Abstract
Histone acetylation regulates gene expression, yet the functional contributions of the numerous histone acetyltransferases (HATs) to gene expression and their relationships with each other remain largely unexplored. The central role of the putative HAT-containing TAF1 subunit of TFIID in gene expression raises the fundamental question as to what extent, if any, TAF1 contributes to acetylation in vivo and to what extent it is redundant with other HATs. Our findings herein do not support the basic tenet that TAF1 is a major HAT in Saccharomyces cerevisiae, nor do we find that TAF1 is functionally redundant with other HATs, including Gcn5, Elp3, Hat1, Hpa2, Sas3, and Esa1, which is in contrast to previous conclusions regarding Gcn5. Our findings do reveal that of these HATs, only Gcn5 and Esa1 contribute substantially to gene expression genome wide. Interestingly, histone acetylation at promoter regions throughout the genome does not require TAF1 or RNA polymerase II, indicating that most acetylation is likely to precede transcription and not depend upon it. TAF1 function has been linked to Bdf1, which binds TFIID and acetylated histone H4 tails, but no linkage between TAF1 and the H4 HAT Esa1 has been established. Here, we present evidence for such a linkage through Bdf1.
Eukaryotic genes are packaged into chromatin that is largely composed of histone proteins. Access to these genes requires mobilization of the histones, which is thought to involve specific lysine acetylation of their amino-terminal tails as well as other types of modifications (7, 38). Cells contain a multitude of histone acetyltransferases (HATs), some of which play important roles in transcriptional activation (43). In addition to mobilizing nucleosomes, histone acetylation provides binding sites for bromodomain proteins, many of which are part of the transcription machinery (32).
The transcription machinery assembles at promoters via two alternative pathways directed by two related subunit complexes, TFIID and SAGA (3, 8, 18, 25, 28, 29). Interestingly, both complexes contain subunits (TAF1 and Gcn5, respectively) that harbor bromodomains and HAT activity (6, 36), thereby linking histone acetylation and recruitment of the transcription machinery. In Saccharomyces cerevisiae, TAF1 lacks bromodomains, which instead appear to be located on the TFIID-interacting protein Bdf1 (34).
Much of what is currently known about the HAT activity of TAF1 stems from studies on higher eukaryotic TAF1. TAF1 was first identified as a HAT through in vitro acetyltransferase assays and was shown to possess substrate specificity similar to that of Gcn5 (36). A naturally occurring mutation (ts13) in mammalian TAF1 renders cells temperature sensitive for both cell cycle progression (15, 41, 42) and expression of the cyclin D1 and cyclin A genes (48, 49). This mutation maps to the TAF1 HAT domain and impairs its HAT activity in vitro (12). Additional mutations that eliminate TAF1 HAT function have been defined, and expression of these mutant TAF1 proteins in vivo leads to defects in H3 acetylation at the cyclin D1 promoter (14). Taken together, these observations provide evidence that TAF1 is a physiologically relevant HAT, at least in higher eukaryotes.
Both TAF1 and Gcn5 acetylate histone H3 at lysine 14 (H3K14) (24, 36, 44, 56), and both TAF1 and Gcn5 have been reported to play functionally redundant roles in yeast (28). At face value, this relationship fits well with the notion that TFIID and SAGA play functionally redundant roles by acetylating the same targets and nucleating assembly of a transcription complex by two alternative pathways. However, many aspects of this relationship have not been rigorously tested. For example, it is not known to what extent histone acetylation in vivo is dependent upon TAF1 versus Gcn5. In addition, yeast TAF1 has not been demonstrated to have robust HAT activity in vitro or to have HAT activity as a full-length protein or when part of TFIID. It is important to clarify the relationship between TAF1 and Gcn5 because their potentially parallel function, if it exists, could be central to the mechanism of transcription complex assembly. Moreover, the putative HAT redundancy between yeast TAF1 and Gcn5 raises the question as to whether other HATs are functionally redundant with TAF1. HATs such as Elp3 and Sas3 have been suggested to be functionally redundant with Gcn5 (17, 52) and thus have the potential to be redundant with TAF1.
TAF1 function might also be intertwined with the HAT Esa1, a subunit of the NuA4 complex, inasmuch as Esa1 acetylates histone H4 tails (2) and the TAF1-containing TFIID complex might bind to acetylated H4 tails via bromodomains contained within TAF1 in higher eukaryotes or Bdf1 in yeast (19, 34). Moreover, TFIID-regulated genes tend to have high levels of H4 acetylation (18). Transcriptional dependencies on Esa1 have not been previously conducted on a genome-wide scale, and so whether gene regulation by TFIID and Esa1 are correlated remains uncertain.
Here, we examine a number of basic tenets of the hypothesis that TAF1 and Gcn5 are functionally redundant in vivo by examining whether each is necessary and sufficient to acetylate H3 in vivo. We further test whether TAF1 is functionally redundant with other HATs in vivo, genome wide. In addition to finding no evidence of TAF1 HAT activity or functional redundancy in yeast, we also find that genome-wide promoter-specific histone acetylation does not require TAF1 or RNA polymerase II (Pol II). We did, however, detect a strong correlation between genes regulated by TAF1 and those regulated by Esa1, which suggests that the two function in the same pathway. Interestingly, Bdf1 occupancy correlates more with H4 acetylation than does TAF1, which is consistent with Bdf1 recruitment being more central to H4 acetylation than TFIID recruitment.
MATERIALS AND METHODS
Immunoblotting.
Cells (1.5 ml for H3 and 3.5 ml for H4) were grown at 25°C to an optical density at 600 nm (OD600) of 0.8 to 0.9 and heat shocked for 45 min at 37°C. Harvested cells were resuspended in cell lysis buffer [0.2 M Tris-acetate, pH 7.5, 0.39 M (NH4)SO4, 1 mM EDTA, 20% glycerol, 2 mM dithiothreitol, 1 complete protease inhibitor tablet (Roche), 0.5 mM phenylmethylsulfonyl fluoride] and lysed with silica/zirconium beads in a mini-bead beater for six sessions, 20 s each, with cooling on ice between sessions. Chromatin was pelleted by centrifugation, washed with cold double-distilled water, and resuspended in sodium dodecyl sulfate (SDS) sample buffer. Samples were electrophoresed on 16.5% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes. Membranes were probed with histone modification antibodies obtained from Upstate and developed by enhanced chemiluminescence.
Microarray analysis.
Strains and plasmids are presented in Table 1. Strains were grown in CSM-His medium at 25°C to an OD600 of ∼0.8. All cultures were shifted to 37°C with an equal volume of heated medium. After 45 min at 37°C, cells were quickly harvested at room temperature. Sample preparation and hybridizations were performed as described previously (9), and data normalization and analysis were performed as described previously (18). Raw data are accessible at the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). Processed data are presented in supplemental material (see Table S1 in the supplemental material).
TABLE 1.
Strains used in this study
| Strain | Chromosomal deletion(s) | Plasmid 1 | Plasmid 2 |
|---|---|---|---|
| yJS4 | taf1Δ gcn5Δ | pSW104-WT TAF1 | |
| yJS5 | taf1Δ gcn5Δ | pSW104-taf1 ts2 | |
| yMD5 | taf1Δ elp3Δ | pSW104-WT TAF1 | |
| yMD6 | taf1Δ elp3Δ | pSW104-taf1 ts2 | |
| yMD7 | taf1Δ hat1Δ | pSW104-WT TAF1 | |
| yMD8 | taf1Δ hat1Δ | pSW104-taf1 ts2 | |
| yMD9 | taf1Δ hpa2Δ | pSW104-WT TAF1 | |
| yMD10 | taf1Δ hpa2Δ | pSW104-taf1 ts2 | |
| yMD11 | taf1Δ sas3Δ | pSW104-WT TAF1 | |
| yMD12 | taf1Δ sas3Δ | pSW104-taf1 ts2 | |
| yMD13 | taf1Δ | pSW104-WT TAF1 | |
| yMD14 | taf1Δ | pSW104-taf1 ts2 | |
| yMD25 | taf1Δ esa1Δ | pSW104-WT TAF1 | pSAPE1 [WT ESA1] |
| yMD26 | taf1Δ esa1Δ | pSW104-taf1 ts2 | pSAPE1 [WT ESA1] |
| yMD27 | taf1Δ esa1Δ | pSW104-WT TAF1 | pSAPE2 [esa1-414] |
| yMD28 | taf1Δ esa1Δ | pSW104-taf1 ts2 | pSAPE2 [esa1-414] |
| RY262a | rpb1-1 |
See reference 37.
chIP-chip.
Cultures were grown in CSM medium at 25°C to an OD600 of 0.8 and then rapidly shifted to 37°C for 45 min. Cells were cross-linked with 1% formaldehyde for 15 min and simultaneously cooled to 25°C. The cross-linking was quenched with 125 mM glycine for 5 min, and cells were subsequently harvested. Cell lysis and sonication of DNA were performed as previously described (54). Sonicated DNA was immunoprecipitated with H3 acetyl (Ac)-K9,14 antibodies to produce enriched DNA. The enriched DNA for each test strain was then cohybridized with enriched DNA from an independent wild-type (WT) strain onto microarrays containing PCR-amplified probes corresponding to ∼6,000 intergenic regions of the yeast genome. Raw data are accessible at the GEO database.
Background signal was subtracted from the intensity for each spot, and values that were less than 1 standard deviation above the background were removed. Intensity values were converted to ratios of mutant over wild type and were log2 transformed.
Computer simulations.
KinTekSim freeware was downloaded from http://www.kintek-corp.com/members/. The input mechanism was A=B; B=C; C=A. Output was C. A, B, and C represent each species illustrated in Fig. 6B, from left to right, respectively. “=” represents a reaction sequence (e.g., A=B means A converts to B). In this simplified simulation, C is synonymous with RNA output. In order to allow the system to reach the steady state, reflecting the physiologic state of the cell, C was allowed to convert back to A (equivalent to the disassembly of the transcription machinery and RNA turnover). The forward rate constants k1 (governing A=B) and k2 (governing B=C) for “wild type” are indicated in Fig. 6B. k1 was reduced by 10-fold when simulating esa1-414, and k2 was reduced by 10-fold when simulating taf1ts2. For all simulations, k3 was set to 1; reverse rate constants were set to 0.01; and starting concentrations of A, B, and C were set to 3, 0, and 0, respectively, Time was set to 100, and f1 (scaling factor) was set to 1. These values were largely arbitrary. The outcome of the simulation was largely independent of these parameters. Values for “C” were obtained at the completion of the time course in which “C” had reached steady state (unchanged over time). In Fig. 6B, abscissa and ordinate values are as follows: 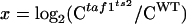 and y = log2(Cesa1-414/CWT).
and y = log2(Cesa1-414/CWT).
FIG. 6.
Esa1 and TAF1 are functionally linked. (A) Data from Fig. 4E (log2 changes in gene expression in wild-type, taf1ts2, esa1-414, and taf1ts2 esa1-414 strains) were filtered for a twofold cutoff and clustered into eight groups by K means (13). Average values for each group in the taf1ts2 and esa1-414 data sets are plotted against each other. (B) A general mechanism describing the potential interrelationship between Esa1 (NuA4) and TAF1 (TFIID) in gene activation. According to the model, recruitment of NuA4 to TATA-less promoters results in H4 tail acetylation, allowing Bdf1 to dock and escort in TFIID. Two potentially rate-limiting steps are shown. Below these steps are arbitrary rate constants in which either NuA4 or TFIID activity, as indicated, is limiting for transcription at five hypothetical genes. The graph represents simulated log2 changes in gene expression (RNA output) in a hypothetical esa1-414 mutant versus a hypothetical taf1ts2 mutant for each of the five genes. See Materials and Methods for a detailed description of the simulation using KinTekSim software.
Microarray accession numbers.
Raw data reported in this paper are accessible at the GEO database (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSM75452 to GSM75463, GSM75470, GSM75471, GSM75473 to GSM75478, GSM75480 to GSM75483, GSM75491 to GSM75504, and GSM75526 to GSM75533.
RESULTS
TAF1 and Gcn5 are not functionally equivalent.
The premise upon which we began our inquiry is that TAF1 is a HAT that targets similar histone residues as Gcn5 and thus is at least in part functionally redundant with Gcn5. Since TAF1 and Gcn5 reportedly acetylate histone H3 tails, we focused our attention on lysine acetylation of H3. If TAF1 and Gcn5 are both major HATs that acetylate the same H3 residues, then the elimination of one or the other should have less of an impact on H3 acetylation than the loss of both. TAF1 was eliminated using a temperature-sensitive taf1ts2 allele, which, upon shifting to 37°C for 45 min, results in the degradation of nearly all of TAF1 (47; data not shown) and at least a partial shutdown of ∼90% of all expressed genes (16, 18). Inasmuch as TAF1 is physically eliminated at the nonpermissive temperature, it is reasonable to assume that any HAT activity within TAF1 is eliminated as well. Gcn5 was eliminated using a gcn5Δ strain. This strain was also shifted to 37°C for 45 min to be consistent with the elimination of TAF1. As shown by the immunoblot in Fig. 1, the loss of TAF1 had no significant effect on bulk H3 acetylation at K9, K14, K18, K23, and K27. In contrast, the loss of Gcn5 resulted in an ∼85% drop in bulk K9, K14, and K27 acetylation and a modest effect at K18, findings that confirm previous results on Gcn5 specificity towards bulk histones at K9, K14, and K18 in vivo (56). Elimination of both TAF1 and Gcn5 had no further effect than elimination of Gcn5 alone.
FIG. 1.
Gcn5, and not TAF1, is important for bulk H3 acetylation levels. All strains were grown in CSM-His medium at 25°C and then shifted to 37°C for 45 min to inactivate the taf1ts2 allele, when present. Crude whole-cell lysates were subjected SDS-polyacrylamide gel electrophoresis and immunoblot analysis using antibodies recognizing the indicated histone H3 modifications or total H3 (bottom immunoblot). Quantitation of three independent replicates is shown.
A number of conclusions can be drawn from these results. (i) Gcn5 is the major HAT operating at bulk H3 K9, K14, and K27 under these growth conditions, which reconfirms similar conclusions regarding H3 K9 and K14 drawn previously (56). This does not exclude smaller contributions from other HATs such as Sas3 (17). (ii) Yeast TAF1 is not a major physiological HAT of bulk H3 histones. (iii) Gcn5 either does not acetylate the bulk of H3 K23 in vivo or does so in a redundant manner with another HAT that is not TAF1. (iv) Ongoing transcription throughout most of the genome, which is lost in the taf1ts2 strain, is not required to maintain bulk H3 acetylation. Collectively, the data indicate that TAF1 is not similar to Gcn5 with respect to bulk histone H3 acetylation.
The immunoblot shown in Fig. 1 examined bulk histone H3 acetylation regardless of its location in the genome. In principle, if acetylation of H3 lysines is spread throughout the genome, including at promoters, within open reading frames and downstream of genes, then it is plausible that putative H3 acetylation by TAF1 might be missed if its HAT activity is concentrated over promoter regions, where it normally binds. This would seem unlikely, since H3 acetylation appears to be concentrated near promoters (30, 39). Nevertheless, to address this possibility, we used genome-wide location analysis (chIP-chip) to determine if promoter-specific acetylation was affected by the loss of TAF1, Gcn5, or both.
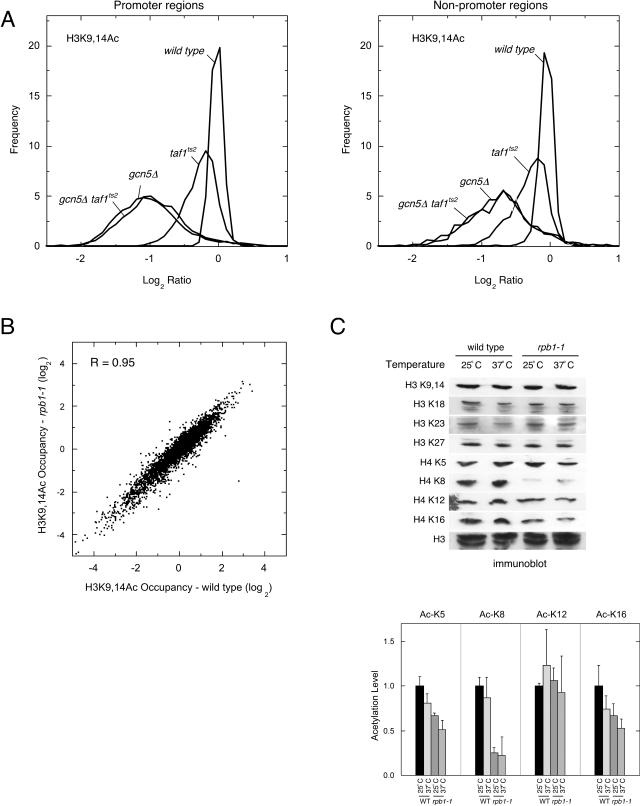
In this analysis, we focused on acetylation at H3 K9,14, a major target of Gcn5. Mutant (gcn5Δ, taf1ts2, and gcn5Δ taf1ts2) and wild-type cells were subjected to formaldehyde cross-linking. The chromatin was sheared and immunoprecipitated with H3 Ac-K9,14 antibodies. Immunoprecipitated DNA was assayed with microarray probes covering approximately 6,000 intergenic regions (including regions that lack promoters). Ratios of mutant-to-wild-type occupancy of H3 Ac-K9,14 were determined, and the data set was centered to the median value of the upper 5th percentile of the data (i.e., mutant-to-wild-type ratios that were the highest). In order to compare data sets, it was necessary to set those genomic regions whose H3 acetylation was truly unaffected by the mutants to be equivalent (i.e., centering). Since it is unknowable from this assay whether any region of the genome meets this criterion, we arbitrary defined those intergenic regions that are in the upper 5th percentile (representing 5% of the data set) as having acetylation levels, if any, that are unaffected by the loss of TAF1 and/or Gcn5. This normalization attempts to steer clear of the 90% of the genome that is at least partially dependent upon TAF1. Implicit in this normalization is that increases in acetylation in the mutants do not occur. This approach makes no assumptions about acetylation in promoter and nonpromoter intergenic regions and thus allows them to be evaluated independently. The conclusions drawn here can be attained from other normalization methods as well (e.g., centering to the nonpromoter region [not shown]).
Log2 ratios for intergenic regions that contain promoters (n = 3,823) were binned and plotted as smoothed frequency distributions in Fig. 2A (left panel). A control cohybridization of two independent wild-type samples resulted in a tight distribution, reflecting intrinsic experimental variance, the peak of which we define as zero (no change). The loss of Gcn5 resulted in a drop in H3 K9,14 acetylation over nearly all promoter regions (manifested by a leftward shift of the profile relative to the wild type). The broad distribution of the data relative to the wild-type control indicates that the drop in acetylation was not uniform at all promoter regions. This profile indicates that Gcn5 contributes to H3 K9,14 acetylation at most promoters but that the contribution is more at some promoters and less at other promoters.
FIG. 2.
Gcn5, and not TAF1, is important for gene-specific H3 acetylation levels. (A) Frequency distribution of H3 Ac-K9,14 occupancy in intergenic regions containing (left panel) or lacking (right panel) a promoter, the latter being intergenic regions located between two convergently transcribed genes. chIP assays were performed on cross-linked, sheared chromatin from WT, taf1ts2, gcn5Δ, and taf1ts2 gcn5Δ strains that were grown at 25°C and then shifted to 37°C for 45 min to inactivate the taf1ts2 allele. Immunoprecipitated DNA from test strains was labeled and cohybridized to intergenic microarrays along with an independent wild-type sample. H3 Ac-K9,14 occupancy levels relative to the wild type were converted to a log2 scale and binned in 0.05 intervals, and the resulting frequency histogram was converted to an interpolated frequency distribution using Kaleidagraph software. (B) Cell growth and chIP were performed as described above (A) using a wild-type or rpb1-1 strain. H3 Ac-K9,14 occupancy data were normalized to a nonspecific immunoprecipitated chIP DNA data set (39), converted to a log2 scale, and centered to the median value for nonpromoter intergenic regions. Shown is a scatter plot comparison between H3 Ac-K9,14 in an rpb1-1 strain versus a wild-type strain at the nonpermissive temperature (37°C). (C) Immunoblots were performed on WT and rpb1-1 strains at the permissive (25°C) and nonpermissive (37°C) temperatures using antibodies against the indicated histone modifications or total H3. Quantitation of three independent H4 modification replicates is shown below the immunoblot.
The loss of TAF1 resulted in a minor leftward shift of the population, which was centered over −0.2 (log2 scale), reflecting a slight (∼1.1-fold) decrease in acetylation. The taf1ts2 gcn5Δ double mutant was indistinguishable from the gcn5Δ single mutant. In both TAF1 mutants (taf1ts2 and taf1ts2 gcn5Δ), it is clear that the loss of TAF1 had no major impact on H3 K9,14 acetylation in promoter regions. Any minor effects are likely to be indirect, since similar changes in H3 K9,14 acetylation were observed in nonpromoter intergenic regions as well (i.e., regions downstream of two convergently transcribed genes) (Fig. 2A, right panel), where TAF1 is not expected to bind. Loss of Gcn5 showed an overall larger decrease in H3 K9,14 acetylation for the promoter regions compared to nonpromoter regions (Fig. 2, compare left and right panels). This observation is consistent with promoter regions having higher H3 K9, 14 acetylation levels (and thus more acetylation to lose) than nonpromoter regions. Nevertheless, Gcn5 contributes to low levels of histone acetylation in nonpromoter regions as well, which is consistent with reports of targeted and nontargeted acetylation by Gcn5 (46).
The following conclusions can be drawn from the chIP-chip data. (i) TAF1 is not a major physiological H3 K9,14 HAT at the vast majority of yeast promoters. (ii) Gcn5 is a physiological HAT at most yeast promoters, which confirms genome-wide Gcn5 occupancy data and genome-wide transcriptional dependencies on Gcn5 (18, 28, 40) but represents the first genome-wide assessment of Gcn5-dependent acetylation. (iii) Loss of TAF-dependent transcription, which occurs upon inactivation of TAF1, leads to little or no changes in H3 K9,14 acetylation in promoter regions, indicating that TFIID occupancy at promoters is not needed to maintain promoter-specific H3 K9,14 acetylation. We conclude from these studies that if yeast TAF1 is a physiological HAT, it lacks the same specificity as Gcn5.
Histone acetylation occurs independently of transcription.
Inasmuch as the SAGA complex can partially compensate for the loss of TFIID in transcription (18, 28), the experiments described above could not ascertain whether transcription was necessary to maintain H3 K9,14 acetylation. To address this, we performed a similar H3 K9,14 chIP-chip experiment in a strain harboring the temperature-sensitive Pol II allele, rpb1-1. In this experiment, H3 K9,14 acetylation levels that exist after a 45-min heat inactivation of the rpb1-1 allele were compared to those of equivalently treated wild-type cells. As shown by the scatter plot in Fig. 2B, promoter occupancy of H3 acetylated at K9,14 in the rpb1-1 strain strongly correlated with occupancy levels occurring in wild-type cells, indicating that transcription is not necessary to maintain H3 K9,14 acetylation in promoter regions. This finding confirms on a genome-wide scale what has been observed at selected loci (24).
Since histone acetylation occurs at a variety of histone sites, we further examined whether sustained transcription is essential for maintaining bulk acetylation at a variety of H3 and H4 residues. As shown in the immunoblot in Fig. 2C, the loss of transcription upon inactivation of the rpb1-1 allele resulted in no observable changes in bulk H3 acetylation at the residues tested, suggesting that ongoing transcription is not necessary for global maintenance of histone H3 acetylation. This does not exclude the possibility of widespread transcription-coupled acetylation in open reading frames that occurs transiently during elongation (21), and thus, sustained transcription is a minor contributor to bulk acetylation.
In contrast, the levels of H4 acetylation at K8 decreased in the rpb1-1 strain, even at the permissive temperature. Little or no effects were observed at H4 K5, K12, and K16. Acetylation at H4 K5, K8, and K12 has been largely linked together as providing additive changes in the overall charge on H4 tails, which incrementally affect transcription (11). Although the basis for the sensitivity of K8 acetylation to the rpb1-1 mutation is unclear, it does suggest a functional link between Pol II and K8 acetylation that might not exist at the other H4 sites. It is intriguing that the Pol II-associated HAT Elp3 specifically acetylates H4K8 (51). Conceivably, H4K8 acetylation might occur via one or more HATs, such as Elp3, that are associated with and dependent upon an elongating Pol II, whereas acetylation at other H4 sites might be less Pol II dependent.
H4K5 and H4K12 hyperacetylation at promoters is linked to transcription (30), yet we find that Pol II is not required to maintain acetylation at K5 and K12, as we observed for H3 acetylation (Fig. 2C). Thus, transcription is not necessary to maintain acetylation.
Functional redundancy reassessed by genome-wide expression profiling.
The previous conclusion that TAF1 and Gcn5 are functionally redundant was based in part on the observation that changes in gene expression for about 25% of the yeast genome was apparent only when both Gcn5 and TAF1 were eliminated (28). Thus far, we have found no evidence to support the basic tenet of this hypothesis. We sought to reconcile this difference by collecting similar genome-wide expression profiles using strains and conditions in our study. Rather than utilizing an arbitrary cutoff (e.g., twofold) for delineating real changes in gene expression, we chose to involve all data in a gene-by-gene comparison. Functional redundancy should be manifested by a larger severalfold change in gene expression in the gcn5Δ taf1ts2 double mutant than can be explained by changes arising from independent effects of single mutants.
In Fig. 3A, log2 changes in gene expression are plotted in terms of a frequency distribution akin to the chIP analysis described in Fig. 2A and elsewhere previously (18). Both gcn5Δ and taf1ts2 single mutants generate a leftward shift of the profile relative to the wild type, and the double mutant generates a further leftward shift. If Gcn5 and TAF1 each make independent contributions to transcription, the loss of both should be equivalent to the multiplicative result (additive on a log scale) of losing each individually. Thus, if gcn5Δ causes a twofold drop in expression and taf1ts2 causes a fourfold drop, then the double mutant should cause an eightfold drop, which was observed. If the two are functionally redundant, the double mutant should result in an effect that is substantially greater than the multiplicative effects of the individual mutants, and the individual mutants should have small effects. The distribution for the gcn5Δ taf1ts2 double mutant, calculated from the single mutants, is shown in Fig. 3. The calculated and observed distributions were not significantly different either in terms of a bulk population distribution (Fig. 3A) or on a gene-by-gene comparison (Fig. 3B), indicating that TAF1 and Gcn5 make independent contributions to gene expression and thus are not functionally redundant. Nonetheless, TAF1 and Spt3, as components of TFIID and SAGA, respectively, display some functional redundancy (18). Thus, TFIID and SAGA appear to be partially redundant with respect to TATA binding protein function but not histone acetylation, at least in yeast.
FIG. 3.
Gcn5 and TAF1 make independent contributions to gene expression. (A) Genome-wide changes in gene expression in WT, taf1ts2, gcn5Δ, and taf1ts2 gcn5Δ strains relative to an independent wild-type strain. Expression changes were determined after cultures were shifted to 37°C for 45 min to inactivate the taf1ts2 allele. Frequency distributions are plotted as described in the legend of Fig. 2. This experiment is a repeat of an experiment described previously (18) but was performed in the context of the experiments described in the legend of Fig. 4. The dashed line represents the calculated distribution for the double mutant obtained by adding the log2 ratios of the single mutants. (B) Gene-by-gene scatter plot relating calculated and observed values for the taf1ts2 gcn5Δ strain.
The previous conclusion (28), arrived at via different methods of analysis, arose because the distribution of values (n-fold changes in gene expression) is largely Gaussian rather than linear. Thus, at a fixed and arbitrary cutoff (e.g., twofold) typically used to establish significance, small lateral shifts in the distribution shown in Fig. 3A can lead to large and nonlinear changes in the number of genes meeting the cutoff criteria. Thus, comparing numbers of genes that meet an arbitrary cutoff should not be used to assess functional redundancy. Arbitrary cutoffs, however, can be valid for other types of statistical analyses.
TAF1 displays no functional redundancy with other HATs.
Since frequency distribution profiles are established with as many as 6,000 data points replicated multiple times, they can provide a robust reflection of overall changes in gene expression and a robust means for assessing functional redundancy. Since we detected no functional redundancy between TAF1 and Gcn5, we asked whether any functional redundancy exists between TAF1 and other cellular HATs. Genome-wide expression profiles were conducted on a variety of yeast HAT deletion strains in the context of either wild-type TAF1 or taf1ts2 after a 45-min shift to 37°C. In one case (Esa1) where the HAT is essential for cell growth, we employed the temperature-sensitive esa1-414 allele. No prior study has reported genome-wide dependencies on these HATs.
Figure 4 displays the distribution profiles for changes in expression for each of the single and double mutants. The same data sets were used for WT and taf1ts2 strains in all plots. Also shown is the predicted distribution for the double mutants calculated from the single mutants. In each case, the observed distribution for the double mutants did not show a significant leftward shift (larger decrease in gene expression) relative to the predicted distribution, reflecting a lack of functional redundancy between TAF1 and each of the tested HATs (Sas3, Elp3, Hpa2, Hat1, and Esa1).
FIG. 4.
TAF1 does not exhibit functional redundancy with a variety of yeast HATs, and many HATs do not make significant contributions to global gene expression or acetylation levels. (A to E) Changes in genome-wide mRNA expression levels were determined in mutant HAT strains in a wild-type or taf1ts2 background, as described in the legend of Fig. 3. Predicted values (dashed line) were calculated for the double mutants by adding the log2 ratios of the single mutants. (F) Changes in bulk H3 or H4 acetylation levels at specific lysine residues in each of the mutant HAT strains were assayed by immunoblotting, as described in the legend of Fig. 1.
Interestingly, the hat1Δ taf1ts2 distribution was significantly shifted to the right of its predicted location. Thus, the loss of Hat1 partially compensated for the loss of TAF1, suggesting that Hat1 contributes to the genome-wide dependency on TAF1. Hat1 acetylates cytoplasmic histones prior to their deposition onto chromosomal DNA (1, 53). However, its contribution to transcription is largely unknown. A plausible, but highly speculative, interpretation of the data is that the loss of TAF1 results in a loss of the major TFIID-directed transcription complex assembly pathway with the consequence of chromatin reassembling at promoters. Such chromatin might antagonize alternative assembly pathways, such as that directed by SAGA. Indeed, histones are more repressive towards the SAGA pathway than towards the TFIID pathway (18). If the accumulation of chromatin depends in part upon Hat1 for deposition, then the loss of Hat1 might keep promoter regions more accessible to SAGA-directed transcription complex assembly and thus less dependent upon TAF1.
With the exception of Gcn5 (Fig. 3A) and Esa1 (Fig. 4E), no other HAT by itself made a broad or substantially unique contribution to gene expression, as evidenced by the largely identical distribution of the expression profiles in the HAT deletion mutants with the wild-type profile (Fig. 4A to D). These HATs might be functionally redundant with one or more other HATs, have physiological roles that are unrelated to transcription under the conditions tested here, or target only a few genes. In fact, <0.3% of the yeast genome is uniquely dependent (twofold or more) upon any one of these HATs (Elp3, Hat1, Hpa2, or Sas3) for expression under the growth conditions studied. Those that were moderately affected showed no particularly distinguishing property, except for having a tendency to be stress induced (data not shown). Interestingly, genes that displayed modest positive regulation by Sas3 tended to be Ntd80-regulated sporulation-induced genes, which are lowly expressed under our growth conditions. Sas3 has not been implicated in the sporulation response, and the growth conditions in this study did not involve sporulation. Therefore, it is conceivable that genes might display greater dependency on Sas3 under conditions of spore formation.
Since the loss of Elp3, Hat1, Hpa2, or Sas3 had little impact on the expression of most genes, we next examined whether this was reflected in bulk histone acetylation states. Bulk acetylation levels were examined at H3 K9, K14, K18, K23, and K27 and at H4 K5, K8, K12, and K16 (Fig. 4F). In no case did the loss of these HATs affect bulk acetylation at specific lysine residues. The lack of an effect could have any number of sources, including (i) having highly selective gene targets, (ii) having lysine specificities other than those tested, (iii) making transient contributions, and (iv) being highly redundant with other HATs.
Genome-wide linkage between Esa1, Bdf1, and TAF1.
In yeast, TFIID interacts with the bromodomain protein Bdf1, and the two function at many of the same genes (18, 34). In higher eukaryotes, BDF1 is fused to TAF1, which physically links Bdf1 function to TFIID (34). Bdf1 interacts with acetylated histone H4 tails (19, 27, 35), and the presence of H4 acetylation is correlated with TFIID function (18). Esa1 is part of the NuA4 (promoter-specific acetylation) and Piccolo (global acetylation) complexes that are thought to be responsible for the majority of histone H4 tail acetylation (2, 5). Together, these findings suggest an intimate relationship between TFIID, Bdf1, Esa1, and H4 acetylation. Given the putative HAT function in TAF1, we explored the relationship between TAF1 and Esa1 by examining whether TAF1 and Esa1 both acetylated histone H4 in vivo. As shown by the immunoblot in Fig. 5, the loss of TAF1 had no effect on bulk H4 acetylation at K5, K8, K12, and K16, indicating that TAF1 is not a major H4 HAT. In contrast, the loss of Esa1 resulted in decreased acetylation at K5, K8, and K12, but not at K16, as previously shown (10, 31). The lack of an effect at K16 is consistent with Sas2, rather than Esa1, acetylation of K16 (45). Changes in the taf1ts2 esa1-414 double mutant were no different than the effects seen from the esa1-414 mutant alone, ruling against the idea that TAF1 and Esa1 are redundant HATs at H4. Taken together, these findings demonstrate that Esa1 is the main HAT for bulk H4 acetylation at K5, K8, and K12 and further suggest that if TFIID is to be recruited to promoters via Bdf1 interactions with acetylated histone H4 tails, it is likely to be Esa1 rather than TAF1 that provides the H4 tail acetylation.
FIG. 5.
Esa1, and not TAF1, is important for bulk H4 acetylation levels. Wild-type, taf1ts2, esa1-414, and taf1ts2 esa1-414 strains were grown and shifted to the nonpermissive temperature as described in the legend of Fig. 1. Blots were probed with the indicated antibody. Quantitation of three independent replicates is shown in the bar graph.
The close working relationship between TFIID and Esa1 was examined in more detail by cluster analysis of genome-wide expression profiles (13). Genes whose expression changed by more than twofold in the taf1ts2 mutant, the esa1-414 mutant, or the double mutant were clustered by K means into eight groups (4,754 total genes). Average values for each group in the taf1ts2 and esa1-414 mutant data sets were plotted against each other in Fig. 6A. As expected, most values were negative (in the lower left quadrant), reflecting losses in gene expression when either TAF1 or Esa1 was inactivated. A finer trend was observed within the entire data set. Gene clusters that depended more on TAF1 tended to depend less on Esa1, as evidenced by the inverse relationship shown on the left side of the graph. The rise on the right side of the graph reflects a trend toward Esa1 independence as genes become more TAF1 independent. These genes tend to be dominated by the alternative SAGA assembly pathway (not shown).
Previous studies have suggested a model for TFIID recruitment where NuA4 hyperacetylates H4, allowing TFIID to dock at promoters via Bdf1 (illustrated in Fig. 6B) (18, 33). We wondered whether the trend observed on the left side of the graph in Fig. 6A could be reconciled in terms of this model. If the acetylation step catalyzed by Esa1 is at least partially rate limiting for the expression of certain genes (Fig. 6B), then transcription will be particularly dependent upon Esa1 and less dependent upon TFIID. At other genes, acetylation may not be limiting. Transcription might instead be limited in part by the recruitment of TFIID. These genes would be more dependent upon TAF1 than Esa1. Nevertheless, all genes regulated by Esa1 and TAF1 would be highly dependent upon both.
To examine the plausibility of a model where genes might be more rate limited by Esa1 and others might be more rate limited by TAF1, we performed a computational simulation of the reaction scheme illustrated in Fig. 6B using KinTekSim software. In this simplified scheme, a chromatin-containing promoter becomes acetylated by Esa1 (NuA4), which is governed by a “rate constant,” k1. Next, TAF1 (TFIID) binds, resulting in transcription (i.e., RNA output) that is governed by a “rate constant,” k2. Arbitrary values for both rate constants were established for five different hypothetical genes, representing two situations where Esa1 is limiting (k1 < k2), two situations where TAF1 is limiting (k2 < k1), and one situation where both are equally limiting (k1 = k2). RNA output from the simulator was recorded. Next, RNA output was measured in a hypothetical esa1-414 mutant, which was computationally modeled by decreasing k1 by 10-fold, reflecting an elimination of 90% of Esa1 activity. The same was done with k2 to simulate the taf1ts2 mutant, reflecting a 90% loss of TAF1 activity. Figure 6B plots the log2-fold change (mutant/WT) in simulated RNA output for each of the five hypothetical genes. The similarity of the response pattern in Fig. 6B to the left side of the graph in Fig. 6A indicates that the simulated model represents a valid, albeit speculative, interpretation of the data. This interpretation is consistent with how Esa1 (NuA4) and TAF1 (TFIID) are thought to work and conceptualizes how different genes may be rate limited by distinct steps in a common assembly pathway.
Previously we have shown by chIP-chip that Bdf1 largely tracks with TAF1 throughout the genome, which is consistent with their functional connection via TFIID (54). However, we noted that Bdf1 comes and goes in many places in a way that is unlinked to TAF1. Bdf1 has been shown to associate with the SWR-C complex (20, 23), and evidence from a recent study has shown that Htz1 deposition by the SWR-C complex is partially Bdf1 dependent (55). These observations raise the question of whether the presence of H4 acetylation is more closely linked to Bdf1 binding or TAF1 binding. To address this, genome-wide promoter occupancy data for TAP-tagged Bdf1 and TAF1 were compared to genome-wide H4 acetylation occupancy data (4, 54) using a sliding-window analysis. As shown in Fig. 7A and B, H4 acetylation was more strongly linked to Bdf1 occupancy than to TAF1 occupancy, but TAF1 nevertheless displayed a strong linkage with Bdf1 (Fig. 7C). A chIP-chip experiment in a strain lacking a TAP tag showed no correlation (Fig. 7D). The strong linkage of Bdf1 to H4 acetylation and TAF1, but weaker linkage between H4 acetylation and TAF1, supports the model in Fig. 6B, where acetylated H4 tails primarily bind Bdf1 which generally (but not always) binds TFIID.
FIG. 7.
H4 acetylation is linked to Bdf1 occupancy, which is linked to TAF1 occupancy. Bdf1-TAP, TAF1-TAP, and “null” (no tag) log2 occupancy levels at 25°C were derived from data reported previously (54). The data were centered to the median value for nonpromoter regions (intergenic regions located between two convergent genes). The H4 tetra-acetylated log2 occupancy levels at 30°C were derived from data reported previously (4) and centered to the genome-wide median. The x-axis data were sorted by value, and 100-gene sliding-window averages (step size = 1) were plotted against the corresponding y-axis sliding-window average.
DISCUSSION
TAF1 may not be a HAT in yeast.
The idea that TAF1 is a HAT is appealing in that an acetyltransferase associated with TFIID might facilitate its binding to promoters by opening up chromatin or generating binding sites for bromodomain proteins such as the TFIID-associated protein Bdf1 (C-terminal domain of TAF1 in higher eukaryotes). TAF1 in TFIID appears in many ways to be functionally similar to Gcn5 in SAGA. Both have HAT activity in vitro and both have bromodomains (in higher eukaryotes) that bind acetylated histone tails. However, the strength of yeast TAF1's HAT activity is weak compared to that of other HATs, and thus, it is not clear whether its HAT activity is sufficiently robust to be of widespread physiological relevance in yeast. We find that the H3 acetylation targets of Gcn5 are not acetylation targets of TAF1 and that previous evidence for functional redundancy between TAF1 and Gcn5 does not withstand alternative methods of analysis. Additionally, we see no evidence for functional redundancy between TAF1 and other yeast HATs. It is unlikely that yeast TAF1's putative HAT function is highly selective for certain genes, inasmuch as chIP-chip assays reveal no significant dependence of H3 Ac-K9,14 on TAF1. We cannot and do not exclude the possibility that TAF1 acetylates histones at residues that were not examined or acetylates proteins other than histones, but there is no evidence for this as of yet. We do exclude the likelihood that TAF1 acetylates histones in a manner equivalent to that of Gcn5.
The fact that the putative HAT region of yeast TAF1 is poorly conserved with higher eukaryotic TAF1 further raises the question of whether yeast TAF1 is a physiological HAT. The evidence for TAF1 being a physiologically important HAT in higher eukaryotes is strong (14). Higher eukaryotic TAF1 has a number of properties, including protein kinase activity, bromodomains, and ubiquitin ligase activity, that have not been shown to be present in yeast TAF1 (50). To this list, we now add histone acetyltransferase. It remains to be answered why higher eukaryotes have accumulated these activities or why yeast has lost them. These features may have evolved to accommodate the increased complexity of gene regulation in higher eukaryotes.
Transcription depends upon acetylation, but acetylation does not depend upon transcription.
Histone acetylation has been linked to transcription (26, 30, 39, 40), yet we find that a shutdown of Pol II transcription via inactivation of either TAF1/TFIID or Rpb1/Pol II has little effect on H3 and H4 acetylation. This suggests that most H3 and H4 acetylation is not absolutely coupled to transcription. However, acetylation does correlate with transcription (30, 39) and the loss of acetylation impairs transcription, which, taken together, suggest that acetylation precedes and facilitates transcription in a way that does not depend upon transcription. More transient acetylation, such as any occurring in open reading frames and linked to a transcribing RNA polymerase, cannot be excluded (51). This type of transcription-coupled acetylation likely represents a small fraction of the steady-state level of acetylated histones.
Gcn5 and Esa1 are major gene regulatory HATs, and Hat1, Elp3, Hpa2, and Sas3 are minor gene regulatory HATs.
Our findings demonstrate that the loss of Hat1, Elp3, Hpa2, or Sas3 does not affect global acetylation levels on bulk nucleosomes or transcription in vivo, suggesting that they do not regulate most genes or are highly redundant with other HATs. Selective redundancy exists between Gcn5 and Elp3 (52) and between Gcn5 and Sas3 (17) but not between Elp3 and Sas3 (22). Thus, Gcn5 has a broad overlapping function with other HATs that are functionally distinct from each other. Our results support the notion that Gcn5 and Esa1 are the major yeast HATs at H3 and H4, respectively. As the major HATs, any redundancy with other HATs would be limited to a relatively small fraction of the genome and thus would not be highly redundant. Our study provides the first genome-wide functional comparison of six yeast HATs plus one putative HAT, allowing their relative contributions to gene regulation to be directly compared.
Mechanisms linking Esa1, H4 acetylation, Bdf1 binding, and TFIID recruitment.
The work presented here strengthens the relationship between Esa1, H4 acetylation, Bdf1, and TAF1. These linkages can be rationalized if a major function of Esa1 is to acetylate H4 tails at K5, K8, and K12 so that TFIID can bind via Bdf1. While each step in this model is supported on a broad genome scale, we do not exclude the likelihood that additional mechanisms contribute to TFIID recruitment. Our finding that H4 acetylation at promoters is more highly linked to Bdf1 than to TAF1 suggests that promoter regions that are H4 acetylated and occupied by Bdf1 are not absolutely committed to TFIID recruitment. Instead, Bdf1 might recruit SWR-C, to which it interacts, to remodel Htz1 nucleosomes, but this remains to be determined.
Supplementary Material
Acknowledgments
We thank S. J. Zanton for conducting the experiment depicted in Fig. 2B, J. Coté for gifts of Esa1 plasmids, J. Reese for several histone modification antibodies, and D. Gilmour, J. Reese, S. Tan, and members of the Pugh laboratory for many helpful discussions.
This work was supported by NIH grant GM059055.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ai, X., and M. R. Parthun. 2004. The nuclear Hat1p/Hat2p complex: a molecular link between type B histone acetyltransferases and chromatin assembly. Mol. Cell 14:195-205. [DOI] [PubMed] [Google Scholar]
- 2.Allard, S., R. T. Utley, J. Savard, A. Clarke, P. Grant, C. J. Brandl, L. Pillus, J. L. Workman, and J. Cote. 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18:5108-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basehoar, A. D., S. J. Zanton, and B. F. Pugh. 2004. Identification and distinct regulation of yeast TATA box-containing genes. Cell 116:699-709. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, B. E., E. L. Humphrey, R. L. Erlich, R. Schneider, P. Bouman, J. S. Liu, T. Kouzarides, and S. L. Schreiber. 2002. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA 99:8695-8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudreault, A. A., D. Cronier, W. Selleck, N. Lacoste, R. T. Utley, S. Allard, J. Savard, W. S. Lane, S. Tan, and J. Cote. 2003. Yeast enhancer of polycomb defines global Esa1-dependent acetylation of chromatin. Genes Dev. 17:1415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 7.Carrozza, M. J., R. T. Utley, J. L. Workman, and J. Cote. 2003. The diverse functions of histone acetyltransferase complexes. Trends Genet. 19:321-329. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, J. X., M. Floer, P. Ononaji, G. Bryant, and M. Ptashne. 2002. Responses of four yeast genes to changes in the transcriptional machinery are determined by their promoters. Curr. Biol. 12:1828-1832. [DOI] [PubMed] [Google Scholar]
- 9.Chitikila, C., K. L. Huisinga, J. D. Irvin, A. D. Basehoar, and B. F. Pugh. 2002. Interplay of TBP inhibitors in global transcriptional control. Mol. Cell 10:871-882. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, A. S., J. E. Lowell, S. J. Jacobson, and L. Pillus. 1999. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol. Cell. Biol. 19:2515-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dion, M. F., S. J. Altschuler, L. F. Wu, and O. J. Rando. 2005. Genomic characterization reveals a simple histone H4 acetylation code. Proc. Natl. Acad. Sci. USA 102:5501-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunphy, E. L., T. Johnson, S. S. Auerbach, and E. H. Wang. 2000. Requirement for TAFII250 acetyltransferase activity in cell cycle progression. Mol. Cell. Biol. 20:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilton, T. L., Y. Li, E. L. Dunphy, and E. H. Wang. 2005. TAF1 histone acetyltransferase activity in Sp1 activation of the cyclin D1 promoter. Mol. Cell. Biol. 25:4321-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisatake, K., S. Hasegawa, R. Takada, Y. Nakatani, M. Horikoshi, and R. G. Roeder. 1993. The p250 subunit of native TATA box-binding factor TFIID is the cell-cycle regulatory protein CCG1. Nature 362:179-181. [DOI] [PubMed] [Google Scholar]
- 16.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 17.Howe, L., D. Auston, P. Grant, S. John, R. G. Cook, J. L. Workman, and L. Pillus. 2001. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 15:3144-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huisinga, K. L., and B. F. Pugh. 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13:573-585. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, R. H., A. G. Ladurner, D. S. King, and R. Tjian. 2000. Structure and function of a human TAF(II)250 double bromodomain module. Science 288:1422-1425. [DOI] [PubMed] [Google Scholar]
- 20.Kobor, M. S., S. Venkatasubrahmanyam, M. D. Meneghini, J. W. Gin, J. L. Jennings, A. J. Link, H. D. Madhani, and J. Rine. 2004. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLOS Biol. 2:E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristjuhan, A., and J. Q. Svejstrup. 2004. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 23:4243-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristjuhan, A., J. Walker, N. Suka, M. Grunstein, D. Roberts, B. R. Cairns, and J. Q. Svejstrup. 2002. Transcriptional inhibition of genes with severe histone H3 hypoacetylation in the coding region. Mol. Cell 10:925-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krogan, N. J., M. C. Keogh, N. Datta, C. Sawa, O. W. Ryan, H. Ding, R. A. Haw, J. Pootoolal, A. Tong, V. Canadien, D. P. Richards, X. Wu, A. Emili, T. R. Hughes, S. Buratowski, and J. F. Greenblatt. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 12:1565-1576. [DOI] [PubMed] [Google Scholar]
- 24.Kuo, M. H., E. vom Baur, K. Struhl, and C. D. Allis. 2000. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6:1309-1320. [DOI] [PubMed] [Google Scholar]
- 25.Kuras, L., P. Kosa, M. Mencia, and K. Struhl. 2000. TAF-containing and TAF-independent forms of transcriptionally active TBP in vivo. Science 288:1244-1248. [DOI] [PubMed] [Google Scholar]
- 26.Kurdistani, S. K., S. Tavazoie, and M. Grunstein. 2004. Mapping global histone acetylation patterns to gene expression. Cell 117:721-733. [DOI] [PubMed] [Google Scholar]
- 27.Ladurner, A. G., C. Inouye, R. Jain, and R. Tjian. 2003. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell 11:365-376. [DOI] [PubMed] [Google Scholar]
- 28.Lee, T. I., H. C. Causton, F. C. Holstege, W. C. Shen, N. Hannett, E. G. Jennings, F. Winston, M. R. Green, and R. A. Young. 2000. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405:701-704. [DOI] [PubMed] [Google Scholar]
- 29.Li, X. Y., S. R. Bhaumik, and M. R. Green. 2000. Distinct classes of yeast promoters revealed by differential TAF recruitment. Science 288:1242-1244. [DOI] [PubMed] [Google Scholar]
- 30.Liu, C. L., T. Kaplan, M. Kim, S. Buratowski, S. L. Schreiber, N. Friedman, and O. J. Rando. 2005. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLOS Biol. 3:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loewith, R., M. Meijer, S. P. Lees-Miller, K. Riabowol, and D. Young. 2000. Three yeast proteins related to the human candidate tumor suppressor p33ING1 are associated with histone acetyltransferase activities. Mol. Cell. Biol. 20:3807-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loyola, A., and G. Almouzni. 2004. Bromodomains in living cells participate in deciphering the histone code. Trends Cell Biol. 14:279-281. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Campa, C., P. Politis, J. L. Moreau, N. Kent, J. Goodall, J. Mellor, and C. R. Goding. 2004. Precise nucleosome positioning and the TATA box dictate requirements for the histone H4 tail and the bromodomain factor Bdf1. Mol. Cell 15:69-81. [DOI] [PubMed] [Google Scholar]
- 34.Matangkasombut, O., R. M. Buratowski, N. W. Swilling, and S. Buratowski. 2000. Bromodomain factor 1 corresponds to a missing piece of yeast TFIID. Genes Dev. 14:951-962. [PMC free article] [PubMed] [Google Scholar]
- 35.Matangkasombut, O., and S. Buratowski. 2003. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol. Cell 11:353-363. [DOI] [PubMed] [Google Scholar]
- 36.Mizzen, C. A., X. J. Yang, T. Kokubo, J. E. Brownell, A. J. Bannister, T. Owen-Hughes, J. Workman, L. Wang, S. L. Berger, T. Kouzarides, Y. Nakatani, and C. D. Allis. 1996. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell 87:1261-1270. [DOI] [PubMed] [Google Scholar]
- 37.Nonet, M., C. Scafe, J. Sexton, and R. Young. 1987. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol. Cell. Biol. 7:1602-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson, C. L., and M. A. Laniel. 2004. Histones and histone modifications. Curr. Biol. 14:R546-R551. [DOI] [PubMed] [Google Scholar]
- 39.Pokholok, D. K., C. T. Harbison, S. Levine, M. Cole, N. M. Hannett, T. I. Lee, G. W. Bell, K. Walker, P. A. Rolfe, E. Herbolsheimer, J. Zeitlinger, F. Lewitter, D. K. Gifford, and R. A. Young. 2005. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122:517-527. [DOI] [PubMed] [Google Scholar]
- 40.Robert, F., D. K. Pokholok, N. M. Hannett, N. J. Rinaldi, M. Chandy, A. Rolfe, J. L. Workman, D. K. Gifford, and R. A. Young. 2004. Global position and recruitment of HATs and HDACs in the yeast genome. Mol. Cell 16:199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruppert, S., E. H. Wang, and R. Tjian. 1993. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature 362:175-179. [DOI] [PubMed] [Google Scholar]
- 42.Sekiguchi, T., Y. Nohiro, Y. Nakamura, N. Hisamoto, and T. Nishimoto. 1991. The human CCG1 gene, essential for progression of the G1 phase, encodes a 210-kilodalton nuclear DNA-binding protein. Mol. Cell. Biol. 11:3317-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suka, N., Y. Suka, A. A. Carmen, J. Wu, and M. Grunstein. 2001. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8:473-479. [DOI] [PubMed] [Google Scholar]
- 45.Sutton, A., W. J. Shia, D. Band, P. D. Kaufman, S. Osada, J. L. Workman, and R. Sternglanz. 2003. Sas4 and Sas5 are required for the histone acetyltransferase activity of Sas2 in the SAS complex. J. Biol. Chem. 278:16887-16892. [DOI] [PubMed] [Google Scholar]
- 46.Vogelauer, M., J. Wu, N. Suka, and M. Grunstein. 2000. Global histone acetylation and deacetylation in yeast. Nature 408:495-498. [DOI] [PubMed] [Google Scholar]
- 47.Walker, S. S., J. C. Reese, L. M. Apone, and M. R. Green. 1996. Transcription activation in cells lacking TAFIIS. Nature 383:185-188. [DOI] [PubMed] [Google Scholar]
- 48.Wang, E. H., and R. Tjian. 1994. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science 263:811-814. [DOI] [PubMed] [Google Scholar]
- 49.Wang, E. H., S. Zou, and R. Tjian. 1997. TAFII250-dependent transcription of cyclin A is directed by ATF activator proteins. Genes Dev. 11:2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wassarman, D. A., and F. Sauer. 2001. TAF(II)250: a transcription toolbox. J. Cell Sci. 114:2895-2902. [DOI] [PubMed] [Google Scholar]
- 51.Winkler, G. S., A. Kristjuhan, H. Erdjument-Bromage, P. Tempst, and J. Q. Svejstrup. 2002. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc. Natl. Acad. Sci. USA 99:3517-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittschieben, B. O., J. Fellows, W. Du, D. J. Stillman, and J. Q. Svejstrup. 2000. Overlapping roles for the histone acetyltransferase activities of SAGA and elongator in vivo. EMBO J. 19:3060-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye, J., X. Ai, E. E. Eugeni, L. Zhang, L. R. Carpenter, M. A. Jelinek, M. A. Freitas, and M. R. Parthun. 2005. Histone H4 lysine 91 acetylation a core domain modification associated with chromatin assembly. Mol. Cell 18:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanton, S. J., and B. F. Pugh. 2004. Changes in genomewide occupancy of core transcriptional regulators during heat stress. Proc. Natl. Acad. Sci. USA 101:16843-16848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, H., D. N. Roberts, and B. R. Cairns. 2005. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123:219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, W., J. R. Bone, D. G. Edmondson, B. M. Turner, and S. Y. Roth. 1998. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 17:3155-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.