Abstract
C/EBPα arrests proliferation of young livers by inhibition of cdk2. In old mice, C/EBPα inhibits growth by repression of E2F-dependent promoters through the C/EBPα-Brm complex. In this paper, we show that cyclin D3-cdk4/cdk6 supports the ability of C/EBPα to inhibit liver proliferation in both age groups. Although cyclin D3-cdk4/cdk6 kinases are involved in the promotion of growth, they are expressed in terminally differentiated cells, suggesting that they have additional functions in these settings. We demonstrate that C/EBPα represents a target for phosphorylation by cyclin D3-cdk4/cdk6 complexes in differentiated liver cells and in differentiated adipocytes. Cyclin D3-cdk4/cdk6 specifically phosphorylate C/EBPα at Ser193 in vitro and in the liver and support growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. We found that cyclin D3 is increased in old livers and activates cdk4/cdk6, resulting in stabilization of the C/EBPα-Brm complex. Old livers fail to reduce the activity of cyclin D3-cdk4/cdk6 after partial hepatectomy, leading to high levels of C/EBPα-Brm complexes after partial hepatectomy, which correlate with weak proliferation. We examined the role of cyclin D3 in the stabilization of C/EBPα-cdk2 and C/EBPα-Brm by using 3T3-L1 differentiated cells. In these cells, cyclin D3 is increased during differentiation and phosphorylates C/EBPα at Ser193, leading to the formation of growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. The inhibition of cyclin D3 blocks the formation of these complexes. Thus, these studies provide a new function of cyclin D3, which is to support the growth-inhibitory activity of C/EBPα.
Quiescence and differentiation of tissues are supported by the Rb family proteins and by certain tissue-specific proteins. C/EBPα is a liver- and adipose-specific protein which maintains quiescence of these tissues (7, 19) and regulates transcription of liver- and adipose-specific genes. Transcriptional and growth-inhibitory activities of C/EBPα are mediated by different mechanisms. While activation of C/EBPα-dependent promoters requires an intact DNA binding domain, the inhibition of cell proliferation is mediated by a direct interaction of C/EBPα with proteins which regulate cell cycle division (9, 11, 13, 14, 19, 22). Liver is a unique tissue which is able to regenerate itself after surgical resections. After partial hepatectomy (PH), the initiation of proliferation requires an orchestrated cascade of alterations of biochemical pathways (6). These alterations result in activation of cell cycle genes and in the neutralization of a negative control of liver proliferation. A key event in the activation of cell cycle genes after PH is the induction of D-type cyclins, which include three members, D1, D2, and D3. Although these cyclins have high levels of homology and might replace each other in knockout mouse models, they seem to play distinct functions in cell cycle progression and differentiation. Cyclin D1 is not detectable in quiescent liver, but it is induced after PH and increases liver proliferation. Moreover, overexpression of cyclin D1 alone is sufficient to initiate proliferation in young livers (12). On the contrary, cyclin D3 is expressed in a number of quiescent tissues (including liver) and perhaps displays functions that support differentiation status of the tissues (16). Protein levels of cyclin D3 are increased during differentiation of myocytes and are involved in the regulation of the MyoD-mediated program of gene expression (2). Moreover, Reichert and Eick showed that cyclin D3 is increased during differentiation of 3T3-L1 adipocytes (15). However, biological functions of cyclin D3 in these differentiated cells and downstream targets of cyclin D3 have not been described.
A second important event in liver proliferation after PH is the neutralization of the growth-inhibitory control in the liver. Rb family and C/EBP family proteins are expressed at high levels in the liver, maintain liver quiescence, and prevent development of tumors (9, 12, 22). Although the roles of Rb and C/EBPα in the inhibition of liver proliferation have been established, the precise mechanisms by which livers regulate their growth-inhibitory activities are not well understood. Recent studies revealed that the activities of both proteins are regulated by phosphorylation of unique residues of these proteins. The growth-inhibitory activity of C/EBPα is significantly enhanced by phosphorylation of Ser193, while the removal of the phosphate from Ser193 eliminates the ability of C/EBPα to cause growth arrest (22, 23). In liver tumors, a PP2A phosphatase accumulates in nuclei and removes a phosphate from Ser193, leading to release of growth-inhibitory activity of C/EBPα (22, 23). We have recently found that partial hepatectomy in young livers also neutralizes growth-inhibitory activity of C/EBPα through activation of the phosphatidyl inositol 3-kinase (PI3K)-Akt pathway, which increases the nuclear concentration of PP2A (22, 23). C/EBPα inhibits proliferation of young livers by interaction with and inhibition of cdk2. Aging changes this pathway to the repression of E2F transcription via an increase in an age-specific C/EBPα-Rb-E2F4-Brm complex (further also called C/EBPα-Brm), which blocks expression of S-phase genes (9). Since this complex represses E2F transcription, the appearance of this complex contributes to the reduced proliferative capacities of old livers. This model is supported by recent work from Rando's group which showed that cross-circulation of young and old mice in parabiotic experiments resulted in decreased C/EBPα-Brm complex formation and increased hepatocyte proliferation in the livers of old mice (3).
In this paper, we show that cyclin D3 regulates the growth-inhibitory activity of C/EBPα in young and old livers and in differentiated 3T3-L1 adipocytes. Cyclin-D3 cdk4/cdk6 specifically phosphorylate C/EBPα at Ser193 in vitro and in vivo. Experiments with the inhibition of cyclin D3 by small interfering RNA (siRNA) showed that cyclin D3-dependent phosphorylation of C/EBPα is required for the formation of growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. We have found that cyclin D3 displays this new activity in two biological processes: during differentiation of 3T3-L1 adipocytes and in maintenance of the quiescent state of young and old livers. We also obtained evidence that aging liver activates cdk4/cdk6 via elevation of cyclin D3, leading to the hyperphosphorylation of C/EBPα at Ser193 and to stabilization of the C/EBPα-Brm complex.
MATERIALS AND METHODS
Antibodies.
Antibodies (Abs) to cyclin D1, D2, and D3, C/EBPα (14AA and N19), C/EBPβ (C-19), cdk6, cdk4 (C-22), cdk2 (M2), and Brm and Rb (C-15) were purchased from Santa Cruz Biotechnology. Antibodies to peroxisome proliferator-activated receptor γ (PPARγ) were purchased from Upstate Biotechnology. Antibodies to PP2A were from Signal Transduction Laboratories. Monoclonal Abs to β-actin were from Sigma. To examine expression of the C/EBPα-Ser193-ph isoform, we generated antibodies which specifically recognize C/EBPα phosphorylated at Ser193. The specificity of these antibodies was examined by Western blotting with wild-type (WT) C/EBPα and with a C/EBPα-Ser193A mutant. These antibodies interact with WT C/EBPα phosphorylated at Ser193 and do not react with the C/EBPα-S193A mutant (see Fig. 1A, below). These Abs specifically recognize C/EBPα phosphorylated in 3T3-L1 cells. Examination of phosphorylated isoforms of C/EBPα in livers was performed using two approaches: immunoprecipitation and Western blotting (IP-Western) and two-dimensional (2D)-Western. The direct Western blotting with ph-Ser193 antibodies does not work with liver nuclear extracts, since these extracts contain immunoreactive bands close to the position of C/EBPα which interact with Ser193-ph antibodies. Therefore, C/EBPα was first immunoprecipitated from liver nuclear extracts with antibodies to total protein and then examined by Western blotting with antibodies to ph-Ser193. To confirm data of the IP-Western with Ser193-ph Abs, we also used a 2D-Western assay as described in our previous papers (22, 23).
FIG. 1.
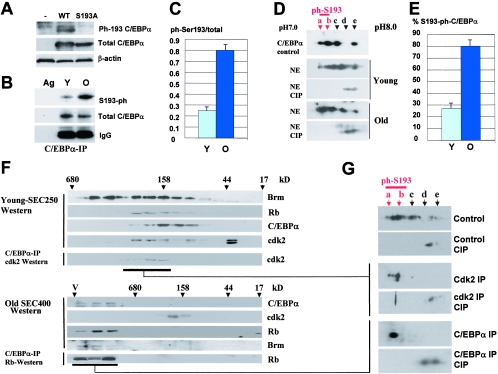
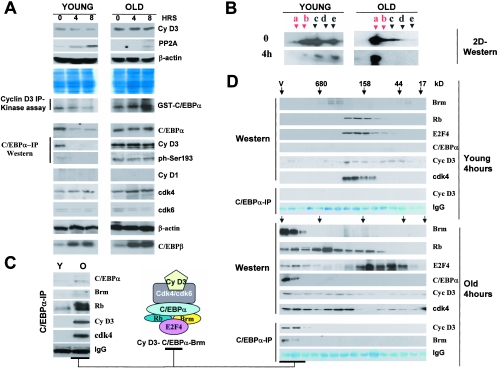
Ser193-phosphorylated isoform of C/EBPα is abundant in C/EBPα-cdk2 and C/EBPα-Brm complexes. A. Characterization of antibodies to the phosphorylated Ser193-C/EBPα isoform. HEK293 cells were transfected with WT and a C/EBPα-S193A mutant, and nuclear extracts were examined by Western blotting with antibodies to ph-Ser193 of C/EBPα and with Abs to total C/EBPα. The filter was reprobed with antibodies to β-actin. B. Western blotting of C/EBPα immunoprecipitates with antibodies to Ser193-ph. C/EBPα was immunoprecipitated from nuclear extracts of young and old livers and examined by Western blotting with Ser193-ph antibodies. The membrane was reprobed with antibodies to total C/EBPα. Immunoglobulin G detected on the same filter showed an equal addition of antibodies to total C/EBPα. C. Phosphorylation of C/EBPα at Ser193 is increased in old livers. Bar graphs show a summary of three experiments with the IP-Western approach. A ratio of signals of ph-Ser193 to total C/EBPα was determined by densitometry. D and E. Examination of C/EBPα isoforms in young and old livers by 2D-Western. The upper image shows a 2D analysis of a control sample of C/EBPα which was overexpressed in cultured cells. Untreated and CIP-treated nuclear proteins were separated by 2D electrophoresis and examined by Western blotting with antibodies to C/EBPα. The percentage of Ser193 isoforms was calculated by densitometry as a ratio to the total amount of C/EBPα isoforms (E). F. Isolation of C/EBPα-cdk2 and C/EBPα-Brm complexes for examination of phosphorylation of C/EBPα. Upper image: nuclear extracts from young livers were fractionated by size exclusion chromatography on an SEC250 column. Identical fractions of five HPLC runs were combined, and positions of C/EBPα, Rb, Brm, and cdk2 within fractions were determined by Western blotting. The location of the C/EBPα-cdk2 complex was determined by co-IP (C/EBPα IP, cdk2 Western). Bottom image: localization of the C/EBPα-Brm complex within fractions of the size exclusion chromatography column with nuclear extracts from old livers. Identical fractions of five runs of old livers on the SEC400 column were combined and examined by Western blotting with antibodies to Brm, cdk2, C/EBPα, and Rb. C/EBPα was immunoprecipitated from each fraction, and Rb was detected in these IPs (C/EBPα-IP, Rb-Western; bottom image). G. 2D analysis of C/EBPα within cdk2 complexes and within the age-specific C/EBPα-Brm complex. C/EBPα complexes were immunoprecipitated from fractions containing the complexes and examined by 2D gel electrophoresis. One half of each IP was treated with alkaline phosphatase (CIP) and examined in a parallel run. Positions of Ser193-phosphorylated isoforms are shown by red arrows.
Animals and liver regeneration.
In these studies, we used mice ages 3 to 4 months (young) and 22 to 24 months (old). Experiments with quiescent livers were performed with eight animals of each age group. Data in the paper summarize multiple experiments. Liver regeneration and examination of protein expression were performed as described in our previous paper (9). In this paper, we examined expression of proteins at earlier time points after PH, which included 0, 4, and 8 h after surgery.
Protein isolation and Western blotting.
Nuclear extracts were isolated as described in previous papers (9, 24, 25). Proteins (50 to 100 μg) were loaded on gradient (4-to-20% or 8-to-16%) polyacrylamide gels (PAAG), transferred on the membrane, and probed with antibodies to C/EBPα, C/EBPβ, PPARγ, cdk2, cdk4, cyclins D1 and D3, Rb, E2F4, and Brm. To verify protein loading, each filter was reprobed with β-actin and then stained with Coomassie.
Gel filtration analysis of protein-protein complexes in mouse liver.
The detailed procedure for the analysis of protein-protein complexes is described in our previous papers (9, 22). Nuclear extracts were isolated from young and old livers as described elsewhere (20) and fractionated by size exclusion columns SEC-250 or SEC-400 (high-performance liquid chromatography [HPLC]; BioLogic HR, Bio-Rad) with monitoring of optical density profiles. Gel filtration fractions were loaded on denaturing gradient (4-to-20%) PAAG, blotted onto membranes, and probed with antibodies to cdk2, C/EBPα, Rb, Brm, and E2F4 or with antibodies to cdk4, cdk6, and cyclin D1, D2, and D3. For examination of phosphorylation status of C/EBPα within C/EBPα-cdk2 (young livers) and C/EBPα-Brm (old livers) complexes, five HPLC runs were performed for each age group and combined for further studies. The locations of C/EBPα-cdk2 and C/EBPα-Brm complexes were determined by coimmunoprecipitation (co-IP) as shown in Fig. 1, below. The complexes were precipitated from corresponding fractions using specific Abs. One half of each precipitate was treated with alkaline phosphatase (CIP). Untreated and CIP-treated IPs were separated by 2D gel electrophoresis and examined by Western blotting with antibodies to C/EBPα.
2D gel Western blotting.
2D gel analysis was performed as described in our previous papers (22, 23). Briefly, nuclear lysates from young and old livers were separated by 2D gel electrophoresis (Protean IEF; Bio-Rad), transferred on the membrane, and probed with antibodies to C/EBPα.
Coimmunoprecipitation.
C/EBPα was immunoprecipitated from nuclear extracts with polyclonal antibodies (14AA; Santa Cruz), and the presence of Rb, Brm, E2F4, cyclin D3, cdk4, and cdk2 in C/EBPα IPs was examined by Western blotting with monoclonal antibodies to the mentioned proteins.
Kinase assay.
The conditions for in vitro kinase assays are described in our previous publications (24, 25). Briefly, baculovirus-expressed purified cdk4-cyclin D1 and cdk6-cyclin D3 complexes or cdk4/cdk6/cyclin D3 IPs were incubated with glutathione S-transferase (GST)-Rb or GST-C/EBPα substrates in the kinase reaction mixtures with [γ-32P]ATP. After 30-min incubations, kinase reaction mixtures were loaded on gradient 8-to-16% PAAG, transferred on a nitrocellulose filter, and exposed with X-ray film. After exposure, the filter was stained with Coomassie blue to verify the loading of the substrates.
Differentiation of 3T3-L1 adipocytes was initiated as described in a previous paper (21). Nuclear extracts were isolated at 0, 1, 3, 5, and 7 days after initiation and analyzed by Western blotting with antibodies to cyclin D3, cdk4, and cdk6 as described above. The proper differentiation was confirmed by examining expression of markers of differentiation, PPARγ, C/EBPβ, and C/EBPα. In experiments involving the inhibition of cyclin D3 expression, 3T3-L1 cells were transfected with siRNA to cyclin D3 (DHARMACON RNA Tech) or with a control siRNA with random sequence a day before initiation of differentiation. We have found that this timing gives a maximum inhibition of cyclin D3 for the whole course of differentiation. Data in the manuscript present a summary of three independent experiments.
RESULTS
Phosphorylation of C/EBPα at Ser193 is increased in old livers.
C/EBPα inhibits proliferation of young and old livers through different mechanisms. In young livers, C/EBPα interacts with and inhibits cdk2 (24), while in old livers C/EBPα represses E2F transcription via the C/EBPα-Brm complex (3, 9). A recent work by Rando's group demonstrated that the disruption of the C/EBPα-Brm complex in a young environment does not change protein levels of Brm and C/EBPα, suggesting that posttranslational modifications are involved in the formation of the complex (3). Consistent with this suggestion, our previous investigations in cultured cells showed that phosphorylation of C/EBPα at Ser193 is required for the interaction of C/EBPα with cdk2 and with Brm (22). Therefore, we examined if phosphorylation of C/EBPα is required for the formation of the C/EBPα-cdk2 (young animals) and the C/EBPα-Brm (old animals) complexes in the liver. We initially determined the phosphorylation status of C/EBPα in old and young livers. Two approaches were used for these studies. We generated antibodies which specifically recognize C/EBPα phosphorylated at Ser193, as described in Materials and Methods. Figure 1A shows the specificity of these antibodies as determined by Western blotting with WT C/EBPα and with a C/EBPα-Ser193A mutant. These studies showed that the Ser193-ph antibodies interact with WT C/EBPα phosphorylated at Ser193 and do not react with the C/EBPα-S193A mutant. Examination of these antibodies with nuclear extracts containing endogenous C/EBPα showed that these Abs recognize a Ser193-ph isoform of C/EBPα in 3T3-L1 cells (see Fig. 5, below). In the liver, however, these Abs interact with several cross-reactive proteins located close to the position of C/EBPα, creating difficulties in interpreting direct Western blot assays. To solve this problem, we first immunoprecipitated C/EBPα from nuclear extracts of young and old livers with antibodies to total C/EBPα and then performed Western blotting with antibodies to Ser193-ph. Figure 1B shows that the amount of the ph-Ser193 isoform of C/EBPα is increased in old livers. After reprobing the filter with antibodies to total C/EBPα, calculation of the Ser193-ph isoform to total protein showed an approximately 2- to 2.5-fold induction of this isoform in old livers (Fig. 1C).
FIG. 5.
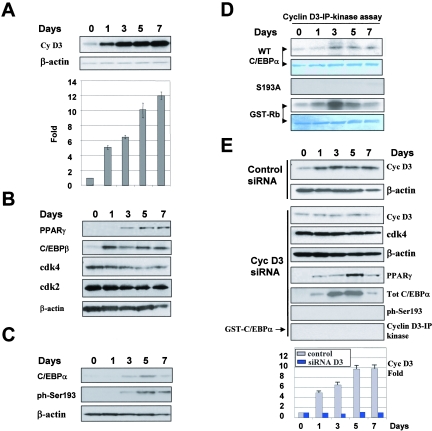
Induction of cyclin D3 in differentiated 3T3-L1 cells is required for the phosphorylation of C/EBPα at Ser193. A. Protein levels of cyclin D3 are increased in 3T3-L1 after induction of differentiation. Nuclear extracts were isolated from 3T3-L1 cells at different days after initiation of differentiation (shown on the top) and examined by Western blotting with antibodies to the proteins shown on the left. Protein levels of cyclin D3 were calculated as ratios to β-actin and are shown in the bar graph. B. Expression of markers of 3T3-L1 differentiation, PPARγ and C/EBPβ, and cyclin-dependent kinases. Western blotting was performed with nuclear extracts isolated from 3T3-L1 differentiated cells as described in Materials and Methods. C. Phosphorylation of C/EBPα during differentiation of 3T3-L1 cells. Nuclear extracts from differentiated 3T3-L1 cells were examined by Western blotting with antibodies to total C/EBPα and to the ph-S193 isoform of C/EBPα. D. Examination of activities of kinases associated with cyclin D3. Cyclin D3 was immunoprecipitated from nuclear extracts and added into an in vitro kinase assay mixture with GST-C/EBPα and GST-Rb substrates. The membranes were stained with Coomassie to visualize substrates. E. Inhibition of cyclin D3 expression by siRNA blocks phosphorylation of C/EBPα at Ser193. Control siRNA lanes: expression of cyclin D3 in 3T3-L1 cells which were treated with control (random sequence) siRNA. The filter was reprobed with β-actin to verify protein loading. Cyclin D3 siRNA lanes: siRNA to cyclin D3 was transfected into 3T3-L1 cells 1 day before initiation of differentiation. Proteins extracts were isolated from cyclin D3 siRNA-treated 3T3-Ll cells at different days and examined by Western blotting with antibodies to cyclin D3, cdk4, PPARγ, total C/EBPα, and the Ser193-ph isoform of C/EBPα. Each membrane was reprobed with Abs to β-actin. The bar graph (bottom) presents levels of cyclin D3 in control 3T3-L1 cells (treated with control siRNA) and in 3T3-L1 cells treated with siRNA to cyclin D3 and is a summary of three independent experiments. Cyclin D3 levels were calculated as ratios to β-actin and then as ratios to levels at day 0. Cyclin D3-IP kinase lanes: examination of the cdk4/cdk6 activities in cyclin D3 IPs using GST-C/EBPα as substrate.
Western blotting with ph-Ser193-specific Abs showed the relative induction of Ser193 phosphorylation in old livers but could not be used for the calculations of the portion of C/EBPα phosphorylated on Ser193, since antibodies to total C/EBPα and to the Ser193-ph isoform have different titers. To examine a portion of C/EBPα phosphorylated on Ser193, we applied the 2D-Western blotting approach. Untreated nuclear lysates and CIP-treated proteins were separated by 2D gel electrophoresis and probed with antibodies to C/EBPα. We have previously shown that 2D analysis detected five major isoforms of C/EBPα in the liver (a, b, c, d, and e) and that isoforms a and b are not detectable with the Ser193A mutant of C/EBPα (22, 23). As can be seen in Fig. 1D, Ser193-phosphorylated isoforms of C/EBPα (a and b) are observed in both young and old livers, and the amounts of these isoforms are higher in old livers. The treatment of nuclear extracts with CIP shifted isoforms a, b, and c to the alkali region. Densitometric calculations revealed that around 75 to 80% of the C/EBPα is hyperphosphorylated at Ser193 in old livers, while in young livers, Ser193-ph isoforms represent 35 to 40% of the total amount of C/EBPα (Fig. 1E). These data are consistent with results obtained by the IP-Western approach with ph-Ser193-specific Abs. Taking into account the role of Ser193 in the interactions with cdk2 and Brm (22), we suggested that the phosphorylation of C/EBPα might be involved in the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes in the liver.
The Ser193-phosphorylated isoform of C/EBPα is a major isoform within growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes in the liver.
To examine the phosphorylation status of C/EBPα within the C/EBPα-cdk2 and C/EBPα-Brm complexes, we isolated C/EBPα from these complexes using an HPLC-based size exclusion-IP procedure as described in our previous papers (9, 22). cdk2-C/EBPα and Brm-C/EBPα complexes were enriched by size exclusion chromatography of proteins from young and old livers, using SEC-250 and SEC-400 columns, respectively. Examination of C/EBPα, cdk2, Brm, and Rb in these size exclusion fractions showed that C/EBPα colocalizes with cdk2 in fractions of young livers but does not colocalize with cdk2 in fractions of proteins from old livers. Consistent with previous findings, C/EBPα colocalizes with Rb and Brm in high-molecular-weight (MW) fractions of the samples from old livers (9). The location of C/EBPα-cdk2 and C/EBPα-Brm complexes in corresponding fractions was confirmed by Western blotting and by co-IP as shown in Fig. 1F. The complexes were isolated from corresponding fractions by immunoprecipitation with antibodies to cdk2 (young livers) and to C/EBPα (old livers), and C/EBPα isoforms were examined by 2D-Western analysis as described in previous studies (9, 22). Half of each IP was treated with an excess of CIP. As can be seen in Fig. 1G, although C/EBPα is observed in livers as five isoforms, only the Ser193-ph isoform of C/EBPα is detected in the complexes with cdk2 or in the C/EBPα-Brm complex. The presence of the Ser193-ph isoform in the complexes strongly suggested that the phosphorylation of Ser193 of C/EBPα is an important event in the formation of the growth-inhibitory C/EBPα complexes in the liver.
cdk4 and cdk6 phosphorylate C/EBPα at Ser193 in vitro and in liver.
We next searched for a liver-specific kinase that might phosphorylate C/EBPα in young and old livers. Given the interactions of C/EBPα with cdk4 and cdk2 (22, 24, 25), we examined activities of cdk2, cdk4, and cdk6 in young and old livers. These kinases were immunoprecipitated from liver nuclear extracts, and their activities were measured in an in vitro kinase assay using as substrates histone H1 (for cdk2) and Rb and C/EBPα (for cdk4 and cdk6). cdk2 kinase activity was undetectable in both young and old livers (data not shown), but the activities of cyclin D3, cdk4, and cdk6 were found in young and old livers. Figure 2A shows a typical result of these studies. In this experiment, cyclin D3, cdk4, and cdk6 were immunoprecipitated from liver nuclear extracts of young and old livers, and their abilities to phosphorylate C/EBPα were examined in an in vitro kinase assay. As one can see, these IPs phosphorylate C/EBPα. This phosphorylation is specific, since a mock agarose control showed no phosphorylation of C/EBPα. A comparison of the kinase activities of cyclinD3-cdk4/cdk6 reproducibly demonstrated the induction of cdk4 and cdk6 activities in old livers. This induction correlates with a high level of Ser193-ph isoform in old livers (Fig. 1). Taken together, these data show that cyclin D3-cdk4 and cyclin D3-cdk6 are active in livers and that these kinases phosphorylate C/EBPα.
FIG. 2.
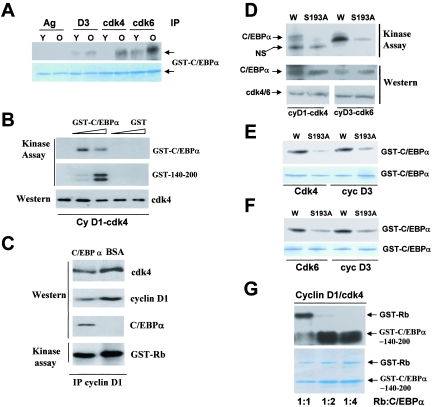
cdk4 and cdk6 phosphorylate Ser193 of C/EBPα in vitro and in vivo. A. Cyclin D3-cdk4 and cyclin D3-cdk6 phosphorylate C/EBPα in liver. cdk4, cdk6 and cyclin D3 were immunoprecipitated from young and old livers and examined in a kinase assay with GST-C/EBPα substrate. Ag, agarose control for absorption. The bottom part shows a Coomassie stain of the filter to verify loading of the GST-C/EBPα substrate. B. Baculovirus-expressed, purified cdk4-cyclin D1 phosphorylates C/EBPα. Full-length GST-C/EBPα or GST-140-200 (growth-inhibitory region of C/EBPα) was incubated with the cyclin D1-cdk4 complex in a kinase assay mixture containing [γ-32P]ATP. Proteins were transferred on the membrane and exposed. The bottom image shows Western blotting of the same membrane with antibodies to cdk4. C. C/EBPα forms a stable complex with cyclin D1-cdk4 in the kinase reactions. C/EBPα was incubated with cdk4-cyclin D1 in a kinase reaction, cyclin D1 was immunoprecipitated, and cdk4 and C/EBPα in IPs were examined by Western blotting. A control experiment was performed with bovine serum albumin (BSA) instead of C/EBPα. An aliquot from each reaction mixture was incubated with the GST-Rb substrate in the presence of [γ-32P]ATP (bottom part). D. Baculovirus-expressed, purified cdk4-cyclin D1 and cdk6-cyclin D3 phosphorylate Ser193 of C/EBPα. GST-WT-C/EBPα (W) and a C/EBPα-S193A mutant (S193) were incubated with cdk4-cyclin D1 and with cdk6-cyclin D3 in a kinase assay. Positions of C/EBPα and a nonspecific band (NS for cdk4-cyclin D1) are shown. The filter was reprobed with Abs to C/EBPα, cdk4, and cdk6 (Western). E. cdk4-cyclin D3 phosphorylates Ser193 of C/EBPα. WT and S193A mutant GST-C/EBPα were incubated with cdk4 and cyclin D3 IPs from old livers. The bottom image shows GST-C/EBPα stained with Coomassie. F. cdk6-cyclin D3 phosphorylates Ser193 of C/EBPα. WT and S193A mutant GST C/EBPα were incubated with cdk6 and cyclin D3 IPs from old livers. The bottom image shows GST-C/EBPα stained with Coomassie. G. Examination of efficiency of phosphorylation of C/EBPα by cdk4 relative to phosphorylation of Rb. GST-Rb (0.5 μg) and increasing amounts (0.5, 1, and 2 μg) of the growth-inhibitory region of C/EBPα (GST-C/EBPα-140-200) were added into kinase reaction mixtures with purified cyclin D1-cdk4 and [γ-32P]ATP. The reaction mixtures were loaded on a denaturing PAAG, transferred on membrane, and exposed to X rays. The bottom part shows a Coomassie stain of the gel loaded with the corresponding ratios of the proteins.
We next performed a detailed characterization of these activities using baculovirus-expressed, purified cdk4-cyclin D1 with the C/EBPα as substrate in an in vitro kinase assay. GST-C/EBPα was added into the kinase reaction mixtures in excess to cyclin D1-cdk4 (1 μg/reaction mixture). A typical picture of these experiments is shown in Fig. 2B. As can be seen, GST-C/EBPα is phosphorylated by cyclin D1-cdk4. Examination of a short growth-inhibitory region of C/EBPα (amino acids [aa] 140 to 200) as a substrate for cdk4 showed that cdk4 also phosphorylates the growth-inhibitory region of C/EBPα. The region aa 140 to 200 contains only one Ser (aa 193) and two tyrosines. Since cdk4 phosphorylates Ser and Thr residues, these data suggested that cdk4 phosphorylates C/EBPα at Ser193. We next examined if cyclin D1 and cdk4 are observed in the complex with C/EBPα in the kinase reaction mixtures. IP of cyclin D1 from the kinase reactions, following Western blotting with antibodies to cdk4, cyclin D1, and C/EBPα, revealed that C/EBPα is in the complex with cdk4 and cyclin D1 (Fig. 2C). A parallel examination of the cdk4 activity in aliquots from these mixtures using Rb as substrate showed that the kinase activity of cdk4 is only slightly reduced in mixtures with C/EBPα, perhaps due to competition for cdk4 with the GST-Rb substrate (see below).
We next determined an amino acid residue of C/EBPα which is phosphorylated by cdk4 and cdk6. WT C/EBPα and the mutant C/EBPα-Ser193A (23) were incubated with baculovirus-expressed, purified cyclin D1-cdk4 and cyclin D3-cdk6 in the presence of [γ-32P]ATP. As seen in Fig. 2D, both kinases phosphorylate wild-type C/EBPα; however, the mutation of S193A abolishes cdk4-mediated phosphorylation of C/EBPα and significantly reduces cdk6-mediated phosphorylation. To examine if cdk4/cdk6-cyclin D3 complexes from livers specifically phosphorylate C/EBPα at Ser193, we performed a similar kinase assay with these kinases immunoprecipitated from old livers using WT GST-C/EBPα and GST-C/EBPα-S193A as substrates. As seen in Fig. 2E and F, the mutation of Ser193 to Ala abolishes phosphorylation of C/EBPα by cdk4 IPs, while cdk6 and cyclin D3 IPs show significantly reduced, but still detectable, phosphorylation of the S193A mutant. Thus, these studies demonstrated that cdk4-cyclin D3 and cyclin D3-cdk6 complexes specifically phosphorylate Ser193 of C/EBPα in livers.
Since these experiments identified C/EBPα as a new substrate for cdk4/cdk6, we asked whether the efficiency in phosphorylation of C/EBPα by cdk4/cdk6 is comparable to phosphorylation of Rb. To address this question, GST-Rb and GST-C/EBPα-140-200 (a fragment of C/EBPα which interacts with cdk4 and contains Ser193 [24, 25]) were simultaneously added in different ratios to an in vitro kinase assay mixture with purified cyclinD1-cdk4 complex. The truncated form of C/EBPα was used in these experiments, because the full-length C/EBPα migrates close to GST-Rb and is not separated well from Rb by electrophoresis. At a ratio of Rb and C/EBPα of 1:1, the levels of phosphorylation of C/EBPα were identical to those of Rb (Fig. 2G, lane 1). At ratios of Rb and C/EBPα of 1:2 and 1:4, the phosphorylation of C/EBPα was increased, while the phosphorylation of Rb was decreased. These results show that the efficiency of phosphorylation of C/EBPα by cdk4 is comparable with that of Rb and that C/EBPα might compete with Rb for the interaction with cdk4 in the kinase reactions.
Cyclin D3-cdk4/cdk6 are associated with C/EBPα in young and old livers.
Given the induction of cdk4/cdk6 activities in old livers (Fig. 2), we next examined mechanisms by which aging increases activities of these kinases. Western blotting with antibodies to cyclins D1, D2, and D3 showed that while cyclins D1 and D2 are not detectable in both young and old livers, cyclin D3 is expressed in quiescent livers and is dramatically increased in old animals. Figure 3A shows examination of young and old animals by Western blotting with antibodies to cyclin D3. As can be seen, old animals contain high levels of cyclin D3 compared to those in young livers. Reprobing the membrane with β-actin and Coomassie staining the filter revealed equal protein loadings (Fig. 3A). Densitometric calculations of cyclin D3 as a ratio to β-actin of multiple Western blotting experiments from six animals of each age group showed a four- to fivefold increase of protein levels of cyclin D3 in old livers (Fig. 3B). Interestingly, real-time PCR analysis with mRNA from young and old livers showed no difference in the levels of cyclin D3 mRNA (data not shown). This observation suggests that aging may increase translation or stability of the cyclin D3 protein.
FIG. 3.
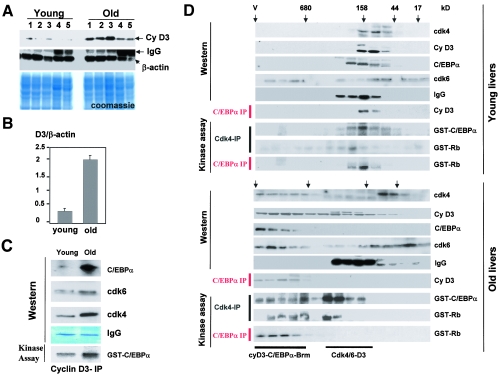
Aging liver increases activities of cdk4 and cdk6 by elevation of cyclin D3. A and B. Protein levels of cyclin D3 are increased in old livers. Nuclear extracts from five livers of young and old animals were examined by Western blotting with antibodies to cyclin D3. The membrane was reprobed with Abs to β-actin. Coomassie stain shows staining of a parallel gel loaded with the same proteins. Bar graphs (B) represent a summary of multiple experiments. Levels of cyclin D3 were calculated as a ratio to β-actin. C. C/EBPα is observed in complexes with cyclin D3 in the liver. Cyclin D3 was immunoprecipitated from young and old livers, and cdk4, cdk6, and C/EBPα were examined by Western blotting in cyclin D3 IPs. IgG lanes, heavy chains of immunoglobulin G were detected by Coomassie staining of the membrane. An aliquot from each reaction mixture was incubated with GST-C/EBPα substrate in the presence of [γ-32P]ATP (kinase assay, bottom part). D. cdk4-cyclin D3 are associated with the C/EBPα-Brm complex in old livers. Nuclear extracts from young and old livers were fractionated by size exclusion chromatography on an SEC-400 column as described in the legend to Fig. 1. Positions of molecular mass markers are shown on the top. Western lanes: examination of cdk4, cdk6, cyclin D3, and C/EBPα in the gel filtration fractions by Western blotting. C/EBPα-IP lanes, C/EBPα was immunoprecipitated from fractions and C/EBPα IPs were probed with antibodies to cyclin D3. Kinase assay lanes: cdk4 and C/EBPα were immunoprecipitated from each fraction and examined in a kinase assay with GST-Rb and with GST-C/EBPα substrates. Positions of cyclin D3-cdk4/cdk6 complexes are shown on the bottom.
Since the in vitro kinase assay showed that C/EBPα is the substrate for phosphorylation by cyclin D3-activated cdk4/cdk6, we asked if cyclin D3 is physically associated with C/EBPα in the young and old livers. Cyclin D3 was precipitated from nuclear extracts of young and old livers, and cdk4, cdk6, and C/EBPα were examined in these IPs. We observed that cdk4, cdk6, and C/EBPα are associated with cyclin D3 in both young and old livers; however, amounts of these proteins were increased in cyclin D3 IPs from old livers (Fig. 3C). A parallel examination of the cdk4/cdk6 activity in aliquots from these IPs using GST-C/EBPα as substrate showed that the kinase activity of cdk's, associated with cyclin D3 and C/EBPα, was also increased in old livers. Thus, cyclin D3-cdk4/cdk6 complexes are associated with C/EBPα in old livers and are able to phosphorylate C/EBPα. These findings suggested that C/EBPα is a substrate for cdk4/cdk6 in quiescent livers.
Cyclin D3-cdk4/cdk6 are observed in high-MW C/EBPα-Brm complexes in old livers.
The presence of C/EBPα in cyclin D3-cdk4/cdk6 complexes suggested that cyclin D3-cdk4/cdk6 might be associated with C/EBPα and/or Rb within a large C/EBPα-Rb-E2F4-Brm complex. To test this suggestion and to examine kinase activities of cyclin D3-cdk4/cdk6 complexes, we utilized the previously established size exclusion chromatography approach (9, 22, 24). Protein extracts from young and old livers were fractionated by size exclusion chromatography, and the composition of cdk4/cdk6-cyclin D3 complexes was examined in different fractions of the chromatography column by Western blotting and co-IP-kinase assays. In young animals, cyclin D3, cdk4, and C/EBPα colocalized in fractions with molecular masses around 160 kDa (Fig. 3D). The immunoprecipitation of C/EBPα from size exclusion fractions and examination of cyclin D3 in C/EBPα IPs revealed that C/EBPα is present in these 160-kDa cyclin D3-cdk4/cdk6 complexes. The size of this complex suggests that the complex consists of cyclin D3, cdk4, and C/EBPα. Immunoprecipitation of cdk4 and C/EBPα, following examination of kinase activity, showed that the 160-kDa cyclin D3-cdk4 complex is active. The results of size exclusion chromatography are consistent with data obtained with a simple coimmunoprecipitation assay (Fig. 3C).
Examination of nuclear extracts from old livers by size exclusion chromatography showed different results. Although cdk6- and cdk4-cyclin D3 complexes were observed in extracts from old livers in fractions ranging from 44 to 160 kDa, significant portions of cdk4, cdk6, and cyclin D3 were shifted to the position of high-MW complexes. Western blotting with antibodies to C/EBPα revealed that this position corresponds to that of the C/EBPα-Brm complex. To examine if cyclin D3-cdk4/cdk6 are associated in these fractions with the C/EBPα-Brm complex, the complex was immunoprecipitated with antibodies to C/EBPα and cyclin D3 was examined by Western blotting. As one can see, cyclin D3 is detectable within the C/EBPα-Brm complex (Fig. 3D). This result is consistent with data obtained in the coimmunoprecipitation assay (Fig. 3C). We next examined activities of cyclin D3-cdk4/cdk6 complexes in size exclusion fractions from old livers. cdk4 was immunoprecipitated from size exclusion fractions and examined in a kinase assay with Rb and C/EBPα substrates. These data showed that old livers contain two active cyclin D3-cdk4/cdk6 complexes. One active complex was observed in the fractions ranging from 160 to 300 kDa, while the second active complex colocalized with the C/EBPα-Brm complex. Kinase activity of cdk4 toward both Rb and C/EBPα was dramatically increased in these high-MW fractions compared to corresponding fractions of young livers. To confirm that the high-MW active cdk4-cyclin D3 is associated with the C/EBPα-Brm complex, the latter was immunoprecipitated with antibodies to C/EBPα and kinase activity of these IPs was examined with Rb substrate. These experiments clearly demonstrated that the high-MW cyclin D3-cdk4 complex is associated with C/EBPα-Brm. Taking together the direct co-IP studies (Fig. 3C) and examination of the complexes by size exclusion chromatography, we conclude that the active cdk4/cdk6-cyclin D3 complexes are associated in quiescent old livers with the age-specific C/EBPα-Brm complex. Since the ph-193 C/EBPα isoform is the major one in the complex, the presence of active cyclin D3-cdk4/cdk6 in the C/EBPα-Brm complex might be necessary to keep high levels of phosphorylation of C/EBPα at Ser193.
High levels of cyclin D3 support the growth-inhibitory C/EBPα-Brm complex in old livers after PH.
The C/EBPα-Brm complex is not reduced after PH in old mice (9). Our previous work and data in this paper identified a phosphatase, PP2A (22), and cdk4/cdk6 kinases as enzymes that regulate the dephosphorylation-phosphorylation of Ser193 and the formation of the age-specific complex. To elucidate the molecular basis for the failure of old livers to reduce the complex after PH, we examined the expression of these enzymes after PH in young and old livers. Since the difference between young and old livers in phosphorylation of C/EBPα is observed at 4 h after PH (Fig. 4B), we focused our studies on the early time period after PH, within 8 h after surgery. Protein levels of cyclin D1 are not detectable within this time period, since the induction of cyclin D1 is observed at 12 to 24 h after PH (16). Although cyclin D3 expression is not changed in either age group, the relative amounts of cyclin D3 during this time period remain stably higher in old livers compared to young (Fig. 4A). Examination of kinase activity associated with cyclin D3 revealed that the cyclin D3-cdk4/cdk6 complex is more active in old livers at earlier time points after PH (Fig. 4A, cyclin D3 IP kinase assay). These data show that the cdk4/cdk6-cyclin D3 pathway is not altered, but cdk4/cdk6-cyclinD3 complexes remain at high levels in old livers at 8 h after PH. Western blotting analysis with antibodies to catalytic subunit C of PP2A indicated that, although the induction of PP2A was observed in both age groups, PP2A levels were 8- to 10-fold increased in young livers at 4 h, while only a 3- to 4-fold induction of PP2A was observed in old livers at 8 h after PH. To examine if the cyclin D3 is associated with C/EBPα in young and old livers after PH, C/EBPα was precipitated by specific antibodies and cyclin D3 was examined in these IPs. These studies revealed that cyclin D3 is associated with C/EBPα after PH (Fig. 4A, co-IP). Analysis of these IPs with ph-Ser193-specific antibodies showed that the ph-Ser193 isoform of C/EBPα is not detectable after PH in young livers, while amounts of Ser193-ph isoforms are abundant in old livers after PH. Examination of C/EBPα and C/EBPβ in livers showed that patterns of their expression were consistent with previously published results: C/EBPβ was increased in both age groups, while C/EBPα was reduced in young livers but not in old livers. We next performed a quantitative examination of C/EBPα isoforms after PH in young and old livers using a 2D-Western technique. Figure 4B shows that, in young livers, PH eliminated growth-inhibitory Ser193-ph isoforms of C/EBPα at 4 h after surgeries. However, the amounts of these isoforms were not altered in old livers after PH. The lack of dephosphorylation of C/EBPα in old livers after PH correlates with a higher activity of cyclin D3-associated kinases and with a weaker induction of PP2A.
FIG. 4.
Old livers fail to dephosphorylate C/EBPα at Ser193 after partial hepatectomy and contain a high-MW cyclin D3-cdk4-C/EBPα-Rb-E2F4-Brm complex. A. Expression of cyclin D3 and PP2A in young and old livers at early time points after partial hepatectomy. Nuclear extracts were isolated at 0, 4, and 8 h after PH and examined by Western blotting with antibodies to cyclin D3, cyclin D1, cdk4, cdk6, PP2A, C/EBPα, and C/EBPβ. Each membrane was reprobed with antibodies to β-actin. β-Actin controls are shown for cyclin D3 and C/EBPα membranes. Coomassie stain shows staining of the membrane for cyclin D3. Cyclin D3 IP-kinase assay: examination of cdk4/cdk6 activities in cyclin D3 IPs during early steps of liver proliferation. Cyclin D3 was immunoprecipitated from nuclear extracts isolated at 0, 4, and 8 h after PH. The activities of cdk4 and cdk6 associated with cyclin D3 were examined in a kinase assay with GST-C/EBPα substrate. C/EBPα-IP-Western: C/EBPα was immunoprecipitated from nuclear extracts with antibodies to total C/EBPα. IPs were probed with Abs to cyclin D3 and with Abs to the Ser193-ph isoform of C/EBPα. B. The growth-inhibitory Ser193-ph isoform of C/EBPα is abundant in old livers after PH. C/EBPα was examined by 2D gel electrophoresis in nuclear extracts from young and old livers at 0 and 4 h after PH. Positions of five major isoforms of C/EBPα are shown on the top. Ser193-ph isoforms (a and b) are shown in red. C. Examination of C/EBPα-Brm complex in young and old livers after PH by the co-IP approach. C/EBPα was immunoprecipitated from nuclear extracts isolated from young and old livers at 4 h after PH, and IPs were examined by Western blotting with antibodies to C/EBPα, Brm, Rb, cyclin D3, and cdk4. IgG, heavy chains of immunoglobulin G. The composition of the cyclin D3-C/EBPα-Brm complex is shown on the right. D. Examination of the cyclin D3-C/EBPα-Brm complex in nuclear extracts isolated 4 h after PH by size exclusion chromatography. Nuclear extracts from young and old livers were examined by HPLC-based gel filtration as described in the legend to Fig. 3. C/EBPα-IP lanes: C/EBPα was immunoprecipitated from each fraction, and the presence of cyclin D3 and Brm was examined in these IPs by Western blotting. IgG lanes: heavy chains of immunoglobulin G were determined on the corresponding filters by Coomassie staining.
Cyclin D3 is associated with C/EBPα-Brm complexes in old livers after PH.
HPLC-based examination of C/EBPα-cyclin D3 complexes in quiescent old livers showed that this complex colocalizes with Brm and Rb (Fig. 1 and 3), suggesting that cyclin D3-cdk4/cdk6 might be associated with high-MW C/EBPα-Rb-E2F4-Brm complexes after PH. To examine this suggestion, we performed coimmunoprecipitation and size exclusion chromatography studies of nuclear extracts isolated from young and old livers at 4 h after PH. Figure 4C shows that immunoprecipitation of C/EBPα from young and old livers at 4 h after PH pulled down Brm, Rb, cdk4, and cyclin D3 in old livers, while only Rb was detectable in C/EBPα IPs from young livers, suggesting that old livers contain the cyclin D3-cdk4-C/EBPα-Brm complex after PH. To verify this result and to precisely determine the composition of this complex, protein extracts from young and old livers isolated at 4 h after PH were fractionated on an SEC-400 column. Size exclusion chromatography fractions were analyzed by Western blotting and by co-IP (as described above for Fig. 3). Since C/EBPα levels are reduced in young livers after PH (Fig. 4A), C/EBPα signals after fractionation are very weak or not detectable. In young livers, Rb, E2F4, cyclin D3, and cdk4 were observed in fractions with molecular masses ranging from 100 to 300 kDa. Consistent with simple co-IP experiments, cyclin D3 was also not detectable in C/EBPα complexes by co-IP from size exclusion fractions (Fig. 4D). Examination of size exclusion fractions from old livers at 4 h after PH showed a quite-different result. While Rb, E2F4, cyclin D3, and cdk4 were distributed throughout many fractions, the major amounts of C/EBPα and Brm were observed in very-high-MW fractions. Precipitation of the C/EBPα-Brm complex with antibodies to C/EBPα and examination of cyclin D3 and Brm in these IPs showed that both cyclin D3 and Brm are associated with C/EBPα in old livers at 4 h after PH. Thus, co-IP and gel filtration studies demonstrated that cyclin D3-cdk4 are bound to the C/EBPα-Brm complex, suggesting that this association phosphorylates C/EBPα at Ser193.
Phosphorylation of C/EBPα at Ser193 by cyclin D3-cdk4/cdk6 is required for the formation of C/EBPα complexes with cdk2 and Brm.
To further examine the role of cyclin D3-cdk4/cdk6 in the regulation of growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes, we utilized a tissue culture system involving differentiation of 3T3-L1 cells, in which C/EBPα plays a key role in the inhibition of proliferation (18). 3T3-L1 cells were selected for these studies since endogenous C/EBPα inhibits proliferation of 3T3-L1 by formation of C/EBPα-cdk2 complexes (24) and, at later stages of differentiation, C/EBPα interacts with Brm and forms a C/EBPα-Brm complex (9). Because expression of cyclin D3 is also increased during differentiation of 3T3-L1 adipocytes (15), we suggested that the formation of the C/EBPα complexes might be controlled in 3T3-L1 cells by cyclin D3-cd4/cdk6. To examine this possibility, we first determined expression of cyclin D3 in 3T3-L1 cells after initiation of differentiation under our conditions. Figure 5A shows that protein levels of cyclin D3 were increased after initiation of differentiation (day 1) and stayed at high levels in fully differentiated cells (day 7). Densitometric calculations of cyclin D3 as a ratio to β-actin showed that protein levels of cyclin D3 were 10- to 12-fold higher at days 5 to 7 than those in predifferentiated cells (Fig. 5A, bar graph). Examination of markers of adipocyte differentiation, PPARγ and C/EBPβ, revealed that the genetic program of differentiation was properly initiated in 3T3-L1 cells (Fig. 5B). Consistent with previous observations, protein levels of cdk2 and cdk4 were not significantly changed during differentiation of 3T3-L1 cells (15, 21). Examination of C/EBPα in differentiated 3T3-L1 cells with Abs to total C/EBPα and to Ser193-ph showed that expression of C/EBPα was increased at days 3 to 7 after initiation of differentiation and that C/EBPα was phosphorylated at Ser193 at days 3, 5, and 7 (Fig. 5C). This phosphorylation of Ser193 correlates with the time of induction of cyclin D3 in differentiated 3T3-L1 cells.
We next examined if cyclin D3-cdk4/cdk6 complexes in 3T3-L1 cells are able to phosphorylate C/EBPα. Cyclin D3 was immunoprecipitated from nuclear extracts of differentiated cells and examined in an in vitro kinase assay with C/EBPα and Rb substrates. Figure 5D shows a typical result of these studies. Cyclin D3 activity toward C/EBPα and Rb was detectable in predifferentiated cells. At day 1, the ability of cyclin D3 to phosphorylate Rb was increased, while the activity of cyclin D3 toward C/EBPα was not changed. However, the ability of cyclin D3 to phosphorylate C/EBPα was increased at day 3 and remained high during the later days of differentiation. This timing correlates with the phosphorylation of endogenous C/EBPα at Ser193. Taken together, these studies suggested that the induction of cyclin D3 is involved in the phosphorylation of C/EBPα in differentiated adipocytes.
Inhibition of cyclin D3 blocks the phosphorylation of C/EBPα at Ser193 and reduces the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes.
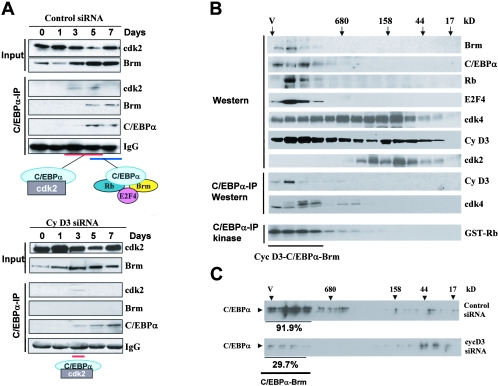
To examine if cyclin D3 is required for the phosphorylation of C/EBPα at Ser193 and for the formation of growth-inhibitory complexes of C/EBPα, the expression of cyclin D3 was inhibited in 3T3-L1 cells by specific siRNA. In these experiments, we obtained around 70 to 80% efficiencies of transfection. As one can see in Fig. 5E, cyclin D3 siRNA significantly reduced levels of cyclin D3 in preadipocytes and blocked the induction of cyclin D3 after initiation of differentiation, while in control cells treated with siRNA of random sequence, cyclin D3 was increased during differentiation. Although we could not inhibit expression of cyclin D3 completely, we obtained a 15- to 20-fold difference in the levels of cyclin D3 in control and cyclin D3 siRNA-treated 3T3-L1 cells at days 3 to 7 of differentiation (Fig. 5E, bar graphs). This inhibition is sufficient to block the phosphorylation of Ser193-ph of C/EBPα, as was shown by Western blotting with specific antibodies. Consistent with the lack of phosphorylation of endogenous C/EBPα on Ser193, phosphorylation of C/EBPα by cyclin D3 IPs from cells transfected with cyclin D3 siRNA was also not detectable in an in vitro kinase assay (Fig. 5E). To examine the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes in control cells and in cells with inhibited expression of cyclin D3, coimmunoprecipitation studies and HPLC-based size exclusion analyses were performed. Figure 6A shows results of co-IP studies. In control cells, C/EBPα formed complexes with cdk2 at days 3 and 5, while C/EBPα-Brm complexes were abundant at days 5 and 7 of differentiation. On the contrary, in 3T3-L1 cells in which cyclin D3 was inhibited, C/EBPα-Brm complexes were not detectable at any stage of differentiation. Although we could detect C/EBPα-cdk2 complex at day 3, this complex was very weak at day 3 and was not detectable at day 5 of differentiation of 3T3-L1 cells, while in control 3T3 cells the complex was strong and was observed at days 3 and 5 (Fig. 6A). These studies demonstrated that the inhibition of cyclin D3 during differentiation of 3T3-L1 cells prevents the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes.
FIG. 6.
Phosphorylation of C/EBPα by cyclin D3-cdk4/cdk6 is required for the formation of the C/EBPα-cdk2 and the C/EBPα-Brm complexes. A. Examination of C/EBPα-cdk2 and C/EBPα-Brm complexes during differentiation of 3T3-L1 cells treated with control siRNA (upper) and with siRNA to cyclin D3 (bottom). Input lanes: Western blotting of nuclear extracts with antibodies to Brm and cdk2, showing the levels of these proteins in extracts used for the co-IP. C/EBPα-IP lanes: C/EBPα was immunoprecipitated from nuclear extracts, and the IPs were examined by Western blotting with antibodies to cdk2, Brm, and C/EBPα. Heavy chains of immunoglobulin G (IgG) are shown for each IP. B. Examination of the cyclin D3-C/EBPα-Brm complex in differentiated 3T3-L1 cells by size exclusion chromatography. Nuclear extracts from differentiated 3T3-L1 cells (day 7) were fractionated with an SEC-400 column. Fractions were analyzed by Western blotting with antibodies to the proteins shown on the right. C/EBPα-IP Western lanes: examination of cyclin D3 and cdk4 in C/EBPα IPs from size exclusion fractions was performed with specific antibodies as described in the legends to Fig. 3 and 4. Kinase activity of cyclin D3-cdk4 was examined in C/EBPα IPs by an in vitro kinase assay with Rb substrate (C/EBPα-IP kinase). C. Size exclusion chromatography of nuclear extracts isolated from 3T3-L1 cells treated with control siRNA and with cyclin D3 siRNA. Proteins were isolated at day 7 after initiation of differentiation and fractionated on an SEC-400 column. Size exclusion fractions were examined by Western blotting with antibodies to C/EBPα. Amounts of C/EBPα were calculated in fractions containing C/EBPα-Brm complexes relative to total amounts of C/EBPα throughout the gel filtration.
The cyclin D3-cdk4-C/EBPα-Rb-E2F4-Brm complex is abundant in differentiated 3T3-L1 cells.
In old livers, the elevation of cyclin D3 leads to the formation of a very-high-MW cyclin D3-cdk4/cdk6-C/EBPα-Rb-E2F4-Brm complex (Fig. 3 and 4). We next asked if this complex is also formed in differentiated 3T3-L1 cells containing high levels of cyclin D3. Protein extracts were isolated from 3T3-L1 cells at day 7 of differentiation and examined by size exclusion chromatography. As seen in Fig. 6B, C/EBPα, Brm, Rb, E2F4, cdk4, and cyclin D3 colocalized in high-MW fractions of gel filtrations of differentiated 3T3-L1 cells. To examine if cyclin D3 and cdk4 are associated with the C/EBPα-Brm complex in these fractions, C/EBPα was immunoprecipitated from each fraction of the gel filtration and IPs were analyzed with antibodies to cyclin D3 and to cdk4. Both cyclin D3 and cdk4 were observed in the C/EBPα IPs (Fig. 6B). These data show that differentiated 3T3-L1 cells contain the high-MW cyclin D3-cdk4-C/EBPα-Rb-E2F4-Brm complex. To examine if cyclin D3-cdk4 is active within this complex, aliquots of these IP mixtures were used in an in vitro kinase assay with GST-Rb substrate. This analysis showed that cyclin D3-cdk4 within the C/EBPα-Brm complex is active and phosphorylates Rb. Using this HPLC technique, we next examined the effects of inhibition of cyclin D3 on the C/EBPα-Brm complexes in differentiated 3T3-L1 cells. In these experiments, we used Western blotting with antibodies to C/EBPα as a measurement of the C/EBPα-Brm complex, followed by densitometric calculations of the portion of C/EBPα in the position of the complex. 3T3-L1 cells transfected with control siRNA contained more than 90% of C/EBPα in the position of the C/EBPα-Brm complex. In 3T3-L1 cells with reduced expression of cyclin D3, the amounts of C/EBPα in the position of the Brm complexes were reduced to 30%. Since efficiencies of transfection in these experiments were 70 to 80%, the remaining C/EBPα-Brm complexes perhaps came from untransfected cells. Taking together co-IP data and HPLC results, these studies showed that cyclin D3 is required for the formation of both C/EBPα-cdk2 and C/EBPα-Brm complexes and that cyclin D3-cdk4 is observed in high-MW C/EBPα-Brm complexes.
DISCUSSION
C/EBPα is a new target for cyclin D3-cdk4/cdk6 in differentiated cells.
Searching for a pathway which positively regulates the growth-inhibitory activity of C/EBPα in the liver, we found that cdk4/cdk6 phosphorylates C/EBPα at Ser193 and that these kinases are activated in the liver by cyclin D3. Although cyclin D3 clearly promotes cell cycle progression in some models, this protein is expressed in numerous nonproliferating tissues, including quiescent liver (1, 4, 16). Indeed, increased cyclin D3 expression appears to participate in cell cycle withdrawal in muscle cells through induction of the transcription factor MyoD, and overexpression of cyclin D3 suppresses reactivation of DNA synthesis in differentiated myotubules (2, 5). Although these observations were described long ago, molecular targets of cyclin D3 in differentiated settings have not been found. Data in this paper provide evidence that, in differentiated livers and adipocytes, cyclin D3-cdk4/cdk6 phosphorylates C/EBPα and supports the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes that inhibit proliferation. We have shown in our previous studies that C/EBPα can interact with cdk4; however, this interaction had a minor effect on the kinase activity of cdk4 (24, 25). A slight reduction of the phosphorylation of Rb by cdk4 in the presence of C/EBPα, rather, reflected a competition, since under conditions of the in vitro kinase assay, C/EBPα and Rb were in excess to cdk4. Under conditions of the in vitro kinase assay, both cyclin D1-cdk4 and cyclin D3-cdk4 complexes phosphorylated C/EBPα, suggesting that these complexes can also phosphorylate C/EBPα in vivo. In quiescent livers, however, cyclin D3 is a major activator of cdk4/cdk6, since the levels of cyclin D3 are very high while cyclin D1 is not detectable (reference 16 and this study). Although both cdk4 and cdk6 phosphorylate C/EBPα, cdk4 seems to be more specific for Ser193, since it does not phosphorylate the C/EBPα-S193 mutant, while the phosphorylation of C/EBPα-S193A by cdk6 is weaker but still detectable (Fig. 2).
Cyclin D3 supports growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes in differentiated livers and in adipocytes.
C/EBPα is a very strong inhibitor of cell proliferation and is expressed in highly differentiated tissues such as liver and adipose tissues. On the other hand, there are several biological situations in which cells proliferate and express high levels of C/EBPα (22, 23). In these cells, C/EBPα activity is eliminated by dephosphorylation of C/EBPα at Ser193 (22). In this paper, we identified cyclin D3-cdk4/cdk6 as enzymes which phosphorylate Ser193, leading to the formation of growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. A hypothetical model for the role of cyclin D3 in supporting the growth-inhibitory activity of C/EBPα in adipocytes and quiescent livers is shown in Fig. 7. Both the liver and differentiated adipocytes express high levels of C/EBPα (21, 23, 25) and high levels of cyclin D3 (Fig. 3 and 4) (15, 16). Different approaches revealed that, in quiescent livers, cyclin D3 is associated with C/EBPα and stabilizes C/EBPα-cdk2 (young liver) and C/EBPα-Brm (old liver) complexes by phosphorylation of C/EBPα at Ser193. Additional evidence for the role of cyclin D3-cdk4 in supporting growth-inhibitory activities of C/EBPα came from experiments with differentiated 3T3-L1 adipocytes. In these cells, C/EBPα is induced at later stages of differentiation and is required for growth arrest and differentiation (18). We showed that cyclin D3/cdk4 phosphorylates C/EBPα at Ser193 at days 3 to 7 of differentiation, resulting in the formation of C/EBPα-cdk2 and C/EBPα-Brm complexes. The inhibition of cyclin D3 expression by siRNA leads to failure of C/EBPα to form growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. In agreement with our data, a recent paper by Sarruf et al. showed that the inhibition of cyclin D3 expression of 3T3-L1 blocks differentiation of 3T3-L1 adipocytes (17).
FIG. 7.
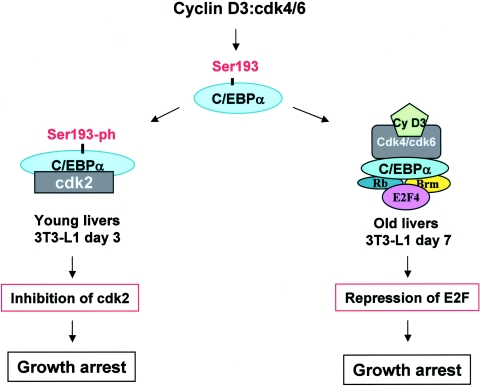
Hypothetic model suggesting that cyclin D3 supports quiescence of the liver and differentiated adipocytes by stabilizing growth-inhibitory C/EBPα-cdk2 and C/EBPα-Brm complexes. Cyclin D3 activates cdk4/cdk6 kinases, which specifically phosphorylate Ser193 of C/EBPα. The Ser193-ph isoform of C/EBPα forms complexes with cdk2 (young livers) and with Brm, Rb, and E2F4 (old livers), which inhibit cell proliferation.
Although our data (this paper and reference 16) and those of others (1, 2, 5) suggest an antiproliferative role for cyclin D3, data in other systems clearly suggest that this protein can promote cell cycle progression. For example, ectopic expression of cyclin D3 promotes progression through the G1 phase in rat fibroblasts (6). Furthermore, transient overexpression of cyclin D3 in young (2-month) livers promotes a modest proliferative effect in hepatocytes (L. Mullany, C. Nelsen, and J. Albrecht, personal communication). These data indicate that cyclin D3 can have either a proliferative or inhibitory effect, presumably depending upon the intracellular environment and levels of cyclin D3 and its downstream targets which mediate inhibition or promotion of proliferation.
Aging liver activates cdk4/cdk6 by increasing cyclin D3 levels and by forming high-MW complexes.
Activities of both cdk4 and cdk6 are increased in old livers by the elevation of cyclin D3. Our examination of enzymatic activities of cyclin D3-cdk4/cdk6 complexes by size exclusion chromatography provided new observations suggesting an additional pathway for the regulation of cyclin D3-cdk4/cdk6. Previous studies had shown that in proliferating cultured cells, active cyclin D3/cdk6 complexes are approximately 170 kDa in size (10). Consistent with these observations, in young livers the active cyclin D3-cdk4/cdk6 complexes range from 100 to 160 kDa and a portion of these complexes is associated with C/EBPα, suggesting that C/EBPα is a substrate for cyclin D3-cdk4/cdk6. In aged liver, however, the major cdk4/cdk6 kinase activity was associated with higher-MW complexes, one of which is the cyclin D3-C/EBPα-Brm complex. Interestingly, partial hepatectomy in old livers failed to reduce the cyclin D3-C/EBPα-Brm complex, suggesting that this complex might be involved in the inhibition of liver proliferation. Examination of high-MW complexes in growth-arrested, differentiated 3T3-L1 cells presented additional evidence for the role of the cyclin D3-C/EBPα-Brm complex in the inhibition of cell proliferation. These data suggest that the recruitment of cyclin D3 into higher-MW complexes may result in a change in its role in cell cycle control.
In this paper, we identified C/EBPα as a new target of cyclin D3. Recent publications suggest that there might be more targets of cyclin D3 which are involved in the differentiation-specific functions of cyclin D3. When our manuscript was under review, Sarruf et al. published observations that cyclin D3-mediated phosphorylation of PPARγ is also involved in the differentiation of adipocytes (17). In addition to the role of cyclin D3 in differentiation of 3T3-L1 cells, protein levels of cyclin D3 are also elevated in differentiated myocytes at the stage of growth arrest (2, 4), suggesting a putative involvement of cyclin D3 in the inhibition of these cells. Although C/EBPα is not expressed in myocytes, experimental work from Paterson's group showed that cdk4 (which is activated by cyclin D3) interacts with the muscle-specific transcription factor MyoD and that this interaction promotes growth arrest and terminal differentiation (26, 27). It would be interesting to examine if cyclin D3 has a positive effect on this interaction and on terminal differentiation of myocytes.
Acknowledgments
This work was supported by National Institutes of Health grants AG20752, CA10070, and GM55188 (N.A.T.) and DK54921 (J.H.A.).
REFERENCES
- 1.Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1998. Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 17:1027-1037. [DOI] [PubMed] [Google Scholar]
- 2.Cenciarelli, C., F. De Santa, P. L. Puri, E. Mattei, L. Ricci, F. Bucci, A. Felsani, and M. Caruso. 1999. Critical role played by cyclin D3 in the MyoD-mediated arrest of cell cycle during myoblasts differentiation. Mol. Cell. Biol. 19:5203-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conboy, I. M., M. J. Conboy, A. J. Wagers, E. R. Girma, I. L. Weisman, and T. A. Rando. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 43:760-764. [DOI] [PubMed] [Google Scholar]
- 4.de La Serna, I. L., K. Roy, K. A. Carlson, and A. N. Imbalzano. 2001. MyoD can induce Cell cycle arrest but not muscle differentiation in the presence of dominant negative SWI/SNF chromatin remodeling enzymes. J. Biol. Chem. 276:41486-41491. [DOI] [PubMed] [Google Scholar]
- 5.Doglioni, C., C. Chiarelli, E. Macri, A. P. Dei Tos, E. Meggiolaro, P. Dalla Palma, and M. Barbaresch. 1998. Cyclin D3 expression in normal, reactive and neoplastic tissues. J. Pathol. 185:159-166. [DOI] [PubMed] [Google Scholar]
- 6.Fausto, N. 2000. Liver regeneration. J. Hepatol. 32:19-31. [DOI] [PubMed] [Google Scholar]
- 7.Flodby, P. C., H. Barlow, L. Kalefjord, L. Ahrlund-Richer, and K. G. Xanthopolous. 1996. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/Enhancer binding protein α. J. Biol. Chem. 271:24753-24760. [DOI] [PubMed] [Google Scholar]
- 8.Herzinger, T., and S. I. Reed. 1998. Cyclin D3 Is rate-limiting for the G1/S phase transition in fibroblasts. J. Biol. Chem. 273:14958-14961. [DOI] [PubMed] [Google Scholar]
- 9.Iakova, P., S. S. Awad, and N. A. Timchenko. 2003. Aging reduces proliferative capacities of liver by switching pathways of C/EBPα growth arrest. Cell 113:495-506. [DOI] [PubMed] [Google Scholar]
- 10.Mahony, D., D. A. Parry, and E. Lees. 1998. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene 16:603-611. [DOI] [PubMed] [Google Scholar]
- 11.Muller, C., C. F. Calkhoven, X. Sha, and A. Leutz. 2004. C/EBPα requires a SWI/SNF complex for proliferation arrest. J. Biol. Chem. 279:7353-7358. [DOI] [PubMed] [Google Scholar]
- 12.Nelsen, C. J., D. G. Rickheim, N. A. Timchenko, M. W. Stanley, and J. H. Albrecht. 2001. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 61:8564-8668. [PubMed] [Google Scholar]
- 13.Pederson, T. A., E. Kowenz-Leutz, A. Leutz, and C. Nerlov. 2001. Cooperation between C/EBPα, TBP/TFIIB and SWI/SNF recruiting domains is required for adipocyte differentiation. Genes Dev. 15:3208-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porse, B. T., T. A. Pederson, X. Xu, B. Lindbergh, U. M. Wewer, L. Fris-Hansen, and C. Nerlov. 2001. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell 107:247-258. [DOI] [PubMed] [Google Scholar]
- 15.Reichert, M., and D. Eick. 1999. Analysis of cell cycle arrest in adipocyte differentiation. Oncogene 18:459-466. [DOI] [PubMed] [Google Scholar]
- 16.Rickheim, D. G., C. J. Nelsen, J. T. Fassett, N. A. Timchenko, L. K. Hansen, and J. H. Albrecht. 2002. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology 36:30-38. [DOI] [PubMed] [Google Scholar]
- 17.Sarruf, D. A., I. Iankova, A. Abella, S. Assou, S. Miard, and L. Fajas. 2005. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 25:9985-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao, D., and M. A. Lazar. 1997. Peroxisome proliferator activated receptor γ, CCAAT/enhancer binding protein α, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 272:21473-21478. [DOI] [PubMed] [Google Scholar]
- 19.Timchenko, N. A., T. E. Harris, M. Wilde, T. A. Bilyeu, B. L. Burgess-Beusse, M. J. Finegold, and G. J. Darlington. 1997. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol. Cell. Biol. 17:7353-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timchenko, N. A., M. Wilde, and G. J. Darlington. 1999. C/EBP alpha regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol. Cell. Biol. 19:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timchenko, N. A., M. Wilde, P. Iakova, J. H. Albrecht, and G. J. Darlington. 1999. E2F/p107 and E2F/p130 complexes are regulated by C/EBPα in 3T3-L1 adipocytes. Nucleic Acids Res. 27:3621-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, G.-L., P. Iakova, M. Wilde, S. S. Awad, and N. A. Timchenko. 2004. Liver tumors escape negative control of proliferation via PI3K/Akt-mediated block of C/EBPα growth inhibitory activity. Genes Dev. 18:912-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, G.-L., and N. A. Timchenko. 2005. Dephosphorylated C/EBPα accelerates cell proliferation through sequestering retinoblastoma protein. Mol. Cell. Biol. 25:1325-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, H., P. Iakova, M. Wilde, A. Welm, T. Goode, W. J. Roesler, and N. A. Timchenko. 2001. C/EBPα arrests cell proliferation through direct inhibition of cdk2 and cdk4. Mol. Cell 8:817-828. [DOI] [PubMed] [Google Scholar]
- 25.Wang, H., T. Goode, P. Iakova, J. H. Albrecht, and N. A. Timchenko. 2002. C/EBPα triggers proteasome-dependent degradation of cdk4 during growth arrest. EMBO J. 21:930-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, J.-M., X. Zhao, Q. Wei, and B. M. Paterson. 1999. Direct inhibition of G1 cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J. 18:6983-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, J.-M., Q. Wei, X. Zhao, and B. M. Paterson. 1999. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 18:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]