PeproTech) in the absence or presence of U0126 (10 μM; Promega) for 16 h. Medium from the wells was collected after stimulation, and the amount of secreted FGF-2 was analyzed by an FGF-2 enzyme-linked immunosorbent assay (ELISA) (DuoSet; R&D) according to the manufacturer's instructions; the experiment was performed in triplicate.
RESULTS
GDNF receptor components are expressed on RMG in porcine retina.
In order to clarify whether GDNF-mediated PR rescue effects are exerted directly or involve indirect retinal pathways, we first analyzed the expression patterns of GDNF receptor components in porcine retina.
Expression of transmembrane receptor RET was found in all retinal layers (Fig. 1A and B). Similar expression patterns were found for GFRα-1 (C and D), GFRα-2 (E and F), and GFRα-3 (G and H), thus indicating the possibility of receptor expression on membranes of RMG, since cell bodies of RMG are located in the INL and extensive RMG processes penetrate through all retinal layers. Costaining with the cellular RMG marker GS (Fig. 1A, C, E and G) supported this hypothesis. In order to localize precisely the site of expression, sections were additionally costained with antibodies against GLAST, a membrane glutamate receptor expressed in RMG (39, 43). Costaining with GLAST produced exact colocalization in overlay images, indicating that receptor components for GDNF, NRTN, and ARTN are indeed confined to the RMG membrane (Fig. 1B, D, F, and H). The fluorescence signal observed for PR inner and outer segments is due to autofluorescence and was revealed by comparison with negative controls taken at similar exposure times as pictures of RET and GLAST immunolabelings (omission of primary antibodies, Fig. 1I and K).
FIG. 1.
Detection of GDNF family receptors on tissue sections of porcine retina. Porcine eyes were fixed and embedded in paraffin and sectioned (5 μm), and nuclei were stained with DAPI. The Nomarski image and DAPI stain were overlaid to visualize retinal structures (left picture in each panel). Sections were stained with primary antibodies against RET (A and B), GFRα-1 (C and D), GFRα-2 (E and F), or GFRα-3 (G and H), followed by visualization with red fluorophore (Alexa 546). Additionally, all sections were stained with primary antibodies against RMG-specific proteins, anti-GS (A, C, E, and G) or anti-GLAST (B, D, F, and H), and visualized with green fluorophore (Alexa 488). Overlay images (right picture in each panel) demonstrate complete colocalization of all GDNF family receptor components with the membrane-localized RMG-specific marker, GLAST. Pictures of negative controls (omitted primary antibodies) taken at similar exposure times as pictures of RET and GLAST immunolabeling, respectively, are provided (I and K) to illustrate autofluorescence-derived signals at the level of photoreceptor segments. RPE, retinal pigment epithelium; OS, outer segments of PR; IS, inner segments of PR; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Expression of GDNF receptor components on isolated RMG in vitro.
Since RMG in the whole retina were found to express GDNF receptor components, we further investigated the expression of these receptor components in isolated RMG. We have previously shown that RMG protein expression patterns are markedly altered upon in vitro culturing (23). Therefore, we addressed the question of whether the GDNF receptor components remain expressed in vitro.
RT-PCR analysis of mRNA prepared from porcine retina, freshly isolated RMG, and RMG cultured in vitro for 2 weeks showed that whole retina and freshly isolated RMG express RET, GFRα-1, GFRα-2, and GFRα-3 and that RMG cells retained expression of these components after 2 weeks in culture (Fig. 2A, B, and C). Although GDNF itself was not expressed in freshly isolated RMG, its expression increased after 2 weeks in vitro, indicating the establishment of an autocrine activation loop. No expression of GDNF receptor components was found in freshly isolated PR (Fig. 2D); although those preparations yield highly enriched PR (48), they are contaminated, e.g., by RMG, as can be seen by signals for the RMG markers vimentin, GS, and GLAST. Preparations of RMG yield those cells at a purity of approximately 75% on day 1 (23), and those preparations do show expression of receptor components. Additionally, there is expression of rhodopsin in these preparations, reflecting the residual proportion of photoreceptors. Most interestingly, mRNA for rhodopsin is still detectable in RMG at day 14, when those cells are grown in vitro to a purity of above 97% (23). This underlines the difficulties in preparation of cell-specific mRNA pools from complex mixtures.
FIG. 2.
RT-PCR detection of receptor components. RT-PCR of Ret (lane 1), GFRα-1 (lane 2), GFRα-2 (lane 3), GFRα-3 (lane 4), GDNF (lane 5), rhodopsin (lane 6), vimentin (lane7), glutamine synthetase (lane 8), GLAST (lane 9), and GAPDH (lane 10) in RMG on days 1 (d1) and 14 (A and B, respectively), porcine retina (C), and isolated PR (D). RMG show expression of RET receptor and coreceptors GFRα-1, GFRα-2, and GFRα-3 (lanes 1, 2, 3, and 4, respectively, in panels A and B). Additionally, GDNF expression is induced in RMG at day 14 in vitro (B, lane 5). In contrast to RMG and total retina, isolated PR do not express any of the GDNF family receptors (D). The identities of all PCR products were verified by sequencing.
GDNF phosphorylates RMG RET receptor in vitro.
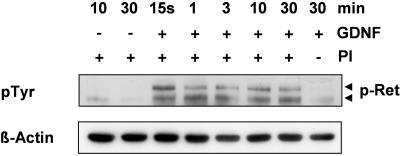
In order to investigate GDNF signaling in RMG in vitro, we first tested GDNF-induced tyrosine phosphorylation. Western blots incubated with an antibody specific for phosphotyrosine showed specific bands of the expected size for the RET receptor (170 and 150 kDa), appearing just 15 s following GDNF stimulation (Fig. 3) and remaining stable for 30 min in the presence of phosphatase inhibitors. In the absence of phosphatase inhibitors, no such phosphorylation could be detected after 30 min. Application of phosphatase inhibitors in the absence of GDNF did not result in higher phosphorylation. Similar results were obtained after stimulation of RMG with ARTN (data not shown).
FIG. 3.
GDNF-induced receptor phosphorylation. GDNF induces rapid phosphorylation (after 15 s) of two bands with the expected size for the RET receptor (150 kDa and 170 kDa), detected with anti-phosphotyrosine antibody (pTyr100; CST) in lysates of RMG. Equal amounts of protein were loaded as demonstrated by reincubation with anti-β-actin antibody. PI, phosphatase inhibitors. The data shown are representative of two independent experiments.
GDNF, neurturin, and artemin activate several RMG signaling cascades in vitro.
Consistent with the expression of coreceptors GFRα-1, GFRα-2, and GFRα-3 in RMG, we found that all three ligands (GDNF, NRTN, and ARTN) could activate signaling cascades in RMG in vitro. Five minutes of GDNF stimulation led to maximum phosphorylation of both MEK and ERK (Fig. 4A). Similar signaling cascades were also activated following stimulation with NRTN and ARTN (Fig. 4B and C). ERK phosphorylation was completely blocked by application of the MEK inhibitor U0126. Whereas GDNF led to strong phosphorylation of SAPK after 5 min, stimulation with ARTN resulted in weaker phosphorylation; NRTN induced SAPK phosphorylation after 10 min. In all three cases, SAPK activation could be partially blocked by the phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor LY294002, indicating that SAPK phosphorylation may be partly induced by active PI 3-kinase. SAPK phosphorylation was completely blocked by the MEK inhibitor U0126. PKB/AKT was phosphorylated by stimulation with NRTN, ARTN, and GDNF at 1, 5, and 10 min, respectively. This phosphorylation was completely blocked by addition of the PI 3-kinase inhibitor LY294002. PKB/AKT phosphorylation was also blocked by the MEK inhibitor U0126.
FIG. 4.
GDNF-, NRTN-, and ARTN-induced signaling in RMG. (A) GDNF induces phosphorylation of both MEK and ERK after 5 min, and the latter can be blocked with MEK inhibitor (U0126). Furthermore, GDNF phosphorylates SAPK/JNK after 5 min, which can be also blocked completely by U0126 and partially by PI 3-kinase inhibitor, LY294002. In addition to activating the MAPK and SAPK pathways, GDNF-induced activation of AKT/PKB was observed after 10 min of treatment, which could be also blocked by PI 3-kinase inhibitor and partially by MEK inhibitor. (B) Similar signaling cascades as described in panel A are induced by stimulation of RMG with NRTN but showing a slightly different time course: SAPK/JNK phosphorylation is observed after 10 min and AKT/PKB after only 1 min of stimulation. (C) Similar signaling cascades as described in panel A are induced by stimulation of RMG with ARTN but showing a slightly different time course: AKT/PKB after 5 min of stimulation. Equal amounts of protein were loaded under each condition as demonstrated by reincubation with antivimentin (Vim) antibody. The data shown are representative for at least three independent experiments of each stimulation set.
GDNF-induced signaling is independent of NCAM in RMG.
It has been reported that alternatively to transmembrane receptor Ret, GDNF can transduce signals into cells via NCAM (40). Therefore, we investigated whether this pathway is utilized by GDNF in the context of RMG. Immunolabeling experiments demonstrate that NCAM is expressed throughout the retina (Fig. 5A), except for photoreceptor inner and outer segments.
FIG. 5.
NCAM in porcine retina. (A) Immunolabeling of NCAM on porcine retinal section (left panel) and visualization of the section with Nomarski optics (right panel). NCAM expression is detected in all retinal layers except for outer and inner segments of photoreceptors (OS/IS); ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (B) GDNF-induced phosphorylation of MEK, ERK, and PKB/AKT is not inhibited by PP2, an inhibitor of intracellular Src family kinases. Equal amounts of protein were loaded under each condition, as demonstrated by reincubation with anti-total ERK antibody. The data shown are representative for two independent experiments.
GDNF-induced signaling via NCAM has been reported to involve the Src family tyrosine kinase Fyn. Thus, we stimulated RMG with GDNF in the presence of 1 μM PP2, an inhibitor of Src family tyrosine kinases, which at this concentration is reported to only minimally inhibit transmembrane tyrosine kinase Ret (14). We found that inhibition of Src family kinases affected neither GDNF-induced phosphorylation of MEK and ERK nor GDNF-induced phosphorylation of AKT (Fig. 5B). However, application of 5 μM PP2, a concentration which is known to block Ret activation, completely abolished activation of MEK/ERK, SAPK, and PKB/AKT signaling cascades (data not shown). We thus concluded that in RMG, GDNF-induced signaling is transduced across the plasma membrane mainly by Ret.
GDNF activates ERK in cells of the inner nuclear layer in retinal explants.
In order to confirm that GDNF is able to induce signaling in intact retina, we dissected fresh porcine retina and immediately treated it with GDNF or medium alone for 20 min. Tissue samples were then fixed, cryopreserved, and sectioned. Immunohistochemical staining for pERK showed intensely positive areas close to nuclei of cells in the INL and additionally in the inner plexiform layer (IPL) and ganglion cell layer (GCL) of GDNF-treated sections compared to untreated sections (Fig. 6A). Coimmunolabeling for glia-specific marker GS reveals a colocalization of all pERK-positive signals with glial cells (Fig. 6B). Quantification of pERK-positive signals within the INL revealed a significant (P < 0.001) increase in response to GDNF stimulation (Fig. 6C).
FIG. 6.
Detection of pERK in retina after stimulation with GDNF. (A) GDNF induces ERK phosphorylation in close proximity to cell nuclei of the inner nuclear layer in intact retina after stimulation for 20 min (right panel), as compared to unstimulated controls (left panel). OS, outer segments of PR; IS, inner segments of PR; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. (B) Colocalization of pERK with glutamine synthetase (GS): all pERK-positive signals after GDNF stimulation (green) could be colocalized with the cytosolic glial cell-specific protein GS (red). Stimulation of ERK (pERK, green) was found in the inner nuclear layer (INL) in close proximity to nuclei (large arrow in upper panels and similar section enlarged in lower panels, counterstained with DAPI for visualization of nuclei). Additionally, pERK-positive signals were found in inner plexiform layer (IPL) and ganglion cell layer (GCL); in all cases, the signals colocalized with GS (open arrows, upper panels). The upper right panel shows visualization of the section with Nomarski optics. OS/IS, outer and inner segments of photoreceptors; ONL, outer nuclear layer. (C) Quantification of pERK-positive cells shows significant increase after GDNF stimulation (P < 0.001).
GDNF upregulates expression of FGF-2 in vitro.
An upregulation of FGF-2 by RMG in response to NT-3 treatment has been demonstrated (21), and FGF-2 has been shown to protect PR in vivo (15). We therefore investigated whether GDNF treatment could increase FGF-2 expression in RMG. Indeed, Northern blot analysis demonstrated that stimulation with GDNF for 24 h increased the FGF-2 transcript level in RMG (Fig. 7A).
FIG. 7.
GDNF, ARTN, and NRTN induce secretion of FGF-2. (A) GDNF-induced upregulation of FGF-2 mRNA. RMG were either incubated in medium alone or stimulated with 100 ng/ml GDNF for 24 h, Northern blot analysis was performed with 5 μg RNA per lane, and FGF-2 transcript was detected with DIG-labeled RNA probe. The experiment was repeated three times. GDNF increases FGF-2 transcript in RMG. Equal loading was controlled by methylene blue staining (data not shown). (B) GDNF-, NRTN-, and ARTN-induced FGF-2 secretion. Shown are the results of the FGF-2 ELISA of culture medium from RMG stimulated for 16 h with GDNF, NRTN, or ARTN in the absence or presence of the MEK inhibitor U0126, as compared to unstimulated RMG. All three factors increase FGF-2 secretion, which is reduced with U0126 below the basic level of FGF-2 secretion from unstimulated RMG (*, P < 0.05; **, P < 0.01).
GDNF, NRTN, and ARTN stimulate secretion of FGF-2.
In order to verify that GDNF-induced upregulation of FGF-2 expression also led to increased secretion of FGF-2 by RMG, we quantified FGF-2 levels in the culture medium following incubation with GDNF, NRTN, and ARTN using an FGF-2 ELISA. All three factors induced secretion of FGF-2 by RMG compared to unstimulated controls. Unstimulated RMG medium contained 33.8 ± 3.8 pg/ml FGF-2, whereas GDNF stimulation resulted in increases of FGF-2 in the medium to 46.1 ± 3.4 pg/ml, NRTN to 48.1 ± 1.7 pg/ml, and ARTN to 47.7 ± 8.8 pg/ml. Moreover, FGF-2 secretion was dependent on active MEK as application of the MEK inhibitor U0126 not only prevented increased secretion (Fig. 7B), but also lowered GDNF-induced secretion of FGF-2 below the level observed for unstimulated RMG. Similar results were obtained for NRTN- and ARTN-induced secretion in the presence of U0126 (data not shown).
FGF-2 prolongs survival of porcine photoreceptors in vitro.
Although the results of our studies indicate that GDNF targets RMG rather than PR in porcine retina, we wanted to investigate direct protective effects of GFLs on PR. GDNF, NRTN, and ARTN were applied to porcine PR in vitro, and survival was monitored by a calcein-esterase assay. In accordance with our hypothesis, neither of the tested GFLs (at concentrations of 100 ng/ml and 500 ng/ml) prolonged survival of PR compared to medium alone (Fig. 8A).
FIG. 8.
FGF-2 prolongs survival of porcine photoreceptors in vitro. Porcine PR were plated in 96-well culture plates, treated as indicated for 5 days in vitro, and cell survival was monitored by an esterase calcein-fluorophore assay. Every value is a mean of two to four wells per experiment, and each experiment was performed in triplicate. (A) GFLs were applied to PR in vitro at either 100 ng/ml or 500 ng/ml. Neither GDNF, NRTN (NTN), nor ARTN (ART) increased survival of photoreceptors compared to medium alone. (B) FGF-2 increased survival of PR in vitro in a concentration-dependent manner: whereas 20 ng/ml did not affect survival, treatment with 100, 500, and 1,000 ng/ml had increasing and significant effects on PR survival (*, P < 0.05; **, P < 0.01).
In order to verify that FGF-2 promotes PR survival, different concentrations of FGF-2 were applied to porcine PR in vitro. We found that FGF-2 significantly increased numbers of surviving PR in a concentration-dependent manner. The survival-supporting effect of FGF-2 was already detectable after 2 days in vitro (data not shown) and was most pronounced after 5 days in vitro (Fig. 8B). Concentrations of 20 ng/ml and 100 ng/ml did not increase survival compared to the negative control, but FGF-2 at 500 ng/ml and 1,000 ng/ml robustly enhanced survival of PR.
DISCUSSION
The large heterogeneity of retinal diseases leading to PR death and consequent loss of vision in patients requires development of alternative therapeutic strategies beyond corrective gene therapy. A promising approach has been to target dying PR with molecules known to act neuroprotective in brain and, in such a manner, delay disease progression. Although this has been achieved with several molecules in animal models of retinal degeneration (reviewed in reference 9), the underlying mechanisms of PR rescue in most of these studies remain unresolved.
Retinal Mueller glial cells as target cells for GDNF-induced signaling in porcine retina.
One of the most effective molecules in rescuing PR function thus far has been GDNF, which rescued PR at both morphological and functional levels in the rd1 mouse model of retinal degeneration (19). The molecular mechanisms of GDNF-induced PR rescue have not been resolved to date but are a fundamental prerequisite for therapeutic application of this molecule. In our study, we examined the expression of GDNF receptor components in the retina. The porcine retina was selected as a model due to its high density of cone cells (24), as well as the close similarity between porcine and human eyes with respect to dimension and anatomical and physiological features (36, 49).
We found that the transmembrane receptor RET, as well as GDNF coreceptor GFRα-1, was not expressed by PR but rather by RMG in porcine retina. In addition to GFRα-1, RMG express the coreceptors necessary for NRTN and ARTN signaling: GFRα-2 and GFRα-3. Studies on the expression of GDNF receptor components in rat retina localized RET and GFRα-1 in PR, the inner nuclear layer, inner plexiform layer, and ganglion cell layer (27), suggesting, among other cells, a localization on RMG, whereas Harada and coworkers (20) have reported expression of GFRα-1 and GFRα-2 receptors mainly in PR. Other evidence has reported GFRα-1 expression in retinal ganglion cells, RMG, and PR (30). These discrepancies have been proposed to reflect specific differences between the rat strains used for the studies (20). Additionally, there are indications that retinae of rodents and other mammals differ in the expression and distribution of neurotrophic factor receptors. As shown for CNTFRα, its expression is absent in PR of adult and developing rodent retinas but rather consistently found in rods and cones of nonrodent mammals, including pigs (6). With regard to GDNF, our findings that GFRα receptors and RET are absent from PR but abundant on RMG lead to the conclusion that, in porcine retina, the neurotrophic effect exerted by GDNF is transduced not directly to PR but indirectly via RMG.
GDNF has been found to signal via NCAM/GFRα-1 complexes in the absence of Ret. Thus, the absence of Ret receptors on PR may still leave the possibility of Ret-independent signaling. Therefore, we investigated here whether GDNF could prolong survival of photoreceptors directly. We found that neither GDNF nor NRTN or ARTN could prolong survival of PR in vitro, even when applied at similarly high concentrations to FGF-2, which in contrast to the tested GFLs was found to prolong survival of PR (Fig. 8). This underlines that even though there is expression of NCAM on PR (Fig. 5A) (5, 16), this receptor does not trigger GFL-induced PR survival in vitro.
There has been increasing evidence for the crucial role of RMG in prolonging PR survival during progressive retinal degenerations (57), and intravitreous injection of brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), or FGF-2 in eyes of wild-type or mutant rats and mice resulted in increased pERK and c-fos immunostaining in cells of the inner retina, particularly RMG but not PR (52, 53). In accordance with activation of RMG by these factors, we propose here that a similar indirect survival-promoting pathway is induced following GDNF application.
GDNF-, NRTN-, and ARTN-induced signaling in RMG.
In order to examine molecular action of GDNF in detail, we used pure primary cell cultures of porcine RMG (23) as a tool to address GDNF-induced signaling exclusively in a GDNF-targeted retinal cell. We found that GDNF, as well as NRTN and ARTN, induced several distinct signaling cascades in primary RMG: MEK/ERK, SAPK, and PKB/AKT, which have been previously described to be activated by those ligands (recently reviewed in reference 26). However, the majority of studies were performed on cell lines, especially those expressing constitutively active RET mutations derived from multiple endocrine neoplasia (MEN2A and MEN2B). Most interestingly, our study revealed considerable cross talk between the activated signaling cascades. Our results with MEK inhibitor U0126 (13) demonstrated that GDNF-, NRTN-, and ARTN-induced MEK activation not only triggers ERK but also regulates SAPK and PKB/AKT phosphorylation (see Fig. 9). This could result from either cross talk or unspecific inhibition by the compound U0126. U0126 has been reported to inhibit other kinases than MEK (10) to some extent (mostly to residual 80% activity), but unspecific inhibition of PI3K has not been examined. Furthermore, as we do not know the upstream kinase involved in phosphorylation of SAPK observed here, we can in both cases not exclude unspecific inhibition by U0126. However, the observed complete inhibition of PKB/AKT and SAPK phosphorylation most likely derives not exclusively from unspecific inhibition alone but additionally from cross talk between activated signaling cascades, because unspecific action alone is not expected to block phosphorylation completely. There has been one report about EGF stimulation of 3T3 cells where inhibition of mitogen-activated protein kinase (MAPK) cascade with U0126 stimulated PI 3-kinase-dependent phosphorylation of glycogen synthase kinase 3 (GSK3) (46), which implies cross talk between the MAPK and PKB/AKT cascades. Communications between the MEK/ERK and SAPK pathways have also been proposed in growth factor-stimulated epithelial layers of the porcine lens. There, MEK inhibition with U0126 led to decreased SAPK phosphorylation, suggesting close association of MEK/ERK with the SAPK cascade (58). Furthermore, in our study, the PI 3-kinase inhibitor LY294002 (50) blocked PKB/AKT phosphorylation completely and reduced SAPK phosphorylation. SAPK may thus be activated by at least two kinases in primary RMG: MEK and PI 3-kinase, whereby a complete block of SAPK phosphorylation occurs only through MEK inhibition; PI 3-kinase inhibition is only able to reduce SAPK phosphorylation. Although reduced SAPK phosphorylation may result from unspecific action of LY294002 (no data exist about possible unspecific inhibition of LY294002 on the classical SAPK-activating kinase MKK4), it may also indicate that MEK acts on an unidentified target kinase, which itself activates PI 3-kinase. GDNF, NRTN, and ARTN all activated similar signaling cascades in this study, which is consistent with previous reports (2, 26), but the time course of activation for phosphorylation of PKB/AKT was remarkably different: stimulation with NRTN, ARTN, and GDNF resulted in phosphorylation of PKB/AKT after 1, 5, and 10 min, respectively. The very rapid phosphorylation of PKP/AKT following NRTN stimulation may indicate recruitment of different adapter proteins to the activated RET receptor, in contrast to the considerably later activation of PKB/AKT through GDNF stimulation. Moreover, we consistently observed GFL-associated different SAPK/JNK phosphorylation intensities: whereas GDNF induced strong phosphorylation, NRTN and ARTN resulted in reduced or very weak phosphorylation of SAPK/JNK, respectively. This indicates differential triggering of signal intensities by different GFLs. Together, these findings underline that although these GFLs signal through the same transmembrane receptor RET (3) and induce phosphorylation at similar tyrosine residues of RET, the intracellular signaling dynamics are indeed distinct.
FIG. 9.
Model of intra- and intercellular signaling induced by GDNF in RMG. RET transmembrane tyrosine kinase is phosphorylated in response to GDNF, NRTN, and ARTN. All ligands are able to induce three intracellular signaling cascades: MEK/ERK, SAPK, and PKB/AKT pathways. Inhibitor experiments demonstrate that MEK acts upstream of PI 3-kinase (PI3-K) to an unidentified target and upstream of SAPK. Both, PI 3-kinase and MEK are able to activate SAPK. Phosphorylated ERK is shuttled into the RMG nucleus, where it induces increased transcription of FGF-2 mRNA. This leads to increased expression and secretion of FGF-2 protein, which then supports survival of PR, which are in close proximity to RMG in intact retina.
GDNF-induced and FGF-2-mediated indirect survival pathway to photoreceptors.
Upon stimulation with GDNF, phosphorylated ERK is shuttled into the nucleus of cells. We found both pERK-positive signals close to nuclei of the INL in intact retina and additional signals in the inner plexiform layer and ganglion cell layer. The pERK-positive cells within the INL show regular spacing, are all located within one row of nuclei in the INL, and are most likely RMG. We could colocalize all pERK-positive labeling with glutamine synthetase, the marker for glial cells in retina (11, 34), thus confirming that ERK is selectively activated in RMG.
Given that GDNF has been shown to rescue PR in the rd1 mouse and our own results indicate that RMG are the target cells of GDNF in the porcine retina, we specifically screened for GDNF-induced expression changes in secreted neuroprotective molecules. We found that RMG indeed respond to GDNF application with an upregulation of FGF-2 expression. This is in agreement with the observations of Harada and coworkers (20), who reported GDNF-induced upregulation of FGF-2 in rat RMG in vitro. Furthermore, subretinal injection of NT-3 prior to light-induced retinal degeneration in mice (21) resulted in upregulation of FGF-2 expression in RMG. This may therefore represent a general pathway for retinal neuroprotection (53, 57). Moreover, we found increased FGF-2 secretion stimulated by GDNF, NRTN, and ARTN, which may support paracrine action of those factors. The increased FGF-2 secretion is entirely dependent on GFL-induced MEK activation, since the presence of U0126 completely abolished enhanced secretion. Since the amount of secreted FGF-2 decreased below control levels in the presence of U0126, this indicates that the presence of RMG-derived GDNF alone triggers basic levels of FGF-2 secretion.
We functionally verified that neither GDNF, NRTN, nor ARTN has a direct survival-supporting effect on PR (Fig. 8), whereas application of FGF-2 prolonged survival of PR in vitro. The ability of FGF-2 to enhance survival of postnatal rat PR has been demonstrated (17), and indications for the long-term survival-promoting effect of FGF-2 (after 2 weeks in vitro) on porcine PR following repeated application (48) also exist. However, the observed GDNF-induced secretion of FGF-2 occurred within 16 h, and we therefore addressed the question of whether in vitro FGF-2 application would show a faster survival-promoting effect in our system. Indeed, we found a pronounced and concentration-dependent effect of FGF-2 on survival of PR in vitro, detectable already 2 days after application and most pronounced after 5 days in vitro. Based on these results, we propose the following model for GDNF-induced neuroprotection of porcine PR. GDNF activates distinct intracellular signaling pathways in RMG, namely, MEK/ERK, AKT/PKB, and SAPK/JNK. Activated MEK results in enhanced FGF-2 transcription, and FGF-2 is released from RMG and subsequently acts as a survival factor for neighboring PR (Fig. 9).
However, for testing the functionality of the suggested paracrine pathway, treatment of retinal explants with GDNF in the absence versus the presence of, e.g., MEK inhibitor U0126 would be necessary to quench the GDNF-induced production of FGF-2. As U0126 targets MEK not only in RMG but in all cells including photoreceptors when applied to whole retina, U0126 application leads to diminished photoreceptor survival as assessed in vitro (data not shown) and in vivo, since FGF-2-induced survival signaling involves MEK in photoreceptors (28). Furthermore, it has been reported that U0126 induces glutamate release from neurons in an ERK-independent manner (41), thus complicating the expected effects of U0126 when applied to whole retina. Another observation in retinal explants is that the explanting procedure alone leads to increased FGF-2 production in the explanted retina (P. Ekstrom, Lund University, personal communication of unpublished results), an event that may be interpreted as an intrinsic survival attempt. Taken together, pharmacological interference experiments performed on intact retina are not expected to further elucidate the GDNF-induced survival mechanism.
Is FGF-2 the survival-promoting molecule in vivo?
The functional importance of this model of PR support through RMG is strengthened by the finding that RMG express three different coreceptor types for GDNF family ligand signaling, thus providing a redundancy also seen with striatal and hippocampal neurons (31). Nevertheless, although FGF-2 is unambiguously upregulated in response to GDNF and does in contrast to GDNF prolong survival of PR in vitro, the question remains open of whether this is the molecule responsible for the GDNF-induced functional rescue of PR in the rd1 mouse (19). We observed here that 1 × 105 RMG secrete approximately 50 pg/ml FGF-2 in 12 h under in vitro conditions (measured by ELISA), whereas the effective amount of exogenous FGF-2 necessary to prolong PR survival was 500 ng/ml. However, this discrepancy could be explained by taking into account that secreted FGF-2 in vitro is diluted into the culture medium (100 μl medium volume per well and 1 × 105 RMG, or 1 nl per cell) by more than a factor of 1,000 when compared to the in vivo situation. In vivo, the extracellular space (interphotoreceptor matrix; IPM) bordering RMG and absorbing the secreted material can be estimated at 0.7 pl per RMG cell. This rather small volume can be calculated based on an estimated number of 1.5 × 107 RMG per porcine retina (24, 44) and on an estimation of total IPM volume for porcine eyes of ∼10 μl. IPM volume for bovine eyes has been calculated at 30 μl (30 to 100 μl) (1) and may thus be expected to be smaller for smaller porcine eyes. Thus, in vivo, RMG-secreted FGF-2 may be concentrated by more than 3 logs compared to the amounts measured by ELISA in vitro. Further, FGF-2 may be accumulated in vivo for longer time periods. If under GDNF stimulation, 50 pg/ml is secreted during 16 h in the in vitro experiment (after 48 h, we observed 230 ± 17.2 pg/ml FGF-2; data not shown), the cumulative secretion after 5 days would be approximately 500 pg/ml. Together, these assumptions may account for large amounts of FGF-2 in vivo, although no definitive proof is available. Additionally, FGF-2 released in vivo into the extracellular matrix (ECM) is likely sequestered by low-affinity FGF receptors (heparan sulfate proteoglycans), thus increasing local concentrations (8, 45), protecting the factor from inactivation (51), and presenting it to high-affinity FGF-receptors (56). However, even if sufficiently high levels of FGF-2 are reached in vivo, direct injections of FGF-2 in animal models of retinal degeneration have so far only been shown to increase PR numbers (33, 47), but failed to improve electroretinogram recordings (32, 54), indicating it is insufficient for functional rescue. Thus, we hypothesize that GDNF modulates the expression of additional molecules in RMG, which in turn enables the preservation of PR function. Identification of these GDNF-induced molecules is a part of our ongoing and future studies, where the porcine retina represents an excellent model, as it is cone rich and it is a tissue in which RMG have been recently demonstrated to secrete a factor specifically promoting cone survival in vitro (4). Although specific information on this factor is presently unavailable, it remains an intriguing candidate for being targeted by GDNF-induced signaling in RMG.
GDNF has been repeatedly suggested in the context of future therapeutic regimens, presented either alone or combined with a cell-based therapy/delivery. Previous studies in gene and cell therapy have revealed that application of such neurotrophic cytokines prior to an in-depth understanding of their mechanism of action bears unpredictable risks for the patients treated (12). Thus, this study contributes to a better understanding of GDNF's mechanism of action on primary retinal cells and tissue and may contribute to a more rationalized risk assessment on this promising factor for future clinical trials.
Acknowledgments
We thank U. Olazabal for critical discussions and P. Hutzler for support with microscopy.
This work was funded by EU grants PRORET QLK6-CT-200000569, PRO-AGE-RET QLK6-CT-2001-00385, RETNET MRTN-CT-2003-504003, EVI-GENORET LSHG-CT-2005 512036, and INTERACTION PROTEOME LSHG-CT-2003-505520 and by funding from the German Federal Ministry of Education and Research (BMBF-Proteomics 031U108A/031U208A).
REFERENCES
- 1.Adler, A. J., and K. M. Severin. 1981. Proteins of the bovine interphotoreceptor matrix: tissues of origin. Exp. Eye Res. 32:755-769. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen, M. S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci 3:383-394. [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen, M. S., A. Titievsky, and M. Saarma. 1999. GDNF family neurotrophic factor signaling: four masters, one servant? Mol. Cell. Neurosci. 13:313-325. [DOI] [PubMed] [Google Scholar]
- 4.Balse, E., L.-H. Tessier, C. Fuchs, V. Forster, J. A. Sahel, and S. Picaud. 2005. Purification of mammalian cone photoreceptors by lectin panning and the enhancement of their survival in glia-conditioned medium. Investig. Ophthalmol. Vis. Sci. 46:367-374. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch, U., F. Kirchhoff, and M. Schachner. 1990. Highly sialylated N-CAM is expressed in adult mouse optic nerve and retina. J. Neurocytol. 19:550-565. [DOI] [PubMed] [Google Scholar]
- 6.Beltran, W. A., H. Rohrer, and G. D. Aguirre. 2005. Immunolocalization of ciliary neurotrophic factor receptor alpha (CNTFRalpha) in mammalian photoreceptor cells. Mol. Vis. 11:232-244. [PubMed] [Google Scholar]
- 7.Bringmann, A., and A. Reichenbach. 2001. Role of Muller cells in retinal degenerations. Front. Biosci. 6:E72-E92. [DOI] [PubMed] [Google Scholar]
- 8.Carey, D. J. 1997. Syndecans: multifunctional cell-surface co-receptors. Biochem. J. 327:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaum, E. 2003. Retinal neuroprotection by growth factors: a mechanistic perspective. J. Cell. Biochem. 88:57-75. [DOI] [PubMed] [Google Scholar]
- 10.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derouiche, A., and T. Rauen. 1995. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J. Neurosci. Res. 42:131-143. [DOI] [PubMed] [Google Scholar]
- 12.Dittrich, F., H. Thoenen, and M. Sendtner. 1994. Ciliary neurotrophic factor: pharmacokinetics and acute-phase response in rat. Ann. Neurol. 35:151-163. [DOI] [PubMed] [Google Scholar]
- 13.Duncia, J. V., J. B. Santella III, C. A. Higley, W. J. Pitts, J. Wityak, W. E. Frietze, F. W. Rankin, J. H. Sun, R. A. Earl, A. C. Tabaka, C. A. Teleha, K. F. Blom, M. F. Favata, E. J. Manos, A. J. Daulerio, D. A. Stradley, K. Horiuchi, R. A. Copeland, P. A. Scherle, J. M. Trzaskos, R. L. Magolda, G. L. Trainor, R. R. Wexler, F. W. Hobbs, and R. E. Olson. 1998. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 8:2839-2844. [DOI] [PubMed] [Google Scholar]
- 14.Encinas, M., M. G. Tansey, B. A. Tsui-Pierchala, J. X. Comella, J. Milbrandt, and E. M. Johnson, Jr. 2001. c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J. Neurosci. 21:1464-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faktorovich, E. G., R. H. Steinberg, D. Yasumura, M. T. Matthes, and M. M. LaVail. 1990. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347:83-86. [DOI] [PubMed] [Google Scholar]
- 16.Fliesler, S. J., G. J. Cole, and A. J. Adler. 1990. Neural cell adhesion molecule (NCAM) in adult vertebrate retinas: tissue localization and evidence against its role in retina-pigment epithelium adhesion. Exp. Eye Res. 50:475-482. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine, V., N. Kinkl, J. Sahel, H. Dreyfus, and D. Hicks. 1998. Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. J. Neurosci. 18:9662-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forster, V., H. Dreyfus, and D. Hicks. 1999. Adult mammalian retina, p. 579-585. In L. W. Haynes (ed.), The neuron in tissue culture, vol. 18. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 19.Frasson, M., S. Picaud, T. Leveillard, M. Simonutti, S. Mohand-Said, H. Dreyfus, D. Hicks, and J. Sahel. 1999. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Investig. Ophthalmol. Vis. Sci. 40:2724-2734. [PubMed] [Google Scholar]
- 20.Harada, C., T. Harada, H.-M. A. Quah, F. Maekawa, K. Yoshida, S. Ohno, K. Wada, L. F. Parada, and K. Tanaka. 2003. Potential role of glial cell line-derived neurotrophic factor receptors in Muller glial cells during light-induced retinal degeneration. Neuroscience 122:229-235. [DOI] [PubMed] [Google Scholar]
- 21.Harada, T., C. Harada, N. Nakayama, S. Okuyama, K. Yoshida, S. Kohsaka, H. Matsuda, and K. Wada. 2000. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron 26:533-541. [DOI] [PubMed] [Google Scholar]
- 22.Harper, J. F. 1984. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput. Biol. Med. 14:437-445. [DOI] [PubMed] [Google Scholar]
- 23.Hauck, S. M., S. Suppmann, and M. Ueffing. 2003. Proteomic profiling of primary retinal Muller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 44:251-263. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickson, A., and D. Hicks. 2002. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp. Eye Res. 74:435-444. [DOI] [PubMed] [Google Scholar]
- 25.Hims, M. M., S. P. Diager, and C. F. Inglehearn. 2003. Retinitis pigmentosa: genes, proteins and prospects. Dev. Ophthalmol. 37:109-125. [DOI] [PubMed] [Google Scholar]
- 26.Ichihara, M., Y. Murakumo, and M. Takahashi. 2004. RET and neuroendocrine tumors. Cancer Lett. 204:197-211. [DOI] [PubMed] [Google Scholar]
- 27.Jomary, C., R. M. Darrow, P. Wong, D. T. Organisciak, and S. E. Jones. 2004. Expression of neurturin, glial cell line-derived neurotrophic factor, and their receptor components in light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 45:1240-1246. [DOI] [PubMed] [Google Scholar]
- 28.Kinkl, N., J. Sahel, and D. Hicks. 2001. Alternate FGF2-ERK1/2 signaling pathways in retinal photoreceptor and glial cells in vitro. J. Biol. Chem. 276:43871-43878. [DOI] [PubMed] [Google Scholar]
- 29.Koeberle, P. D., and A. K. Ball. 1998. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 38:1505-1515. [DOI] [PubMed] [Google Scholar]
- 30.Koeberle, P. D., and A. K. Ball. 2002. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience 110:555-567. [DOI] [PubMed] [Google Scholar]
- 31.Kokaia, Z., M. S. Airaksinen, A. Nanobashvili, E. Larsson, E. Kujamaki, O. Lindvall, and M. Saarma. 1999. GDNF family ligands and receptors are differentially regulated after brain insults in the rat. Eur. J. Neurosci. 11:1202-1216. [DOI] [PubMed] [Google Scholar]
- 32.Lau, D., L. H. McGee, S. Zhou, K. G. Rendahl, W. C. Manning, J. A. Escobedo, and J. G. Flannery. 2000. Retinal degeneration is slowed in transgenic rats by AAV-mediated delivery of FGF-2. Investig. Ophthalmol. Vis. Sci. 41:3622-3633. [PubMed] [Google Scholar]
- 33.LaVail, M. M., D. Yasumura, M. T. Matthes, C. Lau-Villacorta, K. Unoki, C.-H. Sung, and R. H. Steinberg. 1998. Protection of mouse photoreceptors by survival factors in retinal degenerations. Investig. Ophthalmol. Vis. Sci. 39:592-602. [PubMed] [Google Scholar]
- 34.Lewis, G. P., P. A. Erickson, D. D. Kaska, and S. K. Fisher. 1988. An immunocytochemical comparison of Muller cells and astrocytes in the cat retina. Exp. Eye Res. 47:839-853. [DOI] [PubMed] [Google Scholar]
- 35.Lin, L. F., D. H. Doherty, J. D. Lile, S. Bektesh, and F. Collins. 1993. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130-1132. [DOI] [PubMed] [Google Scholar]
- 36.McMenamin, P. G., and R. J. Steptoe. 1991. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J. Anat. 178:65-77. [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan, J. M., H. Navabi, and B. Jasani. 1997. Role of calcium chelation in high-temperature antigen retrieval at different pH values. J. Pathol. 182:233-237. [DOI] [PubMed] [Google Scholar]
- 38.Nosrat, C. A., A. Tomac, E. Lindqvist, S. Lindskog, C. Humpel, I. Stromberg, T. Ebendal, B. J. Hoffer, and L. Olson. 1996. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 286:191-207. [DOI] [PubMed] [Google Scholar]
- 39.Otori, Y., S. Shimada, K. Tanaka, I. Ishimoto, Y. Tano, and M. Tohyama. 1994. Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retinal ischemia. Brain Res. Mol. Brain Res. 27:310-314. [DOI] [PubMed] [Google Scholar]
- 40.Paratcha, G., F. Ledda, and C. F. Ibanez. 2003. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867-879. [DOI] [PubMed] [Google Scholar]
- 41.Pereira, D. B., A. P. Carvalho, and C. B. Duarte. 2002. Non-specific effects of the MEK inhibitors PD098,059 and U0126 on glutamate release from hippocampal synaptosomes. Neuropharmacology 42:9-19. [DOI] [PubMed] [Google Scholar]
- 42.Priglinger, S. G., C. A. May, A. S. Neubauer, C. S. Alge, C. L. Schonfeld, A. Kampik, and U. Welge-Lussen. 2003. Tissue transglutaminase as a modifying enzyme of the extracellular matrix in PVR membranes. Investig. Ophthalmol. Vis. Sci. 44:355-364. [DOI] [PubMed] [Google Scholar]
- 43.Rauen, T., J. D. Rothstein, and H. Wassle. 1996. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res. 286:325-336. [DOI] [PubMed] [Google Scholar]
- 44.Reichenbach, A. 1999. Neuroglia-das andere zelluläre Element im Nervensystem: die Müllersche Gliazelle. Pharmacia & Upjohn GmbH, Erlangen, Germany.
- 45.Schonherr, E., and H. J. Hausser. 2000. Extracellular matrix and cytokines: a functional unit. Dev. Immunol. 7:89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw, M., and P. Cohen. 1999. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 461:120-124. [DOI] [PubMed] [Google Scholar]
- 47.Spencer, B., S. Agarwala, L. Gentry, and C. R. Brandt. 2001. HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol. Ther. 3:746-756. [DOI] [PubMed] [Google Scholar]
- 48.Traverso, V., N. Kinkl, L. Grimm, J. Sahel, and D. Hicks. 2003. Basic fibroblast and epidermal growth factors stimulate survival in adult porcine photoreceptor cell cultures. Investig. Ophthalmol. Vis. Sci. 44:4550-4558. [DOI] [PubMed] [Google Scholar]
- 49.Vestre, W. A. 1984. Porcine ophthalmology. Vet. Clin. N. Am. Large Anim. Pract. 6:667-676. [DOI] [PubMed] [Google Scholar]
- 50.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 51.Vlodavsky, I., H. Q. Miao, B. Medalion, P. Danagher, and D. Ron. 1996. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 15:177-186. [DOI] [PubMed] [Google Scholar]
- 52.Wahlin, K. J., R. Adler, D. J. Zack, and P. A. Campochiaro. 2001. Neurotrophic signaling in normal and degenerating rodent retinas. Exp. Eye Res. 73:693-701. [DOI] [PubMed] [Google Scholar]
- 53.Wahlin, K. J., P. A. Campochiaro, D. J. Zack, and R. Adler. 2000. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Investig. Ophthalmol. Vis. Sci. 41:927-936. [PubMed] [Google Scholar]
- 54.Yamada, H., E. Yamada, A. Ando, N. Esumi, N. Bora, J. Saikia, C. H. Sung, D. J. Zack, and P. A. Campochiaro. 2001. Fibroblast growth factor-2 decreases hyperoxia-induced photoreceptor cell death in mice. Am. J. Pathol. 159:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan, Q., J. Wang, C. R. Matheson, and J. L. Urich. 1999. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF). J. Neurobiol. 38:382-390. [DOI] [PubMed] [Google Scholar]
- 56.Yayon, A., M. Klagsbrun, J. D. Esko, P. Leder, and D. M. Ornitz. 1991. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841-848. [DOI] [PubMed] [Google Scholar]
- 57.Zack, D. J. 2000. Neurotrophic rescue of photoreceptors: are Muller cells the mediators of survival? Neuron 26:285-286. [DOI] [PubMed] [Google Scholar]
- 58.Zatechka, S. D., Jr., and M. F. Lou. 2002. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp. Eye Res. 74:703-717. [DOI] [PubMed] [Google Scholar]