Abstract
Smooth muscle cells (SMCs) display remarkable phenotypic diversity and plasticity and can readily switch between proliferative and differentiated states in response to extracellular cues. In an effort to identify novel transcriptional regulators of smooth muscle phenotypes, we compared the gene expression profiles of arterial and venous SMCs by microarray-based transcriptional profiling. Among numerous genes displaying distinct expression patterns in these two SMC types, we discovered an expressed sequence tag encoding a previously uncharacterized zinc finger protein belonging to the PRDM (PRDI-BF1 and RIZ homology domain) family of chromatin-remodeling proteins and named it PRISM (PR domain in smooth muscle). PRISM is expressed in a variety of smooth muscle-containing tissues and displays especially robust expression in the cardiac outflow tract and descending aorta during embryogenesis. PRISM is localized to the nucleus and contains an amino-terminal PR domain and four Krüppel-like zinc fingers at the carboxy terminus. We show that PRISM acts as a transcriptional repressor by interacting with class I histone deacetylases and the G9a histone methyltransferase, thereby identifying PRISM as a novel SMC-restricted epigenetic regulator. Overexpression of PRISM in cultured primary SMCs induces genes associated with the proliferative smooth muscle phenotype while repressing regulators of differentiation, including myocardin and GATA-6. Conversely, small interfering RNA-mediated knockdown of PRISM slows cell growth and induces myocardin, GATA-6, and markers of SMC differentiation. We conclude that PRISM acts as a novel epigenetic regulator of SMC phenotypic plasticity by suppressing differentiation and maintaining the proliferative potential of vascular SMCs.
Smooth muscle (SM) cells (SMCs) display remarkable phenotypic diversity and plasticity. SMCs of the venous and arterial systems, for example, can be distinguished by their contractile properties, morphologies, and patterns of gene expression (34). Similarly, visceral SMCs of the lungs, bladder, intestine, and other internal organs display distinct properties that reflect their unique functions. SMCs also modulate their phenotypes in response to extracellular cues. In the absence of growth signals, arterial SMCs withdraw from the cell cycle and express a set of contractile protein genes. Conversely, growth factor signaling provokes SMCs to reenter the cell cycle with a consequent activation of genes involved in proliferation and a concomitant down-regulation of contractile transcripts (35). Such phenotypic plasticity is responsible for abnormalities in SMC growth and differentiation in a variety of disorders, including atherosclerosis, vascular restenosis following angioplasty, hypertension, and smooth muscle tumors (10, 36, 45, 51).
Several transcription factors have been implicated in the control of SMC differentiation (35). The best-characterized regulator of smooth muscle gene expression is serum response factor (SRF), which binds a DNA sequence known as a CArG box and recruits members of the myocardin family of coactivators to activate smooth muscle contractile protein genes (29, 48). In addition to myocardin, GATA-6, a member of the GATA family of zinc finger transcription factors (31), has been shown to promote the contractile or differentiated SM phenotype through the induction of the cyclin-dependent kinase inhibitor p21(Cip1) (37). Recent studies have also highlighted the potential importance of chromatin modifications in the modulation of SMC phenotypes (3, 25), although much remains to be learned about the transcriptional targets of such modifications and how they might be regulated in response to extracellular cues. It is also unclear whether smooth muscle-restricted chromatin remodeling proteins exist or if chromatin configurations on smooth muscle genes are dependent upon the recruitment of ubiquitously expressed chromatin-remodeling enzymes by smooth muscle-specific transcriptional partners.
In an effort to identify transcriptional regulators involved in the phenotypic modulation of SMCs, we compared the gene expression profiles of arterial and venous SMCs by microarray-based transcriptional profiling. Among numerous genes expressed preferentially in one SMC type or the other, we identified an expressed sequence tag (EST) encoding a novel zinc finger protein belonging to the PRDM (PRDI-BF1 and RIZ homology domain) family of transcriptional repressors (16) and named it PRISM (PR domain in smooth muscle). PRISM contains a modified SET domain [named for the Drosophila melanogaster proteins Su(var)3-9, Enhancer-of-zeste, and Trithorax (reviewed in reference 22)] followed by clusters of Krüppel-like zinc fingers at the carboxy terminus. PRISM is expressed preferentially in SMCs of the cardiac outflow tract, the descending aorta, the lung, and the lamina propria of the developing bladder. PRISM is localized to the nucleus and is capable of acting as a transcriptional repressor through interaction with a variety of chromatin-remodeling enzymes. Further, gain- and loss-of-function studies demonstrate that PRISM modulates the phenotypic switch between proliferative and differentiated SMCs.
MATERIALS AND METHODS
RNA isolation and microarray analysis.
Mouse tissue, including the aortae and inferior vena cava samples used for microarray analyses, was harvested from 4-week-old SV129 mice. Care and treatment of animals were in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at UT Southwestern. Total RNA was isolated by standard procedures, transcribed into cDNA, and amplified using an Atlas SMART probe amplification kit (Clontech, Palo Alto, CA) per the manufacturer's instructions. Amplified cDNA was subsequently labeled using a Bioprime DNA-labeling kit (Invitrogen Corporation, Carlsbad, CA) and hybridized to a microarray containing 27,648 murine cDNA elements. For the PRISM overexpression studies, total RNA from control and adenoviral PRISM-infected primary smooth muscle cells was harvested, labeled, and hybridized to rat expression array 230 2.0 (Affymetrix Inc., Santa Clara, CA) as previously described (2).
RNA analyses.
Tissue distribution reverse transcription-PCR (RT-PCR) (see Fig. 2) was performed using a SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) per the manufacturer's instructions with primers flanking a portion of the PR/SET domain through the N-terminal-most zinc finger, corresponding to positions 670 to 1100 of the full-length PRISM transcript. Real-time RT-PCR was performed essentially as described previously (23) by use of primers which flank the N-terminal-most zinc finger, corresponding to positions 999 to 1101 of PRISM. Briefly, the relative induction or repression was determined using the comparative threshold cycle method and normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). We note that PRISM levels following gain- and loss-of-function studies were evaluated by use of a real-time RT-PCR primer set. Additional primer and probe sequences are available upon request. Northern blot analyses were performed using a radiolabeled fragment of PRISM corresponding to nucleotides 670 to 1100 hybridized either to a FirstChoice mouse blot 1 (Ambion Inc., Austin, TX) or to a human cardiovascular system multiple-tissue Northern blot (Clontech, Mountain View, CA). All blots were hybridized in Rapid-hyb buffer (Amersham Biosciences, Piscataway, NJ) with 1× 106 cpm/ml of probe. For the in situ section hybridizations, thin sections of mouse embryos were prepared and hybridized to antisense PRISM probes as described elsewhere (43).
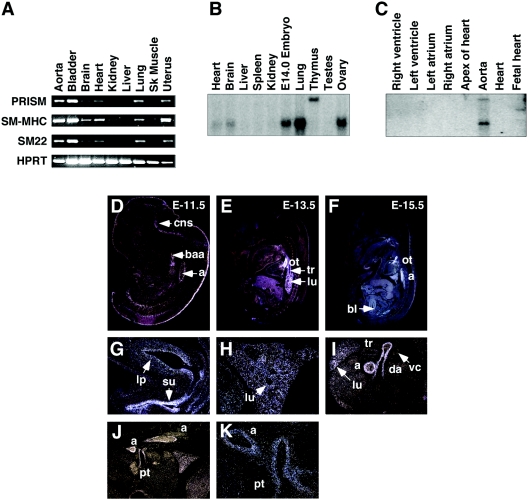
FIG. 2.
Tissue distribution of PRISM. (A) RT-PCR analysis of PRISM. RT-PCR was performed using RNA isolated from adult mouse tissues as described in Materials and Methods. (B) Northern blot analysis of PRISM. Mouse multiple-tissue Northern blots (Ambion) were probed with a radiolabeled PRISM cDNA fragment. Expression of PRISM in whole E14.0 embryo, adult lung, ovary, heart, brain, and thymus is shown. (C) Expression of human PRISM. A human cardiovascular blot (Clontech) was probed with mouse PRISM. Cardiovascular expression of PRISM is specific to the aorta. PRISM expression was detected in sagittal and transverse sections of E11.5 (D), E13.5 (E), and E15.5 (F, G, H, J, and K) mouse embryos. (I) Transverse section of E18.5 mouse embryo exposed to PRISM riboprobes. Antisense probes corresponding to unique PRISM sequences were used as described in Materials and Methods. Staining of the second branchial arch artery (baa), aorta (a), outflow tract (ot), trachea (tr), lung (lu), pulmonary trunk (pt), ductus arteriosus (da), bladder (bl), bladder lamina propria (lp), suburethral space (su), and central nervous system (cns) is depicted.
Expression constructs, cell culturing, transfection, adenoviral infection, and growth curves.
Full-length or truncated PRISM constructs were generated using Expand high-fidelity PCR kits (Roche Applied Science, Indianapolis, IN) and subcloned into amino-terminally FLAG- and myc-tagged pCDNA3.1 (Invitrogen Corp., Carlsbad, CA) expression vectors as described elsewhere (56). COS-7 cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 2 mM l-glutamine, and penicillin-streptomycin. Cells (1.5 × 105) were transfected with 1 μg of FLAG- and/or myc-tagged expression plasmids containing either full-length or truncated forms of the following: PRISM, FLAG-histone deacetylase (HDAC) 1 (HDAC1), FLAG-HDAC2, FLAG-HDAC3 (kind gifts of Edward Seto), FLAG-Hp-1, hemagglutinin (HA)-p300, or HA-G9a (a kind gift of Kenneth Wright) using FuGENE 6 reagent (Roche Applied Science, Indianapolis, IN).
For small interfering RNA (siRNA) studies, a final concentration of 100 nM of SMARTpools, individual siRNAs (Dharmacon Inc., Lafayette, CO) directed against PRISM, or siCONTROL nontargeting RNAs was transfected into rat primary smooth muscle cells by use of Lipofectamine 2000 (Invitrogen Corp., Carlsbad CA) per the manufacturer's instructions. For the adenoviral overexpression of PRISM, the full-length cDNA was subcloned into pVQ-CMV-K-NpA, and virions were generated by Viraquest Inc. (North Liberty, IA). A multiplicity of infection of 50 was used for all studies.
For the growth curves following siRNA treatment, cells cultured in 96-well plates were incubated with WST-1 reagent for 2 h followed by colorimetric analysis at 492 nm per the manufacturer's instructions (Roche Applied Science, Indianapolis, IN). Experiments at all time points were performed in triplicate.
Immunoprecipitations and immunoblotting.
Following transfection, cells were cultured for 24 to 48 h. For immunohistochemistry, transfected cells were washed twice with phosphate-buffered saline, fixed in 3.7% paraformaldehyde, and processed as previously described (27) using a 1:500 dilution of monoclonal FLAG M2 antibody (Sigma-Aldrich, St. Louis, MO) and 1:500 fluorescein isothiocyanate-labeled goat anti-mouse secondary antibody (Vector Laboratories Inc., Burlingame, CA). For immunoprecipitations, cell lysates were collected by scraping in lysis buffer (ELB) containing 10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and a cocktail of protease inhibitors (complete EDTA-free; Roche). Cellular debris was removed by centrifugation, and lysates were subjected either to immunoblotting directly or to immunoprecipitation. For immunoprecipitation, lysates were incubated with 1 μg of anti-FLAG M2 (Sigma-Aldrich, St. Louis, MO), anti-HA, or anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 to 2 h and subsequently incubated with protein G-agarose (Zymed Laboratories Inc., South San Francisco, CA). After incubation, pelleted protein complexes were washed extensively with ELB and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by electrotransfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA) and immunoblot analysis using anti-FLAG (1:5,000), anti-myc (1:1,250), or anti-HA (1:1,250) primary antibodies. After the washing step, the membranes were incubated with either goat anti-rabbit or goat anti-mouse secondary antibodies (Bio-Rad Laboratories, Hercules, CA) at 1:5,000 and subjected to enhanced chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA).
Transcriptional repression and histone methyltransferase assays.
Luciferase measurements using the pGL2-5× GAL4-SV40 luciferase reporter containing five copies of the GAL4-upstream activation sequence (UAS) upstream of the SV40 promoter were taken per the manufacturer's instructions (Promega U.S., Madison, WI). All assays were performed in duplicate in the case of the trichostatin A (TSA) studies or in triplicate, and data were normalized to the mass (micrograms) of cellular protein. Data are shown as relative luciferase expression levels compared to that of cells cotransfected with the reporter plus GAL4 DNA binding domain (DBD) alone. For the methyltransferase assays, immunoprecipitates from transfected COS-7 cell lysates were incubated with 20 μM S-adenosylmethionine (New England Biolabs Inc., Beverly, MA) and 2 μg recombinant histone H3 (Upstate Cell Signaling Solutions, Charlottesville, VA) in histone methyltransferase (HMT) buffer (50 mM Tris HCl, pH 9.0, 0.5 mM dithiothreitol) for 1 h at 30°C. All reactions were stopped by the addition of 6× Laemmli buffer, and the products were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10 to 20% gradient gels followed by electrotransfer to polyvinylidene difluoride membranes. Membranes were incubated with anti-dimethyl-histone H3 (Lys 9) (Upstate Cell Signaling Solutions, Charlottesville, VA) primary antibodies at 1:2,000, followed by incubation with goat anti-rabbit secondary antibodies and subsequent chemiluminescence.
RESULTS
Transcriptional profiling of arterial versus venous SMCs.
In an effort to identify novel regulators of smooth muscle transcription, we compared the gene expression profiles of arterial SMCs of the thoracic aorta and venous SMCs of the inferior vena cava of adult mice by microarray analysis. This approach allowed us to minimize the endothelial contribution and to identify genes which might be expressed in the medial or SM layer of the blood vessel. Candidate genes enriched in the aortic RNA set were then subjected to database interrogation and screened for tissue distribution and specificity by RT-PCR, Northern blot, and RNA in situ hybridization analyses. A sample of known smooth muscle genes and their relative aortic versus venous expression values are presented in Table 1. As can be seen, a variety of SM-specific transcripts, including those encoding calponin, SM myosin heavy chain (MHC), myocardin, and integrin alpha-8, as well as aortic preferentially expressed gene 1 (APEG1), were enriched in the aorta.
TABLE 1.
Representative clones enriched in the aorta
| Symbol | Gene or gene product (description) | Aorta vs veina |
|---|---|---|
| Itga8 | Integrin alpha-8 | 98.9 |
| Cnn1 | Calponin 1 | 28.9 |
| Hoxa7 | Homeobox A7 | 7.8 |
| Hoxb6 | Homeobox B6 | 7.3 |
| Afp | Alpha fetoprotein | 6.3 |
| Myln | Myosin light chain (alkali, nonmuscle) | 6.1 |
| PRDM6 | PR-domain Zn finger protein 6 | 5.9 |
| Apeg1 | Aortic preferentially expressed gene 1 | 5.7 |
| Csrp2 | Cysteine-rich protein 2/smooth muscle LIM protein | 4.2 |
| Mbnl | Muscleblind-like protein | 3.1 |
| Fhl1 | 4.5 LIM domains 1 | 2.8 |
| Myh11 | Myosin heavy chain 11 | 2.8 |
| Mycd | Myocardin | 2.2 |
Expression in aorta versus that in vein.
PRISM, a smooth muscle-restricted member of the PRDM family.
Among the arterial enriched transcripts was an EST representing the unigene cluster of a predicted member of the PRDM family of transcription factors, PRDM6 (Mm. 297645). Based on the PSI-BLAST searches of the human genome done to date (16), a total of 17 PRDMs have been predicted to exist, few of which have been fully characterized. As shown in Table 1, this transcript demonstrated a sixfold enrichment within aortic RNAs, suggesting a bias for arterial versus venous SM. Given the potential enrichment of this sequence, we further characterized the structure, expression pattern and function of the encoded protein, which we named PRISM (PRDM in smooth muscle).
Using the original EST sequence found on our microarrays, 5′ and 3′ rapid amplification of cDNA ends revealed a 2.2-kb transcript encoding a 395-amino-acid protein with high homology to members of the PRDM family of SET proteins (Fig. 1). Found in Su(var)3-9, Enhancer-of-zeste, and Trithorax, SET proteins contain a 130-amino-acid motif that, when adjacent to Cys-rich regions, is capable of directing histone methylation (18). PRISM contains an AWS (associated with SET) domain located amino terminally to a PR domain which is followed by four carboxy terminal zinc fingers.
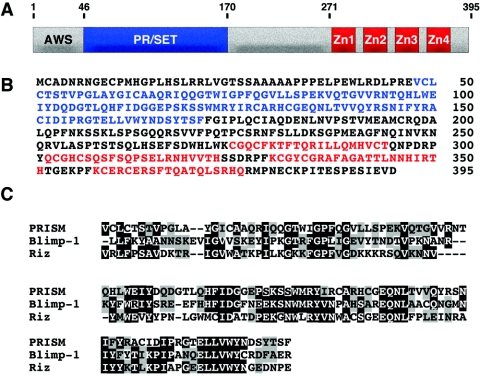
FIG. 1.
Deduced amino acid sequence and homology of PRISM with other PR proteins. (A and B) Schematic and deduced amino acid sequence of PRISM. The PR domain is shown in blue and the Krüppel-like zinc fingers (Zn) are shown in red. The amino acid positions of the deletion constructs are indicated. (C) Comparison of PRISM with other proteins from the PR family showing homology through the PR/SET domain.
The mouse PRISM gene contains seven exons located on chromosome 18. We identified two alternative first exons of PRISM from rapid amplification of cDNA ends reactions; both of these alternatives are 5′ to the PR/SET motif and the initiating methionine found within exon 2. One of these exons maintains a continuous open reading frame 5′ of the initiating methionine. Repeated attempts to identify additional sequence containing a closed reading frame were unsuccessful. The other transcript variant contains an exon coding for translation stops in all reading frames upstream of the initiating ATG within exon 2. Both transcripts differ by 81 nucleotides and were not distinguishable in our Northern blots (see below). Additionally, both variants were detected in aorta, lung, and bladder RNAs at equal intensities and were capable of directing translation in reticulocyte lysates with equal efficiencies (data not shown). All data presented here were obtained with the clone containing translation stop codons in all three reading frames preceding the initiating methionine.
SM-restricted expression of PRISM.
Figure 2A shows an RT-PCR panel of RNAs isolated from representative adult tissues by use of primers within the PRISM protein-encoding region. The SM markers, including SM22-α and SM myosin heavy chain, were included as controls. In addition being expressed in the adult aorta, PRISM is expressed in the bladder, lung, heart, and uterus. Northern analyses revealed expression of a single PRISM transcript of ∼2.5 kb within RNA isolated from an embryonic day 14.0 (E14.0) mouse embryo as well as from the adult lung and ovary and also revealed a lower level of expression within the heart and brain (Fig. 2B). We also detected a significantly larger transcript within the thymus. Northern analysis using human cardiovascular tissue also corroborated the aortic expression of PRISM (Fig. 2C).
The embryonic expression pattern of PRISM was further explored by in situ hybridization to mouse embryo sections. As can be seen in Fig. 2D through K, aortic expression of PRISM occurs as early as E11.5. In addition, the second branchial arch artery and ductus arteriosus, transient structures required for proper oxygen and nutrient delivery during embryonic development (14), were marked with the PRISM probe (Fig. 2D and I) As shown in Fig. 2I, PRISM was not detected within the SM layer of the vena cava, providing additional support for the subtraction values shown in Table 1. As depicted in Fig. 2K, PRISM is expressed specifically within the smooth muscle layer of the aorta and pulmonary trunk. During airway development, PRISM expression was observed as early as E13.5 within developing smooth muscle surrounding the bronchi of the lung (Fig. 2E, H, and I) and within the walls of the trachea as early as E13.5. This expression pattern is maintained throughout pseudoglandular lung development (Fig. 2H) and into the canalicular stage (Fig. 2I). In addition, portions of the central nervous system, bladder lamina propria, and suburethral space were stained with the PRISM probe (Fig. 2D and G).
PRISM acts as a nuclear transcriptional repressor.
To test whether PRISM possessed transcriptional activity, we fused it to the DNA binding domain of GAL4 and performed transfection assays with a GAL4-dependent luciferase reporter (11). As shown in Fig. 3A, PRISM reduced expression of the luciferase reporter in a dose-dependent manner.
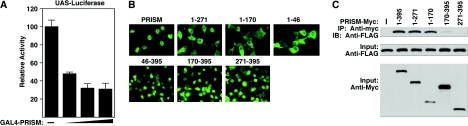
FIG. 3.
Transcriptional repression, nuclear localization, and homodimerization of PRISM. (A) PRISM is a transcriptional repressor. Results for cotransfections of a 5× GAL4-UAS luciferase reporter with the GAL4 DBD alone or with 50, 100, and 150 ng of GAL4-PRISM fusions are shown. All luciferase data are normalized to protein content following cell lysis. (B) Nuclear localization of PRISM. Immunocytochemistry of FLAG-PRISM-transfected COS-7 cells. FLAG-PRISM is directed to the nucleus by the carboxy-terminal Zn fingers. (C) PRISM forms homodimers. Immunoprecipitation (IP) experiments of epitope-tagged full-length PRISM and PRISM deletions in cotransfected COS-7 cells. Input controls are shown below the immunoprecipitation panels. IB, immunoblotting. The amino acid positions of the deletion constructs are indicated.
We next examined the subcellular localization of epitope-tagged PRISM in transfected COS-7 cells and found it to be exclusively nuclear (Fig. 3B), irrespective of the nature or position of the epitope (data not shown). The domains of PRISM responsible for nuclear localization were subsequently mapped by deletion mutagenesis. As shown in Fig. 3B, the carboxy terminal zinc fingers were both necessary and sufficient to direct PRISM to the nucleus. We also examined whether PRISM exists as a monomer or as a multimer. Figure 3C shows PRISM was capable of forming homodimers, as detected by immunoprecipitation, and the PR domain was sufficient to mediate this interaction.
Protein-protein interactions of PRISM.
In an effort to investigate the mechanistic basis for PRISM-mediated transcriptional repression, we performed immunoprecipitation assays with a series of chromatin-remodeling proteins known to interact with HMTs. As shown in Fig. 4, PRISM was capable of interacting with heterochromatin protein-1 (HP1-β) (Fig. 4A) and also with HDAC1, -2, and -3 (Fig. 4B through D). Deletion mapping revealed that the PR domain was sufficient for HDAC binding. However, the HP1-β interaction was lost with any deletion of PRISM, presumably due to a disruption of the tertiary structure required for complex formation. We also observed the sequence between the PR domain and zinc fingers to be capable of associating with HDAC3, consistent with reports for the PR domain protein Blimp-1 and HDAC2 (55).
FIG. 4.
Interaction of PRISM with chromatin-remodeling enzymes. (A) Coimmunoprecipitation assays of PRISM and HP1-β. COS-7 cells were transfected either with HP1-β alone or in combination with full-length p300 and deletions of PRISM. Lysates were immunoprecipitated (IP) with anti-myc antibodies, and immunoblots (IB) were performed with anti-FLAG as described in Materials and Methods. (B through D) Coimmunoprecipitations of PRISM and HDAC1, HDAC2, and HDAC3, respectively. COS-7 lysates from class I HDACs alone or from HDACs plus PRISM transfections were incubated with anti-myc (PRISM) and subjected to immunoblot analysis using anti-FLAG (HDACs) antibodies. Input controls for each experiment are shown below. (E) Coimmunoprecipitation experiment using full-length p300 and deletions of PRISM. Lysates were immunoprecipitated with anti-HA (p300), and blots were probed with anti-FLAG (PRISM). Input controls for each experiment are shown below the immunoprecipitation panels. The amino acid positions of the deletion constructs are indicated.
Studies by Vandel and Trouche indirectly showed that CBP and p300 immunoprecipitates contained HMT activity (46). Further, methylation at histone H3 K4 has been shown to potentiate p300-mediated acetylation at K9, resulting in transcriptional activation (49). To explore the possibility that PRISM might interact with transcriptional activators in addition to repressors, we tested whether PRISM could associate with p300, a potent transcriptional coactivator with intrinsic histone acetyltransferase activity, in transfected COS-7 cells. As shown in Fig. 4E, we observed complex formation between PRISM and p300. This interaction required the PR domain of PRISM, providing additional evidence that the PR domain of this family of proteins functions as a protein interaction domain capable of mediating complex formation with a variety of chromatin-modifying enzymes.
PRISM contains two repressive domains.
In an effort to identify the domains of PRISM responsible for conferring transcriptional repression and to test the functional significance of the interactions shown in Fig. 4, we tested deletions of PRISM using the GAL4 fusion system. As shown in Fig. 3A and 5A, full-length PRISM fused to GAL4 repressed the UAS-luciferase reporter. Further, the PR motif (residues 1 to 170) and the zinc finger domains (residues 271 to 395) of PRISM were sufficient to mediate transcriptional repression on their own. Additionally, as residues 271 to 395 of PRISM were capable of repressing the reporter, PRISM does not require dimerization to mediate transcriptional repression. We also observed that the amino-terminal AWS motif was required for PR-mediated repression, suggesting the importance of this domain for proper PRISM function (see data for deletion mutant 46-395).
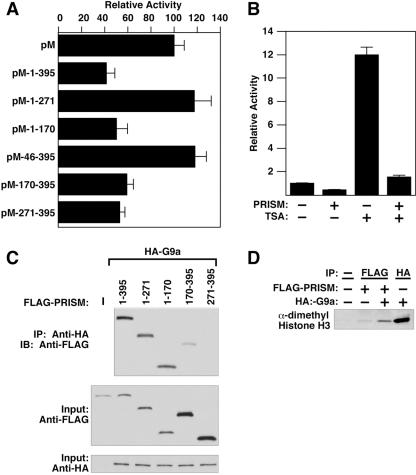
FIG. 5.
PRISM contains two repressive domains and recruits methyltransferase activity. (A) The PR and Zn finger domains of PRISM repress transcription. Cotransfections of the 5× GAL4-UAS luciferase reporter and either the GAL4 DBD alone (pM) or GAL4-PRISM (100 ng) constructs are shown. All data are normalized to protein content as described above. (B) Histone deacetylase inhibitors do not abrogate PRISM-mediated repression. COS-7 cells were cotransfected with 5× GAL4-UAS and either the GAL4 DBD alone or GAL4-PRISM (100 ng) and treated with 100 nM TSA for 24 h prior to harvest. (C) Immunoprecipitations (IP) of PRISM with G9a. Cotransfected lysates were incubated with anti-HA (G9a) antibody followed by immunoblotting (IB) with anti-FLAG (PRISM). (D) PRISM associates with HMT activity. Results from histone methylation assays from transfected cell lysates are shown. Cells were transfected with PRISM, G9a, or both and subjected to immunoprecipitation using the designated antibody and subsequent methyltransferase assays. All samples containing PRISM were immunoprecipitated with anti-FLAG (PRISM) antibodies. The amino acid positions of the deletion constructs are indicated.
We next tested whether PRISM required HDAC activity to mediate transcriptional repression. Figure 5B shows that although the HDAC inhibitor TSA relieved a basal level of transcriptional repression on the reporter plasmid, it did not alleviate PRISM-mediated repression, suggesting that although PRISM interacts with class I HDACs, it does not require HDAC activity to mediate transcriptional repression (Fig. 5B).
Association of PRISM with HMT activity.
The PRDM protein PRDI-BF1 silences the beta interferon promoter by recruiting G9a, a ubiquitous HMT known to dimethylate histone H3 K9 and K27 (13). PRISM, like other PR family members described to date (16), differs from most enzymatically competent SET proteins within the NHSC motif of the PR/SET domain (40). In PRISM, as in all other PRDMs, the essential histidine at position 2 within this motif is changed to a cysteine. As our repeated attempts to detect HMT activity intrinsic to PRISM using a variety of biochemical approaches were unsuccessful (data not shown), and given that HDAC inhibitors did not alleviate PRISM-mediated repression, we explored the possibility that PRISM might recruit G9a in a manner similar to PRDI-BF1 (13) to promote gene silencing. As shown in Fig. 5C, we observed PRISM-G9a complex formation in transfected cells. This interaction was mediated by the PR domain, although we were also able to detect a modest interaction with the adjacent region of PRISM contained between residues 170 and 395.
Figure 5D shows HMT assays using recombinant histone H3 and immunoprecipitates from transfected COS-7 cell lysates. Lane 1 controls for K9 dimethyl specificity of the antibody. Modest HMT activity was observed in immunoprecipitates from cells transfected with PRISM alone, presumably due to interaction with endogenous HMTs in COS-7 cells. HMT activity was significantly increased upon cotransfection with G9a and subsequent PRISM immunoprecipitation, demonstrating a functional consequence for the PRISM-G9a interaction (Fig. 5C). These data suggest that PRISM, although lacking intrinsic HMT activity, is capable of recruiting HMTs and a cohort of chromatin-remodeling enzymes involved in establishing and maintaining transcriptional repression.
Overexpression of PRISM induces markers characteristic of the proliferative phenotype in cultured SMCs.
In an effort to identify SM genes regulated by PRISM, we performed microarray analyses on RNA harvested from primary SMCs infected with PRISM-overexpressing adenovirus. Empty adenovirus was used as a control. Figure 6A shows a list of the most induced and repressed transcripts following PRISM overexpression. Microarray results were confirmed by real-time RT-PCR and are depicted in Fig. 6B. A variety of genes known to be expressed in SM, epithelial, and myoepithelial cells, including those encoding the potent mitogens amphiregulin (19) and Wnt4 (52), were induced, suggesting that PRISM modulates the SM phenotype by regulating the expression of genes associated with activated SM. Consistent with these findings, we observed an induction in cellular retinol binding protein 1 (Rbp1), the gene for which is known to be induced in SMCs following experimental intimal thickening (5).
FIG. 6.
PRISM regulates the expression of SM proliferative and contractile genes. (A and B) Induction of proliferative SM genes in primary SMCs overexpressing PRISM. (A) Microarray data are presented as increases or decreases compared to levels for SMCs infected with empty adenovirus. (B) Representative RT-PCR products and concomitant real-time RT-PCR values validating the overexpression data obtained as shown in panel A. (C) A growth curve following PRISM knockdown in primary SMCs is depicted. Cells from all time points were harvested in triplicate and subjected to colorimetric analyses using WST-1 reagent as described in Materials and Methods (P = 0.03 at 48 h). (D) Representative RT-PCR products following PRISM knockdown are depicted. The SM-MHC panel was generated from RNA harvested 48 h posttransfection. All other panels were from samples harvested at 24 h.
We also observed repression of genes associated with the differentiated SM phenotype, including those encoding myocardin and GATA-6 (Fig. 6B), following PRISM overexpression. As the expression of these genes correlates with the differentiated SM phenotype, these data further implicate PRISM as a component of the activated SMC.
Loss of PRISM induces differentiated markers in SMCs.
To further explore the functions of PRISM, we performed loss-of-function studies using siRNAs. As shown in Fig. 6C and D, a sixfold knockdown of PRISM transcripts reduced the growth rate of primary SMCs compared to that of controls. RT-PCR from PRISM siRNA-transfected SMCs showed an up-regulation of myocardin and a modest increase in GATA-6 expression within 24 h followed by a concomitant induction of SM myosin heavy chain, a marker for differentiated SMCs at 48 h. These data suggest that PRISM regulates the SM phenotype, at least in part, through the induction of growth factors and signaling molecules that prevent or delay SMC differentiation.
DISCUSSION
We describe here the molecular cloning and characterization of PRISM, a novel PR/SET domain protein that displays highly restricted expression in a subset of SMCs. PRISM acts as a transcriptional repressor through interaction with a variety of chromatin-remodeling enzymes. Gain- and loss-of-function approaches in experiments with cultured SMCs point to a role for PRISM in the promotion of proliferation and the suppression of differentiation, which may be dependent, at least in part, on its ability to induce amphiregulin and Wnt4 while repressing the expression of myocardin and GATA-6.
Smooth muscle-restricted expression of PRISM.
PRISM is expressed in a subset of SMCs within the outflow tract and mesenteric arteries in the descending and thoracic aorta and in the second branchial arch artery during embryonic development. PRISM is also detected within the developing smooth muscle of the airway and lung during the pseudoglandular and canalicular stages of lung development and in the bronchi as early as E13.5, suggesting a role in early lung morphogenesis. We also noted robust expression within the lamina propria of the developing bladder and suburethral space. Given its association with the SM proliferative phenotype, it is tempting to speculate about a role for PRISM in neointimal proliferation, bladder neoplasias, and lung pathophysiology.
Association of PRISM with chromatin-remodeling enzymes.
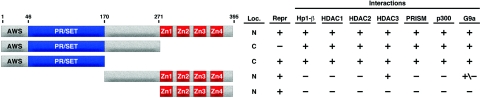
A summary of the known functions of PRISM, including those of the domains responsible for nuclear localization, transcriptional repression, and protein interaction, is shown in Fig. 7. The Krüppel-type zinc fingers located at the carboxy terminus of PRISM are necessary and sufficient for targeting PRISM to the nucleus. As these structures have been shown to bind DNA for other PR family members in addition to nuclear targeting (12, 20, 53), we assayed PRISM for its DNA binding potential using a consensus Blimp-1 binding site (data not shown). Repeated attempts using both transfected cell lysates and in vitro-synthesized PRISM were unsuccessful, suggesting that PRISM either recognizes a unique DNA binding site or lacks high-affinity DNA binding activity.
FIG. 7.
Summary of PRISM domains. A schematic of PRISM, including the amino acid positions of the deletion constructs and their activities, is shown. Repr, transcriptional repression; C, cytoplasmic; N, nuclear.
PRISM homodimerizes and associates with diverse chromatin-remodeling enzymes via its PR domain. We demonstrate that this domain, unlike Riz/PRDM2 (17), is necessary and sufficient for PRISM oligomerization, and we observed no dimerization potential within the carboxy terminus of the protein. Consistent with other SET (6) and PR (55) proteins, PRISM interacts with class I HDACs. Our data, however, differ from studies by Yu et al., who identified two independent Blimp-1 domains capable of interacting with HDAC2 (55). Interestingly, these domains are distinct from the PR motif of Blimp-1, suggesting divergent mechanisms of recruiting chromatin-remodeling proteins for the PRDMs. We also note that the sequence between the PR and Zn finger domains of PRISM was capable of interacting with HDAC3 in a manner similar to the demonstrated interaction of Blimp-1 and HDAC2.
In a further substantiation of its role in transcriptional repression, PRISM was capable of interacting with HP1-β, an H3 K9-specific transcriptional suppressor. Attempts to coimmunoprecipitate PRISM with HP1-α and HP1-γ were unsuccessful (data not shown), underscoring the specificity of the PRISM-HP1-β interaction. Interestingly, the HP1-β interaction was lost with any deletion of PRISM, suggesting a requirement for a complex tertiary structure for the observed interaction. The association of PRISM with HP1-β suggests a mechanism whereby PRISM may be involved in the assembly of facultative chromatin in genomic loci coding for regulators of the differentiated SM phenotype.
Although we detected the association of PRISM with molecules involved in transcription repression, we also found it to interact with p300, a powerful transcriptional coactivator. This observation suggests that PRISM might play a role in the transcriptional activation of genes required for SM function. We observed, however, that PRISM displayed only transcriptional repression activity in transfection assays using a GAL4-dependent reporter (Fig. 4A). We find it more likely that PRISM exists in a multiprotein complex containing both transcriptional repressors and activators. Whether PRISM might activate genes, either alone or in combination with cognate transcription factors, as has been reported for Riz/PRDM2 (4), remains to be determined.
Unlike with SUV39H1 (40), G9a (44), and Riz (21), we were unable to demonstrate intrinsic HMT activity of PRISM. However, we observed an interaction between PRISM and G9a, the ubiquitous H3 K9 and K27 methyltransferase previously demonstrated to effect Blimp-1-mediated transcriptional repression (13). As with all interactions tested, and contrary to what was seen for Blimp-1, the PR domain was sufficient for G9a interaction, although a modest level of interaction was detected in the PRISM170-395 deletion construct (Fig. 5C.). As G9a is a ubiquitous HMT involved in H3 K9 dimethylation and K27 di- and trimethylation, we postulate that PRISM recruits HMT activity to the regulatory regions of genes involved in SM differentiation.
PRDM family.
The functions of PRDM family members are only beginning to be determined. Blimp-1 has been shown to control B-cell differentiation by down-regulating transcription factors important for maintaining B-cell receptor signaling, including c-myc, CIITA, Spi-B, Id-3, and Xbp1 (39, 42). Blimp-1-mediated transcriptional repression requires association with G9a and class I HDACs (13). Recent studies have also described an essential role for Blimp-1 in the specification of slow-twitch skeletal muscle fibers (1) and neural crest progenitors (41) in zebra fish, suggesting a critical role for this transcriptional repressor in cell fate decisions. Whether PRISM plays a similar role in the developing smooth muscle of the lung, blood vessels, and submucosa of the bladder remains to be determined.
Riz/PRDM2 is frequently deleted in human cancers (9, 15) and has been shown to transactivate estrogen-responsive genes in an estrogen-dependent manner through recruitment of p300 (4). Translocations involving the Mds1/EVI1/PRDM3 locus have been associated with human cancer (30). PRDM5 has also been implicated in the control of cell growth, and its promoter is hypermethylated in a variety of human tumors, suggesting a role for this PR family member in oncogenesis (7). The coincidence of neoplasia and PRISM expression within the submucosa of the bladder and airway smooth muscle suggests a potential role for PRISM in tumorigenesis of these tissues.
Transcriptional regulators of smooth muscle gene expression.
Several widely expressed and cell type-restricted transcription factors have been implicated in the control of smooth muscle gene expression (28, 47). In contrast to the advances in understanding the cis-regulatory elements and trans-acting factors involved in SM gene regulation, little is known of the potential involvement of chromatin modifications in the modulation of SMC phenotypes. However, an intriguing recent study revealed a preference for SRF binding to the SM MHC promoter in differentiated versus dedifferentiated SMCs (25). Further, SRF binds preferentially to the SM MHC promoter but not to the skeletal actin promoter in cultured SMCs (24). These studies suggest a role for chromatin remodeling in the regulation of the access of transcription factors to cognate binding sites during SMC differentiation. More recently, our laboratory showed that myocardin can recruit histone acetyltransferase activity to the promoters of SM contractile genes (3), thereby resulting in gene activation. The present work places PRISM in a regulatory cascade which down-regulates the expression of SM contractile genes by repressing the transcription factors necessary for their expression. A determination as to whether PRISM represses myocardin and/or GATA-6 directly will require the identification of the control regions of these genes. Alternatively, PRISM might repress the expression of contractile genes directly. Attempts to place PRISM at the promoters of such genes using chromatin immunoprecipitation and quantitative RT-PCR have been unsuccessful. Elucidating the precise mechanism(s) of PRISM-mediated SM gene modulation is an area of current investigation.
PRISM promotes the transition from differentiated to proliferative SM.
The pathologies of atherosclerosis and restenosis involve multiple cell types and signaling events eventually leading to neointimal proliferation, plaque formation, and a narrowing of the affected vessel. Recent evidence has implicated epidermal growth factors (EGF) in the pathophysiology of this process (8). Moreover, the EGF-related ligand amphiregulin, which we found to be up-regulated by PRISM, has been shown to act through the extracellular signal-related kinase/ELK1-cyclin D1 pathway to stimulate SMC proliferation. Given that SMCs express EGF receptors (54), an autocrine signaling loop in which amphiregulin expression promotes continued proliferation might exist in SMCs, as it does in keratinocytes (38). As PRISM overexpression induced amphiregulin expression in cultured SMCs, it is tempting to place PRISM in this molecular pathway as a key regulator of SMC proliferation. Although Wnt4 is primarily expressed in the kidney (52) and has not been shown previously to be expressed in vascular SMCs, we observed a significant induction of Wnt4 transcripts following PRISM overexpression. As a variety of Wnt receptors (frizzleds) are expressed on SMCs (26, 50), we postulate that like amphiregulin expression, Wnt4 provides a progrowth signal to PRISM-expressing SMCs. Most notably, we observed a dramatic increase in Rbp1 levels following PRISM overexpression (Fig. 6B). As Rbp1 is restricted to dedifferentiated SMCs and given our inability to detect Rbp1 transcripts in control cells, we propose that PRISM can activate the proliferative SM program. Further, as Rbp1 marks SMCs induced to proliferate following endothelial injury in vivo (32), PRISM may serve as a key regulator of disease pathologies associated with vascular SM proliferation.
In addition to the induction of transcripts for growth factors and signaling molecules, we observed a reduction in myocardin and GATA-6 expression in SMCs overexpressing PRISM. We did not, however, observe a reduction in differentiated marker genes, including that encoding SM-MHC, following PRISM overexpression, possibly due to the inherent stability of these transcripts and/or the myocardin and GATA-6 proteins themselves. Conversely, we observed a concomitant induction of transcripts encoding these factors following siRNA-mediated PRISM knockdown. Given that both myocardin and GATA-6 participate in SM differentiation (37, 48), we postulate that PRISM is a component of the molecular switch from cellular quiescence towards SM proliferation. Furthermore, our data suggest that PRISM also modulates the SM phenotype through the coordinated regulation of autocrine growth factors, thereby providing proliferative potential to differentiated SMCs. Taken together, these data allow us to postulate that PRISM functions as a transcriptional repressor that suppresses SMC differentiation while promoting SM proliferation. In addition to the role of PRISM in development, it will be interesting to explore the relationship between PRISM expression and a variety of diseases associated with the aorta, lung and bladder.
Acknowledgments
We thank Kenneth Wright and Edward Seto for the G9a and HDAC expression constructs. We also thank A. Tizenor for assistance with graphics, J. Page for editorial assistance, and M. Hoofnagle and Carolin Laeufle for expert technical assistance.
C.A.D. was supported by an NIH postdoctoral fellowship, M.H. was supported by a grant from the Deutsche Forschungsgemeinschaft (HA 3335/2-1), and work in the laboratory of E.N.O. was supported by grants from the National Institutes of Health, The D. W. Reynolds Clinical Cardiovascular Research Center, and the Robert A. Welch Foundation.
REFERENCES
- 1.Baxendale, S., C. Davison, C. Muxworthy, C. Wolff, P. W. Ingham, and S. Roy. 2004. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat. Genet. 36:88-93. [DOI] [PubMed] [Google Scholar]
- 2.Bush, E., J. Fielitz, L. Melvin, M. Martinez-Arnold, T. A. McKinsey, R. Plichta, and E. N. Olson. 2004. A small molecular activator of cardiac hypertrophy uncovered in a chemical screen for modifiers of the calcineurin signaling pathway. Proc. Natl. Acad. Sci. USA 101:2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, D., Z. Wang, C. L. Zhang, J. Oh, W. Xing, S. Li, J. A. Richardson, D. Z. Wang, and E. N. Olson. 2005. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell. Biol. 25:364-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carling, T., K. C. Kim, X. H. Yang, J. Gu, X. K. Zhang, and S. Huang. 2004. A histone methyltransferase is required for maximal response to female sex hormones. Mol. Cell. Biol. 24:7032-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cremona, O., M. Muda, R. D. Appel, S. Frutiger, G. J. Hughes, D. F. Hochstrasser, A. Geinoz, and G. Gabbiani. 1995. Differential protein expression in aortic smooth muscle cells cultured from newborn and aged rats. Exp. Cell Res. 217:280-287. [DOI] [PubMed] [Google Scholar]
- 6.Czermin, B., G. Schotta, B. B. Hulsmann, A. Brehm, P. B. Becker, G. Reuter, and A. Imhof. 2001. Physical and functional association of SU(VAR)3-9 and HDAC1 in Drosophila. EMBO Rep. 2:915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, Q., and S. Huang. 2004. PRDM5 is silenced in human cancers and has growth suppressive activities. Oncogene 23:4903-4910. [DOI] [PubMed] [Google Scholar]
- 8.Dreux, A. C., D. J. Lamb, H. Modjtahedi, and G. A. Ferns. 30 July 2005, posting date. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed]
- 9.Du, Y., T. Carling, W. Fang, Z. Piao, J. C. Sheu, and S. Huang. 2001. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res. 61:8094-8099. [PubMed] [Google Scholar]
- 10.Farber, H. W., and J. Loscalzo. 2004. Pulmonary arterial hypertension. N. Engl. J. Med. 351:1655-1665. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb, P. D., S. A. Pierce, R. J. Sims, H. Yamagishi, E. K. Weihe, J. V. Harriss, S. D. Maika, W. A. Kuziel, H. L. King, E. N. Olson, O. Nakagawa, and D. Srivastava. 2002. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat. Genet. 31:25-32. [DOI] [PubMed] [Google Scholar]
- 12.Gyory, I., G. Fejer, N. Ghosh, E. Seto, and K. L. Wright. 2003. Identification of a functionally impaired positive regulatory domain I binding factor 1 transcription repressor in myeloma cell lines. J. Immunol. 170:3125-3133. [DOI] [PubMed] [Google Scholar]
- 13.Gyory, I., J. Wu, G. Fejer, E. Seto, and K. L. Wright. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5:299-308. [DOI] [PubMed] [Google Scholar]
- 14.Hiruma, T., Y. Nakajima, and H. Nakamura. 2002. Development of pharyngeal arch arteries in early mouse embryo. J. Anat. 201:15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, S. 1999. The retinoblastoma protein-interacting zinc finger gene RIZ in 1p36-linked cancers. Front. Biosci. 4:D528-D532. [DOI] [PubMed] [Google Scholar]
- 16.Huang, S. 2002. Histone methyltransferases, diet nutrients and tumour suppressors. Nat. Rev. Cancer 2:469-476. [DOI] [PubMed] [Google Scholar]
- 17.Huang, S., G. Shao, and L. Liu. 1998. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J. Biol. Chem. 273:15933-15939. [DOI] [PubMed] [Google Scholar]
- 18.Jenuwein, T. 2001. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol. 11:266-273. [DOI] [PubMed] [Google Scholar]
- 19.Kato, M., T. Inazu, Y. Kawai, K. Masamura, M. Yoshida, N. Tanaka, K. Miyamoto, and I. Miyamori. 2003. Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, A7r5. Biochem. Biophys. Res. Commun. 301:1109-1115. [DOI] [PubMed] [Google Scholar]
- 20.Keller, A. D., and T. Maniatis. 1992. Only two of the five zinc fingers of the eukaryotic transcriptional repressor PRDI-BF1 are required for sequence-specific DNA binding. Mol. Cell. Biol. 12:1940-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, K. C., L. Geng, and S. Huang. 2003. Inactivation of a histone methyltransferase by mutations in human cancers. Cancer Res. 63:7619-7623. [PubMed] [Google Scholar]
- 22.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Z. P., Z. Wang, H. Yanagisawa, and E. N. Olson. 2005. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev. Cell 9:261-270. [DOI] [PubMed] [Google Scholar]
- 24.Manabe, I., and G. K. Owens. 2001. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J. Clin. Investig. 107:823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manabe, I., and G. K. Owens. 2001. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ. Res. 88:1127-1134. [DOI] [PubMed] [Google Scholar]
- 26.Mao, C., O. T. Malek, M. E. Pueyo, P. G. Steg, and F. Soubrier. 2000. Differential expression of rat frizzled-related frzb-1 and frizzled receptor fz1 and fz2 genes in the rat aorta after balloon injury. Arterioscler. Thromb. Vasc. Biol. 20:43-51. [DOI] [PubMed] [Google Scholar]
- 27.McKinsey, T. A., C. L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408:106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miano, J. M. 2002. Mammalian smooth muscle differentiation: origins, markers and transcriptional control. Results Probl. Cell Differ. 38:39-59. [DOI] [PubMed] [Google Scholar]
- 29.Miano, J. M. 2003. Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 35:577-593. [DOI] [PubMed] [Google Scholar]
- 30.Mochizuki, N., S. Shimizu, T. Nagasawa, H. Tanaka, M. Taniwaki, J. Yokota, and K. Morishita. 2000. A novel gene, MEL1, mapped to 1p36.3 is highly homologous to the MDS1/EVI1 gene and is transcriptionally activated in t(1;3)(p36;q21)-positive leukemia cells. Blood 96:3209-3214. [PubMed] [Google Scholar]
- 31.Molkentin, J. D. 2000. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 275:38949-38952. [DOI] [PubMed] [Google Scholar]
- 32.Neuville, P., A. Geinoz, G. Benzonana, M. Redard, F. Gabbiani, P. Ropraz, and G. Gabbiani. 1997. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am. J. Pathol. 150:509-521. [PMC free article] [PubMed] [Google Scholar]
- 33.Oh, J., Z. Wang, D. Z. Wang, C. L. Lien, W. Xing, and E. N. Olson. 2004. Target gene-specific modulation of myocardin activity by GATA transcription factors. Mol. Cell. Biol. 24:8519-8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens, G. K. 1995. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75:487-517. [DOI] [PubMed] [Google Scholar]
- 35.Owens, G. K., M. S. Kumar, and B. R. Wamhoff. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84:767-801. [DOI] [PubMed] [Google Scholar]
- 36.Pasterkamp, G., D. P. de Kleijn, and C. Borst. 2000. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc. Res. 45:843-852. [DOI] [PubMed] [Google Scholar]
- 37.Perlman, H., E. Suzuki, M. Simonson, R. C. Smith, and K. Walsh. 1998. GATA-6 induces p21(Cip1) expression and G1 cell cycle arrest. J. Biol. Chem. 273:13713-13718. [DOI] [PubMed] [Google Scholar]
- 38.Piepkorn, M., M. R. Pittelkow, and P. W. Cook. 1998. Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J. Investig. Dermatol. 111:715-721. [DOI] [PubMed] [Google Scholar]
- 39.Piskurich, J. F., K. I. Lin, Y. Lin, Y. Wang, J. P. Ting, and K. Calame. 2000. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat. Immunol. 1:526-532. [DOI] [PubMed] [Google Scholar]
- 40.Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun, M. Schmid, S. Opravil, K. Mechtler, C. P. Ponting, C. D. Allis, and T. Jenuwein. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593-599. [DOI] [PubMed] [Google Scholar]
- 41.Roy, S., and T. Ng. 2004. Blimp-1 specifies neural crest and sensory neuron progenitors in the zebrafish embryo. Curr. Biol. 14:1772-1777. [DOI] [PubMed] [Google Scholar]
- 42.Shaffer, A. L., K. I. Lin, T. C. Kuo, X. Yu, E. M. Hurt, A. Rosenwald, J. M. Giltnane, L. Yang, H. Zhao, K. Calame, and L. M. Staudt. 2002. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17:51-62. [DOI] [PubMed] [Google Scholar]
- 43.Shelton, J. M., M. H. Lee, J. A. Richardson, and S. B. Patel. 2000. Microsomal triglyceride transfer protein expression during mouse development. J. Lipid Res. 41:532-537. [PubMed] [Google Scholar]
- 44.Tachibana, M., K. Sugimoto, T. Fukushima, and Y. Shinkai. 2001. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276:25309-25317. [DOI] [PubMed] [Google Scholar]
- 45.Trion, A., and A. van der Laarse. 2004. Vascular smooth muscle cells and calcification in atherosclerosis. Am. Heart J. 147:808-814. [DOI] [PubMed] [Google Scholar]
- 46.Vandel, L., and D. Trouche. 2001. Physical association between the histone acetyl transferase CBP and a histone methyl transferase. EMBO Rep. 2:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, K., and A. Takahashi. 2001. Transcriptional regulation of vascular smooth muscle cell phenotype. Z. Kardiol. Suppl. 2:12-16. [DOI] [PubMed] [Google Scholar]
- 48.Wang, D. Z., and E. N. Olson. 2004. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr. Opin. Genet. Dev. 14:558-566. [DOI] [PubMed] [Google Scholar]
- 49.Wang, H., R. Cao, L. Xia, H. Erdjument-Bromage, C. Borchers, P. Tempst, and Y. Zhang. 2001. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol. Cell 8:1207-1217. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z., W. Shu, M. M. Lu, and E. E. Morrisey. 2005. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol. Cell. Biol. 25:5022-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss, S. W. 2002. Smooth muscle tumors of soft tissue. Adv. Anat. Pathol. 9:351-359. [DOI] [PubMed] [Google Scholar]
- 52.Woolf, A. S., and C. M. Cale. 1997. Roles of growth factors in renal development. Curr. Opin. Nephrol. Hypertens. 6:10-14. [DOI] [PubMed] [Google Scholar]
- 53.Xie, M., G. Shao, I. M. Buyse, and S. Huang. 1997. Transcriptional repression mediated by the PR domain zinc finger gene RIZ. J. Biol. Chem. 272:26360-26366. [DOI] [PubMed] [Google Scholar]
- 54.Yamanaka, Y., K. Hayashi, T. Komurasaki, S. Morimoto, T. Ogihara, and K. Sobue. 2001. EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem. Biophys. Res. Commun. 281:373-377. [DOI] [PubMed] [Google Scholar]
- 55.Yu, J., C. Angelin-Duclos, J. Greenwood, J. Liao, and K. Calame. 2000. Transcriptional repression by Blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol. Cell. Biol. 20:2592-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, C. L., T. A. McKinsey, and E. N. Olson. 2002. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 22:7302-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]