Abstract
The insulin-like growth factor 1 receptor (IGF-1R) is a multifunctional receptor that mediates signals for cell proliferation, differentiation, and survival. Genetic experiments showed that IGF-1R inactivation in skin results in a disrupted epidermis. However, because IGF-1R-null mice die at birth, it is difficult to study the effects of IGF-1R on skin. By using a combined approach of conditional gene ablation and a three-dimensional organotypic model, we demonstrate that IGF-1R-deficient skin cocultures show abnormal maturation and differentiation patterns. Furthermore, IGF-1R-null keratinocytes exhibit accelerated differentiation and decreased proliferation. Investigating the signaling pathway downstream of IGF-1R reveals that insulin receptor substrate 2 (IRS-2) overexpression compensates for the lack of IGF-1R, whereas IRS-1 overexpression does not. We also demonstrate that phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1 and 2 are involved in the regulation of skin keratinocyte differentiation and take some part in mediating the inhibitory signal of IGF-1R on differentiation. In addition, we show that mammalian target of rapamycin plays a specific role in mediating IGF-1R impedance of action on keratinocyte differentiation. In conclusion, these results reveal that IGF-1R plays an inhibitory role in the regulation of skin development and differentiation.
The balance between cellular proliferation and differentiation plays a vital role in many physiological processes. In skin, the maintenance of such a balance, i.e., between the proliferation of mitotically active skin epidermal keratinocytes on the one hand and the differentiation of postmitotic skin cells on the other, is extremely important for skin formation and development (11). However, under certain pathological conditions, this equilibrium may be disturbed, leading to impaired wound healing, tumorigenesis, and many other skin pathologies.
Among the well-characterized growth factors and their receptors, insulin-like growth factor 1 (IGF-1) is one of the major regulators of cellular proliferation and differentiation (33). IGF-1 mediates its effects through the IGF-1 receptor (IGF-1R). This receptor belongs to the tyrosine kinase family of growth factor receptors (5). Ligand binding to IGF-1R leads to autophosphorylation of tyrosine residues in the cytoplasmic regions of the receptor β subunits, which is associated with activation of the IGF-1R tyrosine kinase, followed by phosphorylation of downstream signaling pathways. One of the first families of proteins that are phosphorylated by the activated IGF-1R is the insulin receptor substrate (IRS) proteins (39, 40). The activated IRS proteins serve as docking proteins to which several signaling molecules bind and then become activated. This ultimately results in the activation of at least two main signaling pathways: the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide-3 kinase (PI3K)/Akt/p70S6K pathway (10). Upon activation, these downstream molecules mediate a wide variety of intracellular signals in many cells and tissues, including those regulating glucose transport, protein synthesis, cell proliferation, and survival (40).
There are several studies demonstrating the role of IGF-1R and its signaling components in skin. Skin dermal fibroblasts and epidermal keratinocytes express IGF-1R, and IGF-1 stimulation of these cells leads to proliferation and mitogenicity (7, 22). Furthermore, increasing levels of IGF-1 or IGF-1R are associated with increased cell proliferation, skin hyperplasia, and tumorigenesis (18). Moreover, mice with disrupted IGF-1R have a thinner and disrupted epidermis (21).
We have recently shown that IGF-1R is activated and phosphorylated in skin keratinocytes, in response to IGF-1 stimulation, in a differentiation-dependent manner. Moreover, we have found that chronic IGF-1 stimulation inhibits the skin keratinocyte differentiation process (38). However, studies of the role of IGF-1 signaling in skin development and function have been largely limited by the fact that IGF-1R-null mice die soon after birth, and there is therefore no model available for studies on the direct effects of IGF-1R on skin development and function in vivo. Furthermore, the isolation of IGF-1R-null primary epidermal skin cells, as well as their growth in culture, is associated with technical difficulties, due to the thinning of IGF-1R-null skin, the reduced number of cells, the small size of the IGF-1R knockout pups, and the reduced rate of cell division (2, 3).
To overcome these difficulties, we applied two different approaches in the present study. We studied a model of primary skin keratinocytes in which the IGF-1R was inactivated in vitro, using the Cre-lox system (29). In this model, the genetically manipulated keratinocytes can be either further maintained in a proliferative basal cell phenotype, or induced to differentiate, by increasing the Ca2+ concentration in the growth medium. This differentiation process closely follows the maturation pattern of the epidermis in vivo (43). In addition, we developed a unique three-dimensional (3D) organotypic skin coculture model. In this model, primary murine epidermal keratinocytes and dermal fibroblasts can reorganize in vitro into a skin-equivalent structure, with histological characteristics of skin in vivo (12). The 3D organotypic skin model enables us to genetically knock down the epidermal and dermal skin components by using the Cre-lox system prior to establishing the 3D structure and, thus, to identify the direct effects of IGF-1R on skin organization and function. By using these approaches, we reveal an active inhibitory role of IGF-1R and IGF-1 signaling in skin cell differentiation. These results provide insight into processes that control the differentiation phenotype of skin.
(This work was performed in partial fulfillment of the requirements for the Ph.D. degree of Marianna Sadagurski, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.)
MATERIALS AND METHODS
Animals.
Mice homozygous for a floxed allele of exon 3 of IGF-1R were obtained as described previously (15). Animal experimentation was approved by the appropriate institutional committee and conducted in compliance with the rules of animal care of the National Institutes of Health (NIH).
Adenoviral constructs.
For IGF-1R inactivation, cells were infected with a cre recombinase adenoviral construct (a generous gift from S. H. Yuspa, National Cancer Institute, NIH).
For overexpressing proteins, the following adenoviral constructs were used: AxCAp85, encoding a dominant negative (DN) mutant of the p85 subunit of PI3K, and AxCAMyr-p110, encoding a myristoylated p110 subunit of PI3K (both were generous gifts from W. Ogawa, Kobe University School of Medicine [30]), and wild-type (WT) IRS-1 and WT IRS-2 adenoviruses (20). An adenoviral construct containing a luciferase reporter gene or an empty viral vector was used as a control, as indicated in each experiment, to exclude the effects of the infection procedure.
Cell isolation, culture, and infection.
Primary mouse keratinocytes from mice homozygous for a floxed allele of exon 3 of the IGF-1R were isolated and maintained as described previously (37). Briefly, freshly isolated keratinocytes were cultured in Eagle's medium (Sigma Aldrich, St. Louis, MO) with 10% chelexed fetal calf serum (Sigma Aldrich), 1% antibiotics, and Ca2+ (concentration adjusted to 0.05 mM). After 4 days in culture, for in vitro inactivation of the IGF-1R, cells were infected with an adenovirus encoding the desired adenoviral construct. After 1 h, the viral supernatant was replaced with fresh culture medium. Control cells were infected with an empty viral vector.
In experiments where double inactivation was carried out, 24 h after the first infection, cells were infected for the second time with the appropriate adenovirus vectors.
Differentiation of cells was induced by increasing the Ca2+ concentrations as detailed elsewhere (38).
Inhibitors of MAPK and mTOR.
To specifically inhibit MAPK or mammalian target of rapamycin (mTOR), the inhibitor U0126 (20 μM) for extracellular signal-regulated kinase 1 and 2 (ERK1/2) phosphorylation (Calbiochem, San Diego, CA) and rapamycin (20 μM) mTOR inhibitor (Calbiochem), respectively, were employed. The control cells were treated with dimethyl sulfoxide (DMSO). At this time point the differentiation process was induced.
Organotypic skin 3D coculture.
Dermal equivalents for organotypic cocultures were prepared with native type I collagen extracted from rat tail tendons (13). Collagen solution was mixed with 10× Hanks buffered saline, and suspended fibroblasts were added, mixed thoroughly, poured into membrane filter inserts, placed in special, deep six-well trays (Falcon, Becton Dickinson), and allowed to jellify at 37°C. The next day, keratinocytes were seeded on top of the collagen matrix in the Eagle's medium (Biological Industries, Beit Haemek, Israel). The cells were maintained for an additional 48 h, after which the cultures were raised to the air-medium interface.
Histological analysis and immunohistochemistry.
For histological or immunohistochemical analysis, organotypic cocultures were fixed in formaldehyde (3.7%) for at least 24 h and embedded in paraffin. Thereafter, 5-μm sections were stained with hematoxylin and eosin. Immunohistochemistry staining for keratin 14 (K14) and K1 and for loricrin (generous gift from S. H. Yuspa) was performed using the ABC staining kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's recommendations.
Immunoprecipitation, cell lysis, and Western blot analysis.
Cell lysis, Western blotting, and immunoprecipitation were performed as described previously (37). Fifteen to 50 μg of lysate was used in Western blotting. Antibodies used for immunoblotting, as detailed in each experiment, included anti-IGF-1R β subunit polyclonal antibodies and antiactin monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-keratin 14, anti-keratin 1, and antiloricrin polyclonal antibodies (generous gift from S. H. Yuspa), rabbit polyclonal antibodies against the IRS-1 and IRS-2 proteins (Upstate Biotechnology, Inc., Lake Placid, NY), rabbit polyclonal anti-Akt and anti-phospho-Akt antibody recognizing phosphorylated Ser 473 of mouse Akt1, monoclonal anti-ERK1/2, anti-phospho-ERK1/2 (Cell Signaling Inc., Beverly, MA), and a monoclonal mouse antiphosphotyrosine antibody (Upstate Biotechnology, Inc.). Filters were then incubated with the appropriate secondary horseradish peroxidase-conjugated anti-rabbit (Bio-Rad, Hercules, CA) or anti-mouse (Amersham, Piscataway, NJ) antibodies.
Preparation of cytoskeleton protein samples for analysis of keratin expression.
The Triton-insoluble fraction (pellet) obtained as described above (38) was incubated for 30 min in a special lysis buffer containing 20% β-mercaptoethanol and 5% sodium dodecyl sulfate. The samples were homogenized, incubated for 5 min at 100°C, and then spun for 30 min at maximal speed in a microcentrifuge. The lysates were further analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
5′ BrdU labeling and detection.
Cells were plated and grown to confluence on glass coverslips. On the day of the experiment, coverslips were fixed and processed following the manufacturer's protocol (bromodeoxyuridine [BrdU] cell proliferation kit; Chemicon). Cells that incorporate BrdU are detected by immunochemistry, as detailed in the protocol, and their nucleus acquires a brown color.
Fluorescence-activated cell sorter (FACS) analysis.
Primary keratinocytes were isolated and maintained as described previously. At 48 h after the adenoviral infection, nonattached keratinocytes in the medium were collected, attached cells were trypsinized and washed, and both cell populations were incubated with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide in binding buffer at concentrations suggested by the manufacturer (Medical and Biological Laboratories) on ice in the dark for 10 min. Stained cells were analyzed on a FACSort (Beckton Dickinson).
PI3K activity assay.
PI3K activity was measured in the antiphosphotyrosine immunoprecipitates (Upstate Biotechnology, Inc.) by in vitro phosphorylation of PI as previously described (28).
MAPK activity assay.
The MAPK activity assay was carried out on proliferating keratinocytes as described in the manufacturer's protocol for the nonradioactive MAPK activity kit (Upstate Biotechnology, Inc.).
RESULTS
Lack of IGF-1R expression disturbs skin formation and organization in a 3D organotypic coculture model.
In the present study, we were interested in elucidating the role of IGF-1R in skin. Since the skin of IGF-1R-null pups is very thin and delicate (21) and the cells isolated from these mice demonstrate decreased growth rate, it is extremely difficult to isolate, culture, grow, and study their primary keratinocytes. Furthermore, it is impossible to follow the effects of IGF-1R on skin development and maturation in vivo, since the IGF-1R-null pups die at birth.
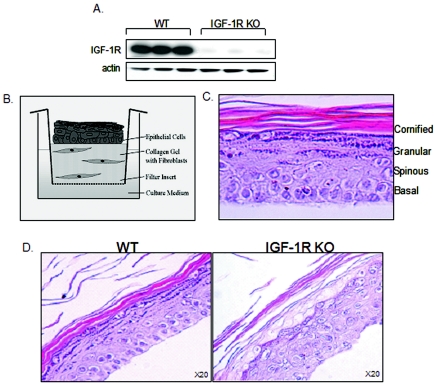
To overcome these technical difficulties and to obtain primary keratinocytes lacking IGF-1R expression, we used cells isolated from IGF-1R flox/flox mice. In these mice, the IGF-1R gene is flanked by lox sequences (15). Thus, infecting primary cultured cells isolated from these mice with an adenoviral construct containing the Cre-recombinase gene (adeno-Cre) results in inactivation of the IGF-1R protein in vitro. Cultures containing the floxed allele of IGF-1R and infected with viral vector lacking the Cre gene served as controls. The success of the inactivation was evaluated by Western blot analysis. As can be seen in Fig. 1A, proliferating keratinocytes infected with the adeno-Cre construct do not express IGF-1R protein.
FIG. 1.
Lack of IGF-1R expression disturbs skin formation and organization in a 3D organotypic coculture model. (A) Keratinocytes isolated from mice containing the floxed allele of IGF-1R (IGF-1R flox/flox) were maintained in culture for 4 days until reaching confluence. Afterwards, cells were infected with an adenovirus-Cre construct as detailed in Materials and Methods (IGF-1R KO). After 1 h, the viral supernatant was replaced with fresh culture medium. Control cells were infected with an empty viral vector (WT). Total protein lysates were analyzed by Western blotting, using antibodies against the murine IGF-1R. Actin levels are shown, demonstrating equal loading. Evaluation of IGF-1R expression was carried out routinely in all experiments to confirm IGF-1R inactivation. (B) 3D organotypic skin cocultures were established as detailed in Materials and Methods, leading to the formation of a skin-like structure, as depicted in the scheme. (C) Cocultures were maintained for 14 days, after which they were fixed, paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E). A typical H&E-stained section of the 3D coculture is shown at high (40×) magnification. Layers of epidermal compartments, as occur in vivo, are shown. (D) Skin fibroblasts and keratinocytes were isolated from mice containing the floxed allele of IGF-1R (IGF-1R flox/flox) and infected with the adenovirus-Cre construct as detailed in Materials and Methods. Cells infected with the empty viral vector served as controls (WT). 3D organotypic skin cocultures were established, maintained, and processed as detailed in Materials and Methods. A representative H&E-stained section is shown at high (20×) magnification.
These genetically manipulated cells, lacking IGF-1R, were further assembled into an in vitro 3D organotypic skin coculture. In this model, epidermal skin keratinocytes are plated on top of a dermal equivalent, which is composed of a fibroblast-containing collagen gel. Within a couple of weeks, the cells reorganize into a skin-equivalent structure, obtaining the histological characteristics of skin in vivo, including formation of the different epidermal layers (Fig. 1B and C).
By using this combination of models, we have found that lack of IGF-1R has devastating effects on skin organization. As can be seen in Fig. 1D, the IGF-1R-null skin cocultures appeared to be completely disorganized, with abnormally disordered layers compared to the control WT organotypic coculture.
Decreased proliferation and increased apoptotic rate of the IGF-1R-null organotypic skin coculture.
The abnormal formation of the 3D skin cocultures lacking IGF-1R suggested the possible deregulation of the cellular proliferation-apoptosis-differentiation balance. To investigate whether a lack of IGF-1R in 3D skin cocultures influences proliferation rate, we examined the expression of PCNA, an auxiliary factor for DNA polymerase δ required for DNA synthesis and an index of cell proliferation. As can be seen in Fig. 2A, positively PCNA-stained nuclei were localized in the basal compartment of the control 3D coculture, as occurs in skin in vivo. In contrast, lack of IGF-1R disturbed the architecture of the formed skin, which was associated with decreased proliferation in the organized cocultures.
FIG. 2.
Decreased proliferation and increased apoptotic rate of the IGF-1R-null organotypic skin coculture. IGF-1R-null (IGF-1R KO) and control (WT) 3D organotypic skin cocultures were established and maintained as detailed in Materials and Methods. (A) Positive PCNA-stained nuclei were detected by immunohistochemistry as described in the text. Photographs of high (40×) magnification are presented. (B) TUNEL staining was detected by immunofluorescence (green [FITC], apoptotic cells; blue [4′,6′-diamidino-2-phenylindole], nuclear counterstaining). For the positive control, normal mice were treated with UVB light and sacrificed 48 h later (b). (a) Nonirradiated mice. (c) Organotypic cocultures of the control (WT); (d) IGF-1R-null cocultures (IGF-1R KO). Percent cell death, determined by tabulating FITC-stained cells, appears in the lower corner (P < 0.05 for WT versus IGF-1R KO). The images demonstrate only the epidermal layer and are representative of results reproduced three to five times.
Our next step was to examine the possibility that the decrease in the proliferation rate observed in the IGF-1R-null cocultures could also result from decreased viability. We followed the effect of the IGF-1R inactivation on the apoptotic rate of skin keratinocytes in the organized skin organotypic cocultures. Apoptotic cells were detected by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. As can be seen in Fig. 2B, a dramatic increase in the apoptotic rate was detected in the epidermis of IGF-1R-null organotypic cocultures, with approximately 30% TUNEL-positive nuclei compared with 3 to 5% in the control cocultures (Fig. 2Bc and d).
Abnormal differentiation pattern of the IGF-1R-null organotypic skin coculture.
Subsequently, we followed the effects of IGF-1R inactivation on the progress of skin differentiation in the organizing cocultures by following the expression of defined markers of skin differentiation. Most of these markers are cytoskeleton proteins whose expression changes during differentiation as detailed herein. Normally, the proliferating cells of the basal layer of the skin express keratins 5 and 14. The induction of differentiation, associated with the upward movement of the cells to the spinous compartment, is accompanied by induction of the expression of keratins 1 and 10. Terminal differentiation, occurring in the granular compartment, is characterized by flattening of cells, enucleation, and finally cell death leading to sloughing of the cells off the skin surface. This process is associated with the induced expression of loricrin, filaggrin, and other proteins (11, 23).
As can be seen in Fig. 3A, lack of IGF-1R in the 3D coculture skin led to a facilitated process of enucleation compared to the control 3D coculture, suggesting an acceleration of the differentiation process. The induction of the expression of differentiation markers in the IGF-1R-null cocultures was abnormal as well (Fig. 3B). The expression of K14, the basal layer marker, was disturbed, and ectopic expression was observed in cells out of the basal layer. The expression pattern of K1, the early differentiation marker, was also abnormal, appearing somehow to our surprise only in the upper layers, close to the skin surface. In contrast, the expression pattern of loricrin, the terminal differentiation marker, was similar to the control, appearing in the uppermost layer of the organized skin. Interestingly, in the IGF-1R-null cocultures, we observed the expression of K6, which is usually not observed in the normal epidermis. K6 is a general marker of several pathological skin conditions (25).
FIG. 3.
Abnormal differentiation pattern of the IGF-1R-null organotypic skin coculture. IGF-1R-null (IGF-1R KO) and control (WT) 3D organotypic skin cocultures were established and maintained as detailed in Materials and Methods. (A) Cocultures were maintained for 14 days, after which they were fixed, paraffin embedded, sectioned, and stained with hematoxylin (upper panels) or stained to demonstrate cell nuclei (lower panels). The ×20 magnification photographs are from a representative experiment. (B) K14, K1, loricrin, and K6 were detected by immunohistochemistry. The high-magnification (×40) photographs are from a representative experiment.
Lack of IGF-1R expression is associated with decreased proliferation and increased apoptotic rate of primary keratinocytes in vitro.
The advantage of the 3D organotypic coculture is that it enables us to follow the 3D organization of skin, as well as to investigate the interactions between the epidermal keratinocytes and dermal fibroblasts. However, to identify the pathophysiological mechanism leading to the abnormalities in skin organization and to further characterize the acceleration on the differentiation process of the IGF-1R-null skin cocultures, we turned to another model system, primary cells in culture.
Initially, we evaluated the effects of IGF-1R inactivation, using the previously described Cre-lox model, on primary skin cell proliferation in vitro. The proliferation rate of primary keratinocytes was estimated by cell counting on the day of infection and 24, 48, and 72 h postinfection. As can be seen in Fig. 4A, there was a significant decrease in cell proliferation rate of the IGF-1R-null cells compared to the WT control. This decrease was observed as early as 24 h postinfection and decreased even further 48 and 72 h after the infection.
FIG. 4.
Lack of IGF-1R expression is associated with decreased proliferation and increased apoptotic rate of primary keratinocytes in vitro. Proliferating IGF-1R flox/flox keratinocytes were grown and maintained as described in the legend for Fig. 1. IGF-1R inactivation was carried as described (IGF-1R KO). Control cells were infected with an empty viral vector (WT). (A) On the day of infection, 24, 48, and 72 h postinfection, live cells were counted; dead cells were excluded by trypan blue staining. (B) The incorporation of BrdU into dividing cells was detected with anti-BrdU antibody as described in Materials and Methods. Cells incorporating BrdU were detected by immunochemistry, as their nucleus acquired a brown color. Photographs of representative fields are shown. Original magnification, ×20. Positively stained nuclei are marked with arrows. (C) The percentage of positively BrdU-stained cells per total number of cells was counted in several fields, arbitrarily chosen, of the WT and IGF-1R KO cultures. *, P < 0.05 for WT versus IGF-1R KO. (D) Primary cultured WT and IGF-1R KO keratinocytes were cultured for 48 h. Apoptotic cells were detected by FACS analysis as described in Materials and Methods using annexin V. The annexin V-positive cell fraction is presented. *, P < 0.05 for WT versus IGF-1R KO. The plot represents an average of three separate experiments.
To confirm these results, we studied cellular proliferation of the primary IGF-1R-null and WT cultures by following the incorporation of BrdU into dividing cells. Figures 4B and C demonstrate a similar decrease to that measured by cell counting in the number of dividing cells in the IGF-1R-null culture compared to control WT cells.
In order to support our results on the role of IGF-1R in skin apoptosis, primary cultured proliferating keratinocytes were examined using flow cytometric analysis of annexin V staining. As can be seen in Fig. 4D, the percentage of FITC-annexin V-positive cells in the IGF-1R null culture was about two times higher than in the control WT culture. Thus, we can conclude that the decrease in cell number in the IGF-1R-null cells results from both decreased proliferation and facilitated cell death.
Lack of IGF-1R expression facilitates the differentiation process of primary keratinocytes in vitro.
We then examined the effects of IGF-1R inactivation on the differentiation of primary cultured keratinocytes in vitro. It is well known that differentiation of primary cultured skin keratinocytes can be induced by increasing Ca2+ levels in the growth medium (44). Primary murine keratinocytes cultured in medium with low Ca2+ levels (0.05 mM) retain a proliferative basal cell phenotype. Elevation of Ca2+ concentrations up to 0.14 mM induces a rapid sequence of events leading to the termination of cell proliferation and the induction of differentiation. Further elevating the Ca2+ concentration to 1.0 mM leads to terminal differentiation. This differentiation of Ca2+-induced keratinocytes in vitro closely resembles the differentiation program occurring in vivo and, similarly, can be evaluated by following the expression of skin differentiation markers, such as K14, a marker for proliferating basal cells, K1, a marker for induction of differentiation, and loricrin, a marker for final differentiation. Using these markers, we followed the effects of IGF-1R inactivation on keratinocyte differentiation in vitro. The most striking finding was that lack of IGF-1R leads to deregulation and acceleration of the differentiation process. As can be seen in Fig. 5A and B, in the WT cells 12 h after the induction of differentiation, K1 was elevated as expected. In contrast, in the IGF-1R-null cells, the expression of K1 had already decreased, while loricrin was already clearly expressed. Later still, 48 h after the induction of differentiation, while loricrin expression was induced in the WT cells, its expression in the IGF-1R-null cells was far more advanced (Fig. 5C and D). Expression of K14, the marker for proliferating keratinocytes, did not change during this process. These results support the IGF-1R-null skin cocultures findings. Therefore, we suggest that IGF-1R acts as an inhibitory factor in the regulation of normal differentiation of epidermal keratinocytes and that removal of its inhibitory effect leads to precocious differentiation.
FIG. 5.
Lack of IGF-1R expression facilitates the differentiation process of primary keratinocytes in vitro. Proliferating IGF-1R flox/flox keratinocytes were grown and maintained as described in the legend for Fig. 1. IGF-1R inactivation was carried as described there. Cells were induced to differentiate by increasing the Ca2+ concentration from 0.05 mM (low [L]) to 0.12 mM (medium [M]), or 1.0 mM (high [H]). Cells were further maintained for 12 (A and B) or 48 (C and D) h. The induction of differentiation markers was identified by Western blot analysis, using antibodies against K14, K1, and loricrin. Actin levels are shown and demonstrate equal loading. Results are representative of six independent experiments. Samples of each independent experiment were run on the same gel and blotted together. The blots of loricrin and K1 were further analyzed by scanning densitometry (B and D). Results are a summary (means ± standard errors) of three separate experiments and are expressed in arbitrary units. *, P < 0.01; **, P < 0.001 for WT versus IGF-1R KO, 48 h.
Differential effects of IRS-1 and IRS-2 proteins on the differentiation process in IGF-1R-null cells.
The specific downstream signals that trigger the observed effects of lack of IGF-1R on keratinocyte differentiation remain unknown. To identify the exact role of proteins of the IGF-1 signaling pathway in this process, we manipulated their expression level or activity. Initially, we studied the expression and phosphorylation of the closely related insulin receptor (IR). However, no compensatory changes were observed in the expression or in the phosphorylation state of the IR in response to IGF-1R inactivation (data not shown).
Next, we followed the involvement of the IRS proteins, the first downstream mediators of IGF-1R. To follow the ability of IRS-1 or IRS-2 to compensate for lack of IGF-1R and prevent the observed precocious differentiation, we overexpressed these proteins in the IGF-1R-null keratinocytes, using an adenoviral construct of WT IRS-1 or IRS-2 proteins (Fig. 6A). IRS-2 overexpression in the IGF-1R-null cells prevented the abnormal elevation in loricrin expression, decreasing it to levels similar to those measured in the control cells (Fig. 6D and E). In contrast, IRS-1 overexpression had no such effect and the IGF-1R-null cells overexpressing IRS-1 exhibited pathologically elevated loricrin levels, similar to the original IGF-1R-null cells (Fig. 6B and C). No significant effects of IRS-1 or IRS-2 overexpression were observed on the expression levels of K1 in the treated IGF-1R null cells compared to the untreated ones (Fig. 6B to E).
FIG. 6.
Differential effects of IRS-1 and IRS-2 proteins on the differentiation process in IGF-1R-null cells. (A) Cells were infected with an adenoviral IRS-1 or IRS-2 construct. Control cells were infected with a luciferase-containing adenoviral construct (Luc). Cell lysates were analyzed by Western blotting with anti-IRS-1 and anti-IRS-2 antibodies, as indicated. IGF-1R inactivation (IGF-1R KO) was carried out as described in the legend for Fig. 1. Control cells were infected with an empty viral vector (WT); at 16 h postinfection, keratinocytes were infected a second time, with an adenoviral IRS-1 (B) or IRS-2 (D) construct. Control cells were infected on both occasions with a luciferase-containing adenoviral construct (Luc); 6 h after the second infection, the cells were induced to differentiate for 48 h as described in the legend for Fig. 5, and analysis of differentiation marker expression was carried out as described there. Actin levels are shown, demonstrating equal loading. Results are representative of three independent experiments. Samples of each independent experiment were run on the same gel and blotted together. The blots of loricrin and K1 were further analyzed by scanning densitometry (C and E). Results are a summary (means ± standard errors) of three separate experiments and are expressed in arbitrary units. **, P < 0.01 for IGF-1R KO luciferase infected versus IGF-1R KO infected by adenoviral IRS-2 construct.
The PI3K/Akt signaling pathway inhibits skin keratinocyte differentiation.
In vitro studies have delineated the PI3K pathway as associated with cellular differentiation mediated by IGF-1 signaling in various tissues (26, 32, 42). Furthermore, the PI3K signaling pathway has been recently shown to inhibit the early phase of differentiation of human skin keratinocytes (30). On the basis of these results, we hypothesized that IGF-1R exerts its effect downstream of the IRS-2 pathway via PI3K.
Interestingly, as can be seen in Fig. 7A, lack of IGF-1R was associated with a decrease in total PI3K activity. Furthermore, a decrease was observed in the activity of one of the downstream targets of PI3K, Akt, in response to IGF-1 (Fig. 7B). These results may imply that decreased PI3K activity is part of the process leading to abnormalities in the differentiation process in IGF-1R-null cells. PI3K is composed of two subunits, the regulatory p85 subunit and the catalytic p110 subunit. Thus, the possible active role of PI3K in the abnormal differentiation of IGF-1R-null cells was examined by manipulating its expression, by either blocking or overactivating these PI3K subunits as follows.
FIG. 7.
The PI3K/Akt signaling pathway inhibits skin keratinocyte differentiation. Cells (A and B) were stimulated with IGF-1 (10−7 M) for 3 min. Cells lysates were immunoprecipitated with antiphosphotyrosine antibody and used for an in vitro PI3 kinase assay (A) or were analyzed by Western blotting with anti-phospho-Akt and anti-Akt antibodies (B). Results are representative of three independent experiments. PIP, inositol triphosphate; ORI, origin. (C) Proliferating keratinocytes were infected with DN PI3K, CA PI3K, or luciferase as a control vector (Luc). Thereafter, cells were induced to differentiate for 48 h as already described and analyzed by Western blotting with anti-phospho-Akt and anti-Akt antibodies. (D and F) IGF-1R inactivation was carried as described in the legend for Fig. 1. Proliferating keratinocytes were then infected with DN PI3K (D), CA PI3K (F), or luciferase (Luc) constructs. Cells were induced to differentiate for 48 h as detailed above. Analysis of differentiation marker expression was carried as described in the legend for Fig. 5. Actin levels are shown and demonstrate equal loading. Results are representative of three independent experiments. Samples of each independent experiment were run on the same gel and blotted together. The blots of loricrin and K1 were further analyzed by scanning densitometry (E and G). Results are a summary (means ± standard errors) of three separate experiments and are expressed in arbitrary units. Symbols in panel E: **, P < 0.01 for WT (L and M) luciferase infected versus WT infected by adenoviral DN PI3K construct; *, P < 0.05 for WT (H) luciferase infected versus WT (H) infected by adenoviral DN PI3K construct. Symbols in panel G: **, P < 0.001 for WT (M and H) luciferase infected versus WT infected by adenoviral CA PI3K construct and IGF-1R KO (H) luciferase infected versus IGF-1R KO (H) infected by adenoviral CA PI3K construct.
We infected normal murine keratinocytes with either a DN form of p85 PI3K (AxCAΔp85), which should inhibit PI3K activity, or with a constitutively active (CA) form of the catalytic p110 PI3K subunit (AxCAMyr-p110), which should increase the total cellular PI3K activity level. As can be seen, 24 h postinfection, the DN form of PI3K almost completely inhibited Akt activity, as expected. In contrast, the CA form of PI3K enhanced Akt activity (Fig. 7C).
Next, control cells and cells lacking IGF-1R expression were infected with the DN and CA forms of PI3K and were then induced to differentiate by elevating the Ca2+ concentration as mentioned earlier. We followed the progress of the differentiation process as already described, by analyzing the expression of the skin differentiation markers loricrin, K1, and K14. PI3K inhibition led to a marked increase in K1 in WT cells, with no significant effect on IGF-1R-null cells (Fig. 7D and E). Furthermore, PI3K inhibition had no effect on loricrin induction, in either the WT or IGF-1R-null cells. These results support Sayama's (30) findings on the role of PI3K in the early stages of differentiation. However, these results also indicate that PI3K is not the sole mediator of the IGF-1R differentiation signal, since the outcome of PI3K inhibition was different from that of IGF-1R inactivation. Furthermore, there seemed to be no additive effects when PI3K was inhibited in the IGF-1R-null cells.
Next, we increased the activity of PI3K by expressing the CA mutant form of the p110 catalytic subunit. To our surprise, overactivation of PI3K overcame the pathological effects of IGF-1R inactivation on the differentiation process. While there was a slight decrease in K1 expression in IGF-1R-null cells, there was a marked decrease in loricrin expression in the IGF-1R-null cells treated with the CA mutant (Fig. 7F and G). This decrease was observed in the infected WT cells as well. Based on these findings, it can be suggested that PI3K plays an important inhibitory role in the various differentiation stages of skin keratinocytes, that it partially regulates the differentiation in parallel with IGF-1R, and that it can partially compensate for IGF-1R inactivation. However, the PI3K pathway does not exclusively mediate IGF-1R signaling in skin differentiation.
Inhibitory role of the MAPK pathway during the differentiation process of skin keratinocytes.
The other main cellular signaling pathway mediating the IGF-1R signal is the ERK1/2 MAPK pathway. To assess the impact of ERK1/2 in mediating the IGF-1R signal, we first determined IGF-1-induced ERK1/2 activation and phosphorylation in WT and IGF-1R-null keratinocytes. As can be seen in Fig. 8A and B, IGF-1 stimulation led to activation of ERK1/2. Furthermore, there was a decrease in the activation and phosphorylation of ERK1/2 in basal as well as IGF-1-stimulated keratinocytes lacking IGF-1R relative to the control (Fig. 8A and B). Next, we investigated the effects of inhibiting the ERK1/2 pathway on the precocious differentiation of IGF-1R-null cells. Keratinocytes lacking IGF-1R expression and WT cells were treated with the ERK1/2 inhibitor U0126 throughout the experiment while being induced to differentiate by increasing Ca2+ concentrations (Fig. 8C and D). U0126 treatment resulted in a significant increase in the expression of the differentiation markers of early and late differentiation, K1 and loricrin, respectively, in both WT and IGF-1R-null cells. Thus, it seems that ERK1/2 has an inhibitory role in mediating cellular differentiation signals throughout, from the early to late differentiation stages, and is a possible protein candidate for the partial mediation of downstream signals of IGF-1R on skin differentiation. However, inhibition of ERK1/2 only partially reversed the effects of IGF-1R inactivation, suggesting that other parallel pathways are involved in mediating this inhibitory signal.
FIG. 8.
Inhibitory role of the MAPK pathway during the differentiation process of skin keratinocytes. Proliferating skin keratinocytes were stimulated with IGF-1 (10−7 M) for 3 min. Cells lysates were immunoprecipitated with anti-phospho myelin basic protein (MBP) antibody and used in an in vitro MAPK activity assay (A) or analyzed by Western blotting with anti-phospho-ERK1/2 or anti-ERK1/2 antibodies (B). (C) IGF-1R inactivation was carried as described in the legend for Fig. 1. Cells were then treated with U0126 (20 μM) and induced to differentiate for 48 h as already detailed. Analysis of differentiation marker expression was carried out as described in the legend for Fig. 5. Actin levels are shown and demonstrate equal loading. Results are representative of three independent experiments. Samples of each independent experiment were run on the same gel and blotted together. The blots of loricrin and K1 were further analyzed by scanning densitometry (D). Results are a summary (means ± standard errors) of three separate experiments and are expressed in arbitrary units. **, P < 0.02 for WT (H) DMSO treated versus WT (H) treated with U0126; *, P < 0.05 for WT (M) DMSO treated versus WT (M) treated with U0126; *, P < 0.05 for IGF-1R KO DMSO treated (M and H) versus IGF-1R KO (M and H) treated with U0126.
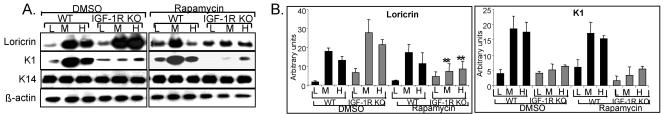
mTOR is a specific mediator of IGF-1R action during keratinocyte differentiation.
Another signaling molecule that we investigated was mTOR, a key downstream mediator of cell growth and survival (27) and essential for the differentiation process in multiple cell types. Thus, we investigated the potential involvement of mTOR in mediating IGF-1R signals. IGF-1R-null and WT keratinocytes were treated with rapamycin, and the expression of the differentiation markers K14, K1, and loricrin was examined. As shown in Fig. 9A and B, mTOR inhibition completely abolished the abnormally increased expression of loricrin in the IGF-1R-null cells, normalizing its expression levels to that of the control cells. In contrast, mTOR inhibition had no effect on K1 decreased expression in the IGF-1R null cells. Interestingly, rapamycin treatment had no significant effect on the expression of the differentiation markers in the WT cells, thus demonstrating the specific active role of mTOR in mediating the effects of IGF-1R on the late differentiation process of skin keratinocytes.
FIG. 9.
mTOR is a specific mediator of IGF-1R action during keratinocyte differentiation. (A) IGF-1R inactivation was carried out as described as described in the legend for Fig. 1. Thereafter, cells were treated with rapamycin (20 nM) and induced to differentiate for 48 h as detailed in the text. Analysis of differentiation marker expression was carried as described in the legend for Fig. 5. Actin levels are shown and demonstrate equal loading. Samples of each independent experiment were run on the same gel and blotted together. The blots of loricrin and K1 were further analyzed by scanning densitometry (B). Results are a summary (means ± standard errors) of three separate experiments and are expressed in arbitrary units. **, P < 0.01 for IGF-1R KO (M and H) DMSO treated versus (M and H) IGF-1R KO treated with rapamycin.
DISCUSSION
Proliferation-differentiation regulation of normal epithelial cells is a complicated and highly dynamic process, triggered by a variety of growth factors. Herein we present a new look at the role of IGF-1R in maintaining those processes in skin. Until now, it was generally accepted that IGF-1R possesses an activating role in the skin proliferation process and, by extension, that its role in the differentiation process was passive. However, in the present study we show that in the epidermis, IGF-1R is a fully active participant in both processes. While its presence is necessary to activate the normal proliferation of skin keratinocytes, at the same time it actively inhibits the differentiation process of those cells: ablation of IGF-1R expression releases the inhibition, resulting in pathologically facilitated differentiation. These findings are in agreement with our previous work, where we showed that Ca2+-induced differentiation of cultured murine keratinocytes leads to decreased IGF-1R signaling (38).
An additional conclusion from our studies is that each of the stages of skin differentiation is regulated by an individual set of signals and that it is not enough just to trigger the differentiation process, for example, by increasing the Ca2+ concentration, and then expect the process to continue to completion. Thus, it seems that the progression from early to late stages of differentiation is regulated as well. This fact has been suggested and is supported by other investigators. For example, PI3K has been suggested as a regulator of the early differentiation of skin keratinocytes (30), epidermal growth factor receptor activation promotes the terminal differentiation of suprabasal epidermal keratinocytes (36), and the activation of certain protein kinase Cs is required for the expression of granular differentiation markers (6).
What is the functional meaning of the inhibitory role of IGF-1R in the skin epidermal differentiation process? One possibility is that the potential counter-play between IGF-1R and the IR determines the balance of keratinocyte proliferation and differentiation. Both receptors are highly homologous, can sometimes even compensate for one another, and presumably coordinate the regulation of their functions by similar cellular processes (1). At the same time, their signals can and do lead to different outcomes. We have been investigating this question for the last few years and have demonstrated that during the Ca2+-induced differentiation of skin cells IGF-1R and IR may exhibit different mechanisms of action (38): both insulin and IGF-1 lead to increased proliferation, but insulin supports the differentiation process whereas IGF-1 inhibits it. Furthermore, IR-null keratinocytes show decreased proliferation and abnormally decreased differentiation. This regulation is opposite to that of IGF-1R, as we demonstrate in the present study. Therefore, these results suggest a possible tight regulation by the actions of IR and IGF-1R on the epidermal proliferation-differentiation equilibrium.
Based on these results, we expected that in the IGF-1R-null organotypic skin cocultures, there would be acceleration in the differentiation process. Indeed, the enucleation process of the IGF-1R-null skin cocultures was facilitated and occurred in earlier layers compared to the control coculture skin. These results were further corroborated by the in vitro studies of primary IGF-1R-null keratinocytes, where a facilitated induction of differentiation markers was observed.
The IGF-1R-deficient skin organotypic tissues also showed disruption in the expression of differentiation markers. K14 expression was limited to the basal proliferative layer in control skin, whereas in the IGF-1R-deficient cocultures, K14-positive cells were present in a random pattern. On the other hand, the late differentiation markers appeared in higher and later layers compared to their expression profile in the control organotypic cocultures. These findings seem to be somehow in contrast to the in vitro studies of the primary IGF-1R-null keratinocytes, where a facilitated expression of the differentiation markers was observed. However, the most likely explanation to this theoretical discrepancy could be that the suprabasal K14 observed in the organotypic IGF-1R skin is not a result of delayed differentiation but actually results from enhanced differentiation and rapid turnover of the basal layer, such that protein degradation lags behind. This would also explain why expression of the late differentiation markers was only found in the upper layer of the organotypic skin: cells progress to the outer layers of the epidermis too rapidly for induction of the expression of the differentiation markers to keep up. This pattern of abnormal in vivo delay in expression of differentiation markers is similar to that described for the keratinization process in psoriasis. In the latter, skin differentiation is facilitated, but expression of the late differentiation markers appears only in the uppermost layer of the skin, because they do not have enough time to be expressed. The maturation of those cells in vivo involves their rapid migration from the basal layer to the skin surface, while keratin expression follows (16). Further studies are required to elucidate the possible regulation of cell migration separately from skin cell differentiation. In cultured cells, on the other hand, the growth conditions are completely different, differentiation is induced by abrupt elevations in Ca2+ concentration, and the cells are further maintained in the presence of this milieu. Under these conditions, terminal differentiation of the IGF-1R-null cells can be followed and maintained, enabling us to detect the abnormally facilitated differentiation.
A difference was also noticed between the IGF-1R-null mouse skin and the IGF-1R-null organotypic skin coculture. The IGF-1R-null skin was much thinner in comparison to the skin of the wild-type littermates (2). In contrast, the IGF-1R-null organotypic skin appears thicker than the control organotypic skin. However, one should keep in mind that many of the aspects of in vivo embryonic development which contribute to the histological structure of skin do not occur in the organization process of the skin organotypic coculture. These aspects include early stages of organogenesis as well as stem cell development. It is plausible that IGF-1R plays a role in the regulation of these early stages of skin development, leading to a more pronounced histopathological appearance of the IGF-1R skin in vivo in comparison to that of the organotypic skin.
The downstream signals leading to keratinocyte differentiation remain poorly understood. In the present paper, we have solved another piece of this puzzle. We demonstrate the importance of some of the known downstream signaling elements in mediating the inhibitory effects of IGF-1R. We show that overexpressing IRS-2 can compensate for a lack of IGF-1R, whereas overexpressing IRS-1 cannot. We also demonstrate that PI3K, ERK1/2, and mTOR take part in mediating the inhibitory signal of IGF-1R on differentiation.
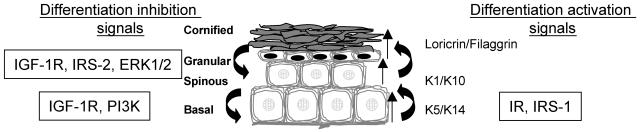
Based on our findings, as well as on previous publications by other investigators, we designed a provisional model which attempts to incorporate the proteins involved in insulin or IGF-1 signaling. It should be emphasized that we did not include proteins from other signaling pathways. In addition, other proteins, such as those of the protein kinase C family, were not included, even though they are part of insulin and IGF-1 signaling; this is because there is so much information regarding these proteins and their roles in skin proliferation and differentiation that they deserve a separate review. This provisional model is shown in Fig. 9. Here, we briefly summarize the information reported by us and other investigators on the role of each of these proteins in the regulation of skin proliferation and differentiation.
IR.
Previously, we found that the induction of keratinocyte differentiation is associated with an increase in IR phosphorylation (38). Furthermore, lack of IR expression resulted in abnormal differentiation of cultured murine skin keratinocytes, as demonstrated by a decrease in the expression of early skin differentiation markers (37). Thus, we suggest that IR activates and supports the initiation of the differentiation process in keratinocytes.
The IRS family of proteins.
The role of the IRS proteins as the main mediators of IGF-1/insulin signaling in differentiation has been shown in a variety of cells (8, 24, 34). In unpublished results from our lab, we found a defect in the induction of early differentiation markers in IRS-1-null cells, similar to the abnormality found in the IR-null cells. Thus, we assume that the initiation of keratinocyte differentiation is mediated by IR, via the IRS-1 protein. On the other hand, the IRS-2 protein plays a different, sometimes contradictory role. From our previous studies on IRS-2-null keratinocytes and from the studies performed on other cell types derived from IRS-2 knockout mice (9, 19, 24, 35, 41), it was found that cells lacking IRS-2 do not exhibit any differentiation deficiencies but have more of a metabolic defect, expressed by impaired glucose uptake (28). However, herein we show that IRS-2 overexpression significantly decreases the differentiation rate of cells lacking IGF-1R. Thus, we suggest that IRS-2 plays an inhibitory role in the IGF-1R-mediated regulation of the late stages of skin differentiation.
PI3K.
PI3K has been recognized as a negative regulator of the early stage of skin differentiation (30). Consistent with these findings, we show here that PI3K activity appears to be necessary for the differentiation process in keratinocytes, that a lack of IGF-1R is associated with decreased PI3K activity, and that overactivity of PI3K inhibits differentiation of WT and IGF-1R-null cells. However, whether PI3K mediates the downstream inhibitory signal initiated by IGF-1R, as a sole mediator or in cooperation with other signaling molecules, remains unclear. In contrast to the results reported by us and others, a recent study has claimed that the PI3K pathway activated early stages of keratinocyte differentiation (4). The different conclusion could be due to differences in the method and length of inhibition as well as the different Ca2+ concentration used.
ERK1/2.
Inhibition of ERK1/2 led to increased differentiation of keratinocytes throughout all stages, in both WT and IGF-1R-null keratinocytes. This is consistent with other studies (14, 17, 31). Thus, we can conclude that ERK1/2 is takes part in the regulation of skin differentiation; however, it does not necessarily mediate directly the IGF-1R effects on differentiation. Here again, additional studies are required to identify its direct involvement in mediating the IGF-1R-inhibitory signal on keratinocyte differentiation.
mTOR.
In recent years, evidence has emerged that the activation of mTOR is essential for differentiation of a variety of cell types (27). However, its role in skin keratinocytes has not yet been established. In the present study, we demonstrate that inhibition of mTOR by rapamycin prevents the precocious differentiation occurring in IGF-1R-null cells, normalizing its level to that of normal control cells, and thus suggesting a specific role of mTOR in the IGF-1R regulation of skin differentiation. At the same time, mTOR inhibition has no effect on differentiation in WT cells and, thus, we did not include it in our model as an important modulator of skin keratinocyte differentiation. Our findings demonstrate, for the first time, the direct involvement of mTOR in IGF-1R-regulated skin keratinocyte differentiation.
In summary, our results suggest a vital function for IGF-1R signaling in epidermal skin development and maintenance. IGF-1R and the downstream signaling molecules actively inhibit epidermal differentiation during its multiple stages, as summarized in the model shown in Fig. 10. These findings shed new light on the regulation of normal skin physiology and may pave the way to the development of innovative treatment modalities for skin diseases that involve altered epidermal differentiation patterns.
FIG. 10.
Schematic model of the roles of IGF-1R and downstream molecules in epidermal differentiation.
Acknowledgments
This work was supported by a Minerva Short-Term Research Grant, an NIH/Tel Aviv University Graduate Partnership Grant, and by grants from the D-CURE Israel foundation, the Russell Berrie Foundation, and the Israeli Ministry of Health. This work was also supported by the Cooperation Program in Cancer Research of the Deutches Krebsforschungzentrum and Israel’s Ministry of Science and Technology.
REFERENCES
- 1.Accili, D., J. Nakae, J. J. Kim, B. C. Park, and K. I. Rother. 1999. Targeted gene mutations define the roles of insulin and IGF-I receptors in mouse embryonic development. J. Pediatr. Endocrinol. Metab. 12:475-485. [DOI] [PubMed] [Google Scholar]
- 2.Baker, J., J. P. Liu, E. J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73-82. [PubMed] [Google Scholar]
- 3.Baserga, R., A. Hongo, M. Rubini, M. Prisco, and B. Valentinis. 1997. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim. Biophys. Acta 1332:F105-F126. [DOI] [PubMed] [Google Scholar]
- 4.Calautti, E., J. Li, S. Saoncella, J. L. Brissette, and P. F. Goetinck. 2005. Phosphoinositide 3-kinase signaling to AKT promotes keratinocyte differentiation versus death. J. Biol. Chem. 280:32856-32865. [DOI] [PubMed] [Google Scholar]
- 5.De Meyts, P., J. Palsgaard, W. Sajid, A. M. Theede, and H. Aladdin. 2004. Structural biology of insulin and IGF-1 receptors. Novartis Found. Symp. 262:160-171. [PubMed] [Google Scholar]
- 6.Denning, M. F., A. A. Dlugosz, C. Cheng, P. J. Dempsey, R. J. Coffey, Jr., D. W. Threadgill, T. Magnuson, and S. H. Yuspa. 2000. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp. Dermatol. 9:192-199. [DOI] [PubMed] [Google Scholar]
- 7.DiGiovanni, J., D. K. Bol, E. Wilker, L. Beltran, S. Carbajal, S. Moats, A. Ramirez, J. Jorcano, and K. Kiguchi. 2000. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 60:1561-1570. [PubMed] [Google Scholar]
- 8.Fasshauer, M., J. Klein, K. M. Kriauciunas, K. Ueki, M. Benito, and C. R. Kahn. 2001. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol. Cell. Biol. 21:319-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasshauer, M., J. Klein, K. Ueki, K. M. Kriauciunas, M. Benito, M. F. White, and C. R. Kahn. 2000. Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J. Biol. Chem. 275:25494-25501. [DOI] [PubMed] [Google Scholar]
- 10.Foulstone, E., S. Prince, O. Zaccheo, J. L. Burns, J. Harper, C. Jacobs, D. Church, and A. B. Hassan. 2005. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J. Pathol. 205:145-153. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs, E., and S. Raghavan. 2002. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 3:199-209. [DOI] [PubMed] [Google Scholar]
- 12.Fusenig, N. E., R. T. Dzarlieva-Petrusevska, and D. Breitkreutz. 1985. Phenotypic and cytogenetic characteristics of different stages during spontaneous transformation of mouse keratinocytes in vitro. Carcinog. Compr. Surv. 9:293-326. [PubMed] [Google Scholar]
- 13.Fusenig, N. E., A. Limat, H. J. Stark, and D. Breitkreutz. 1994. Modulation of the differentiated phenotype of keratinocytes of the hair follicle and from epidermis. J. Dermatol. Sci. 7(Suppl.):S142-S151. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs, R. M., V. Silva-Vargas, R. Groves, and F. M. Watt. 2004. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J. Investig. Dermatol. 123:503-515. [DOI] [PubMed] [Google Scholar]
- 15.Holzenberger, M., G. Hamard, R. Zaoui, P. Leneuve, B. Ducos, C. Beccavin, L. Perin, and Y. Le Bouc. 2001. Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology 142:4469-4478. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka, H., H. Takahashi, M. Honma, and A. Ishida-Yamamoto. 2004. Unique keratinization process in psoriasis: late differentiation markers are abolished because of the premature cell death. J. Dermatol. 31:271-276. [DOI] [PubMed] [Google Scholar]
- 17.Johansen, C., K. Kragballe, M. Westergaard, J. Henningsen, K. Kristiansen, and L. Iversen. 2005. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br. J. Dermatol. 152:37-42. [DOI] [PubMed] [Google Scholar]
- 18.Kanter-Lewensohn, L., A. Dricu, L. Girnita, J. Wejde, and O. Larsson. 2000. Expression of insulin-like growth factor-1 receptor (IGF-1R) and p27Kip1 in melanocytic tumors: a potential regulatory role of IGF-1 pathway in distribution of p27Kip1 between different cyclins. Growth Factors 17:193-202. [DOI] [PubMed] [Google Scholar]
- 19.Kido, Y., D. J. Burks, D. Withers, J. C. Bruning, C. R. Kahn, M. F. White, and D. Accili. 2000. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J. Clin. Investig. 105:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingohr, M. K., L. M. Dickson, J. F. McCuaig, S. R. Hugl, D. R. Twardzik, and C. J. Rhodes. 2002. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes 51:966-976. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59-72. [PubMed] [Google Scholar]
- 22.Martin, P. 1997. Wound healing—aiming for perfect skin regeneration. Science 276:75-81. [DOI] [PubMed] [Google Scholar]
- 23.Mehrel, T., D. Hohl, J. A. Rothnagel, M. A. Longley, D. Bundman, C. Cheng, U. Lichti, M. E. Bisher, A. C. Steven, P. M. Steinert, S. H. Yuspa, and D. R. Roop. 1990. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 61:1103-1112. [DOI] [PubMed] [Google Scholar]
- 24.Miki, H., T. Yamauchi, R. Suzuki, K. Komeda, A. Tsuchida, N. Kubota, Y. Terauchi, J. Kamon, Y. Kaburagi, J. Matsui, Y. Akanuma, R. Nagai, S. Kimura, K. Tobe, and T. Kadowaki. 2001. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol. Cell. Biol. 21:2521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro, J. M., J. Casatorres, and J. L. Jorcano. 1995. Elements controlling the expression and induction of the skin hyperproliferation-associated keratin K6. J. Biol. Chem. 270:21362-21367. [DOI] [PubMed] [Google Scholar]
- 26.Radcliff, K., T. B. Tang, J. Lim, Z. Zhang, M. Abedin, L. L. Demer, and Y. Tintut. 2005. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ. Res. 96:398-400. [DOI] [PubMed] [Google Scholar]
- 27.Rowinsky, E. K. 2004. Targeting the molecular target of rapamycin (mTOR). Curr. Opin. Oncol. 16:564-575. [DOI] [PubMed] [Google Scholar]
- 28.Sadagurski, M., G. Weingarten, C. J. Rhodes, M. F. White, and E. Wertheimer. 2005. Insulin receptor substrate 2 plays diverse cell-specific roles in the regulation of glucose transport. J. Biol. Chem. 280:14536-14544. [DOI] [PubMed] [Google Scholar]
- 29.Sauer, B. 1998. Inducible gene targeting in mice using the Cre/lox system. Methods 14:381-392. [DOI] [PubMed] [Google Scholar]
- 30.Sayama, K., K. Yamasaki, Y. Hanakawa, Y. Shirakata, S. Tokumaru, T. Ijuin, T. Takenawa, and K. Hashimoto. 2002. Phosphatidylinositol 3-kinase is a key regulator of early phase differentiation in keratinocytes. J. Biol. Chem. 277:40390-40396. [DOI] [PubMed] [Google Scholar]
- 31.Scholl, F. A., P. A. Dumesic, and P. A. Khavari. 2004. Mek1 alters epidermal growth and differentiation. Cancer Res. 64:6035-6040. [DOI] [PubMed] [Google Scholar]
- 32.Stitt, T. N., D. Drujan, B. A. Clarke, F. Panaro, Y. Timofeyva, W. O. Kline, M. Gonzalez, G. D. Yancopoulos, and D. J. Glass. 2004. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14:395-403. [DOI] [PubMed] [Google Scholar]
- 33.Valentinis, B., and R. Baserga. 2001. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 54:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentinis, B., M. Navarro, T. Zanocco-Marani, P. Edmonds, J. McCormick, A. Morrione, A. Sacchi, G. Romano, K. Reiss, and R. Baserga. 2000. Insulin receptor substrate-1, p70S6K, and cell size in transformation and differentiation of hemopoietic cells. J. Biol. Chem. 275:25451-25459. [DOI] [PubMed] [Google Scholar]
- 35.Valverde, A. M., D. J. Burks, I. Fabregat, T. L. Fisher, J. Carretero, M. F. White, and M. Benito. 2003. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes 52:2239-2248. [DOI] [PubMed] [Google Scholar]
- 36.Wakita, H., and M. Takigawa. 1999. Activation of epidermal growth factor receptor promotes late terminal differentiation of cell-matrix interaction-disrupted keratinocytes. J. Biol. Chem. 274:37285-37291. [DOI] [PubMed] [Google Scholar]
- 37.Wertheimer, E., N. Spravchikov, M. Trebicz, M. Gartsbein, D. Accili, I. Avinoach, S. Nofech-Mozes, G. Sizyakov, and T. Tennenbaum. 2001. The regulation of skin proliferation and differentiation in the IR null mouse: implications for skin complications of diabetes. Endocrinology 142:1234-1241. [DOI] [PubMed] [Google Scholar]
- 38.Wertheimer, E., M. Trebicz, T. Eldar, M. Gartsbein, S. Nofech-Mozes, and T. Tennenbaum. 2000. Differential roles of insulin receptor and insulin-like growth factor-1 receptor in differentiation of murine skin keratinocytes. J. Investig. Dermatol. 115:24-29. [DOI] [PubMed] [Google Scholar]
- 39.White, M. F. 1997. The insulin signalling system and the IRS proteins. Diabetologia 40(Suppl. 2):S2-S17. [DOI] [PubMed] [Google Scholar]
- 40.White, M. F. 2002. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 283:E413-E422. [DOI] [PubMed] [Google Scholar]
- 41.Withers, D. J., D. J. Burks, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White. 1999. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 23:32-40. [DOI] [PubMed] [Google Scholar]
- 42.Xu, J., and K. Liao. 2004. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J. Biol. Chem. 279:35914-35922. [DOI] [PubMed] [Google Scholar]
- 43.Yuspa, S. H., P. Hawley-Nelson, B. Koehler, and J. R. Stanley. 1980. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 40:4694-4703. [PubMed] [Google Scholar]
- 44.Yuspa, S. H., A. E. Kilkenny, P. M. Steinert, and D. R. Roop. 1989. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J. Cell Biol. 109:1207-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]