Abstract
Accelerated osteoclastic bone resorption plays a central role in the pathogenesis of osteoporosis and other bone diseases. Identifying the molecular pathways that regulate osteoclast activity provides a key to understanding the causes of these diseases and to the development of new treatments. Here we show that mice with inactivation of cannabinoid type 1 (CB1) receptors have increased bone mass and are protected from ovariectomy induced bone loss. Pharmacological antagonists of CB1 and CB2 receptors prevented ovariectomy induced bone loss in vivo and caused osteoclast inhibition in vitro by promoting osteoclast apoptosis and inhibiting production of several osteoclast survival factors. These studies show that the CB1 receptor plays a role in the regulation of bone mass and ovariectomy induced bone loss and that CB1 and CB2 selective cannabinoid receptor antagonists are a novel class of osteoclast inhibitors that may be of value in the treatment of osteoporosis and other bone diseases.
Introduction
Osteoclasts are cells derived from the monocyte – macrophage lineage that play an important role in modelling bone during skeletal growth and in remodelling bone during adult life(1). Increased osteoclast activity or uncoupling of osteoclastic bone resorption from bone formation results in focal or generalised bone loss and is a characteristic feature of bone diseases such as osteoporosis, Paget’s disease of bone, and cancer associated bone disease(2). The importance of osteoclastic bone resorption in the pathogenesis of these disease is reflected by the fact that the most successful drug treatments for bone disease work by inhibiting bone resorption(3). Osteoclastic bone resorption is regulated by a complex interplay between circulating calciotropic hormones like parathyroid hormone, calcitriol and sex hormones; and local regulators of bone cell activity like receptor activator of nuclear factor kappa B ligand (RANKL), macrophage colony stimulating factor (M–CSF) and osteoprotegerin(4). Recent work has shown that neuroendocrine pathways and neurotransmitters also play a key role in the regulation of bone remodelling(5–9). In view of this, we investigated the role of the endocannabinoid system in the regulation of bone mass and bone turnover by studying the skeletal phenotype in mice with targeted inactivation of cannabinoid type 1 (CB1) receptors (CB1 KO mice) and by studying the effects of cannabinoid receptor ligands on bone cell function in vitro and ovariectomy induced bone loss in vivo.
Results
Skeletal Phenotype of CB1 KO mice
We found that CB1 KO mice had significantly increased bone mineral density (BMD) when compared with wild type (WT) littermates. For example, the levels of BMD assessed by dual X–ray absorptiometry (DXA) at the femur were 18% higher in CB1 KO animals than WT and values at the spine were 10% higher (Figure 1, panel a). Further evaluation using peripheral quantitative computed tomography (pQCT) showed that trabecular BMD values at the tibial metaphysis in CB1 KO mice were 16% higher than in WT (Figure 1, panel b) and this difference was clearly evident on low power photomicrographs at this site (Figure 1 panel c). Bone histomorphometry showed that CB1 KO mice had significantly increased trabecular bone volume at the tibial metaphysis compared with wild type mice, consistent with the BMD measurements, but no difference was observed between CB1 KO mice and wild type littermates in terms of osteoclast numbers, eroded surfaces or osteoblast numbers (Supplementary Table 1). This indicates that absence of the CB1 receptor regulates BMD but does not influence bone turnover appreciably in normal adult mice. In order to determine whether the CB1 receptor plays a role in the regulation of bone loss, we studied the effects of ovariectomy in CB1 KO and WT mice. We found that CB1 KO mice were completely resistant to trabecular bone loss induced by ovariectomy when compared with WT; analysis by pQCT showed no significant change in total BMD of the tibial metaphysis in CB1 KO mice following ovariectomy, compared with a 12% reduction in WT (Figure 2, panel a). Analysis by micro–computed tomography (μCT) showed a 40% reduction in trabecular bone volume following ovariectomy in WT mice (Figure 2, panel b); a 12% reduction in trabecular thickness (Figure 2, panel c) and a 30% reduction in trabecular number (Figure 2, panel d) whereas none of these variables changed significantly in CB1 KO mice. Uterine weight (mean ± standard deviation) fell to a similar degree after ovariectomy in WT mice (0.38 ± 0.03 vs. 0.08 ± 0.01 g; p<0.01) and CB1 KO mice (0.43 ± 0.02 vs. 0.09 ± 0.01 g; p<0.01). These data indicate that the CB1 receptor plays an essential role in regulating bone loss that results from estrogen deficiency, but that the gonadal response to ovariectomy is unaffected by CB1 deficiency.
Figure 1. CB1 KO mice have increased bone mass.
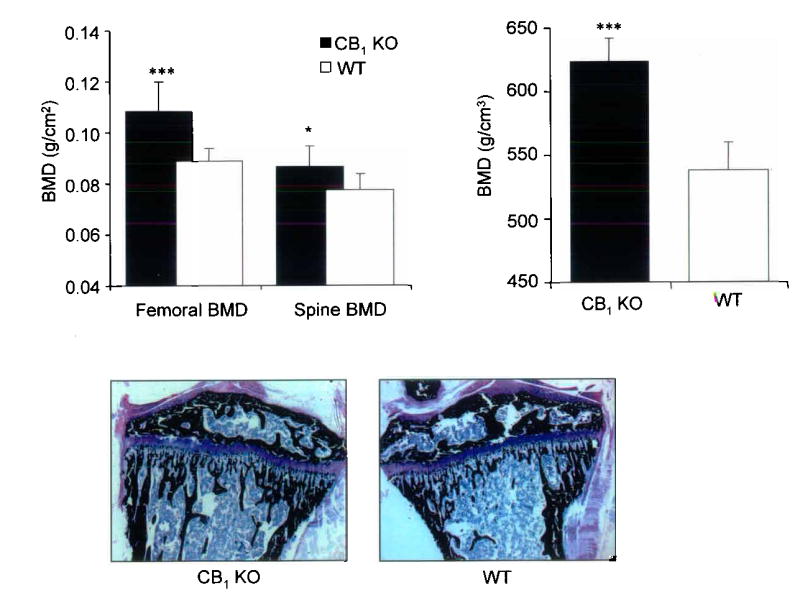
a, Bone mineral density at the spine and femur assessed by DXA in CB1 KO mice and wild type littermates; b trabecular bone mineral density at the tibial metaphysis assessed by pQCT in CB1 KO mice and wild type littermates; c, representative photomicrograph of the proximal tibia from and CB1 KO mice (left panel) and wild type mice (right panel) Values in the bar charts are means and standard errors. Significant differences between CB1 KO and wild type mice are indicated by: ***p<0.001; *p<0.02.
Figure 2. CB1 KO mice are protected against ovariectomy induced bone loss.
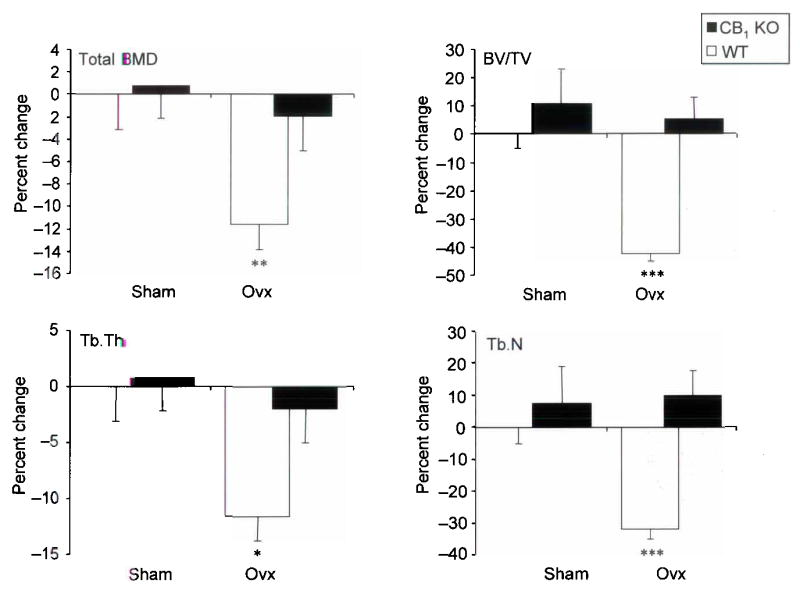
a, Total BMD at the tibial metaphysis in CB1 KO mice and wild type littermates before and after sham operation or ovariectomy (ovx); b, Bone volume / total volume (BV/TV) assessed at the same site by μCT; c, Trabecular thickness (Tb.Th) assessed by μCT; d trabecular number (Tb.N) assessed by μCT. Values in the bar charts are expressed as the percent change relative to the value in sham operated wild type animals and are means and standard errors. Significant differences between CB1 KO and wild type mice are indicated by: *** p<0.001; ** p<0.01.
Effects of CB receptor ligands on osteoclast function
In order to further explore the mechanisms by which the CB1 pathway regulates bone mass and bone loss, we studied the effects of various cannabinoid receptor agonists and antagonists on bone cell function in vitro using primary mouse osteoblast cultures and RANKL–generated mouse osteoclast cultures. None of the CB ligands that we tested significantly affected osteoblast growth or viability at concentrations of up to 20μM (data not shown), but significant effects on osteoclast activity were observed using ligand concentrations in the nanomolar range. The CB1– selective antagonist AM251(10) and the CB2– selective antagonists SR144528 and AM630(10) significantly inhibited osteoclast formation in RANKL and M–CSF stimulated mouse bone marrow cultures in a concentration dependent manner with IC50 values of 700nM for AM251; 850nM for SR144528 and 100nM for AM630 (Figure 3, panel a). Conversely, the endogenous cannabinoid receptor agonist anadamide (AEA) and the non–selective synthetic agonist CP55940 stimulated osteoclast formation in a concentration dependent manner between 100nM and 5 μM (Figure 3, panel a). Representative photomicrographs from cultures treated with vehicle, AEA, AM251 and SR144528 are shown in Figure 3, panel b. Moreover, AEA reversed the inhibitory effect of AM251 on osteoclast formation (Figure 3, panel c), consistent with a receptor mediated effect.
Figure 3. Regulation of osteoclast formation by cannabinoid receptor ligands.
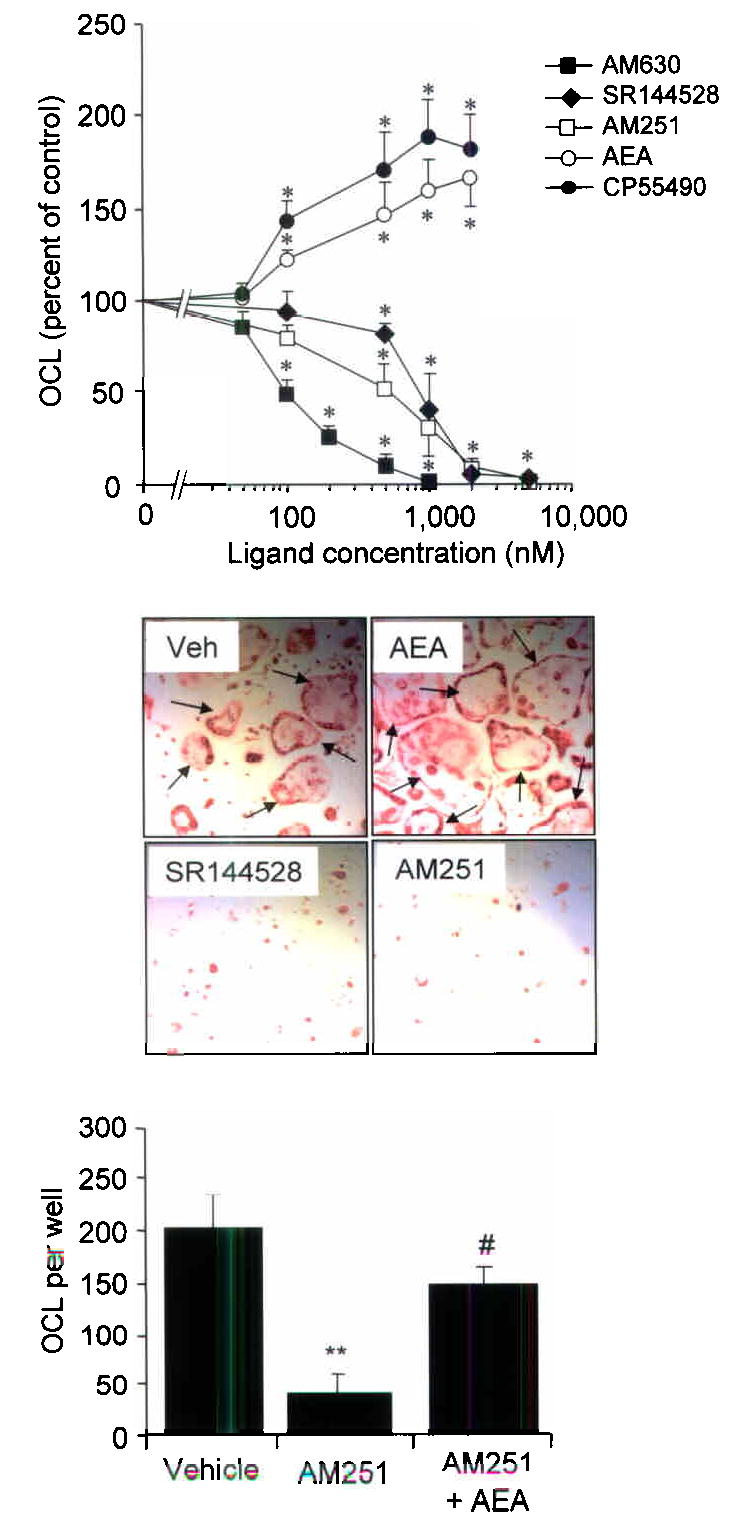
a, Effects of the CB ligands on osteoclast formation in C57BL/6 bone marrow cultures stimulated with RANKL and M–CSF. The data shown are from three independent experiments and are expressed as a percent of values in control cultures.. Significant inhibition or stimulation of osteoclast formation when compared with control is indicated by * p<0.05 or below. b, representative photomicrographs of vehicle treated culture and cultures treated with anandamide (AEA), SR144528 and AM251 stained for TRAcP. Osteoclasts (arrows) were larger and more numerous in the AEA treated cultures when compared with vehicle and were virtually absent from the SR144528 and AM251 treated cultures. c, effect of AM251 alone (1μM) and AM251 in combination with AEA (5μM) on osteoclast formation. Values in the bar charts and line graphs are means and standard deviations. Significant differences are indicated by: *** p<0.001 (vehicle vs. AM251); # p<0.02 (AM251 vs. AM251 + AEA).
Expression and functional role of CB1 and CB2 receptors on osteoclasts
Both the CB1 and CB2 receptor subtypes were expressed on mouse osteoclasts as assessed by immunohistochemical staining (Supplementary Figure 1, panel a), western blotting (Supplementary Figure 1, panel b) and RT–PCR (Supplementary Figure 1, panel c), indicating that CB1, CB2 or both receptors could mediate the effects of CB ligands on osteoclast activity. In order to clarify the relative role of the CB1 and CB2 receptors as targets for the regulation of osteoclast activity, we compared the effects of AM251 and AM630 on RANKL induced osteoclast formation in cultures generated from CB1 KO mice and wild type littermates. Cultures prepared from CB1 KO mice were resistant to the inhibitory effects of AM251 on osteoclast formation when compared with WT cultures (Figure 4, panel a). The CB2 selective antagonist AM630 inhibited osteoclast formation to a similar extent in cultures prepared from CB1 KO and WT mice and was an order of magnitude more potent than AM251 (Figure 4, panel b). Taken together these data indicate that the CB1 receptor contributes in part, to the osteoclast inhibition which occurs in vitro when these cultures are exposed to CB antagonists but that osteoclast inhibition can also occur through antagonism of CB2 receptors.
Figure 4. Osteoclasts generated from CB1 KO mice are resistant to the inhibitory effects of CB1 selective, but not CB2 selective receptor antagonists.
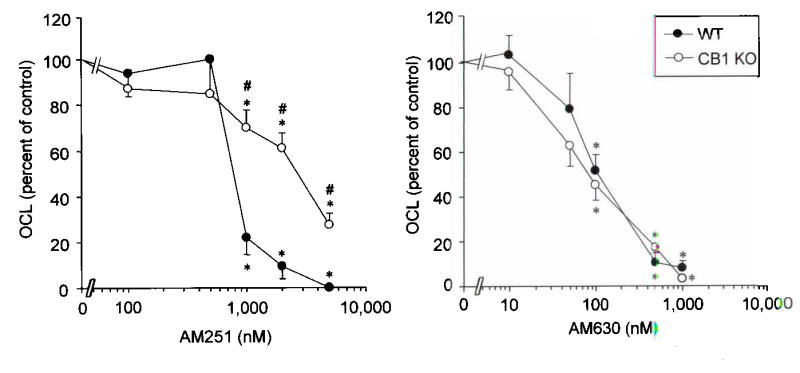
a, effect of AM251 on osteoclast formation in RANKL / M–CSF stimulated bone marrow cultures prepared from CB1 KO mice and wild type littermates. b, effect of AM630 on osteoclast formation in bone marrow mononuclear cells cultured from wild type and CB1 KO mice. Values are means and standard deviations. Significant differences from control cultures are indicated by * p<0.05. Significant differences between CB1 KO and wild type cultures are indicated by # p<0.05.
Effects of CB antagonists on ovariectomy induced bone loss
Since CB1 KO mice were resistant to ovariectomy induced bone loss, we wanted to determine if pharmacological blockade of CB receptors could prevent the bone loss that occurs as the result of ovariectomy. We studied the effects of AM251 in doses of 0.3–3.0 mg/kg/day on ovariectomy induced bone loss in wild type mice and SR144528 at a single dose of 3 mg/kg/day. These doses were chosen on the basis of previous work, which has shown that AM251 and the related compound SR141716 prevent diet induced obesity in wild type mice in doses of 3–30 mg/kg/day(11–13). We found that AM251 protected against ovariectomy induced bone loss in a concentration–dependent manner at doses as low as 0.3 mg/kg/day (Figure 5, panels a – c). Bone histomorphometry showed that osteoclast numbers and active resorption surfaces were increased following ovariectomy in vehicle treated animals, whereas no significant increase in either variable was observed in AM251 treated animals (Supplementary Table 2). Osteoblast numbers were unaffected by AM251 treatment, indicating that the protective effect of CB receptor blockade on ovariectomy induced bone loss was primarily mediated by inhibiting bone resorption, rather than by stimulating bone formation. Administration of SR144528 to ovariectomized mice in a dose of 3 mg/kg/day also completely prevented ovariectomy induced bone loss, with results almost identical to those observed with AM251 at 3 mg/kg/day (data not shown).
Figure 5. Cannabinoid receptor antagonists prevent ovariectomy induce bone loss.
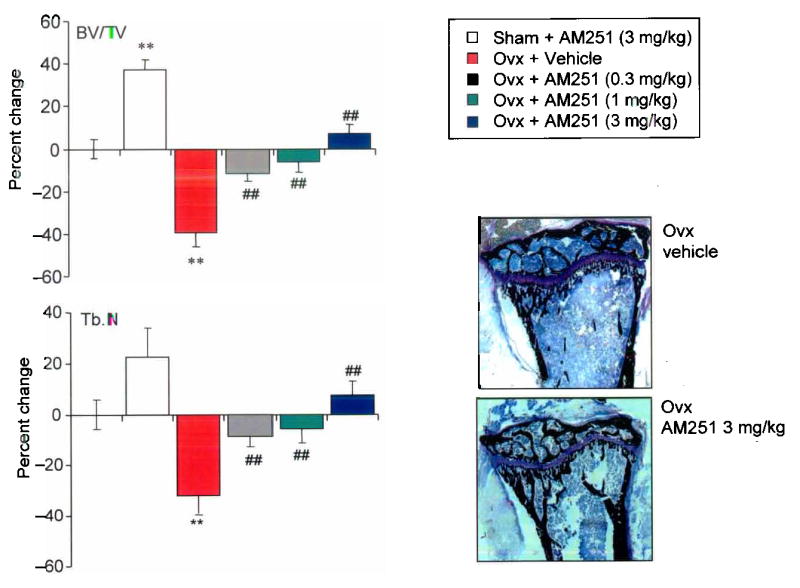
a. Effects of AM251 on trabecular bone density in sham operated (sham) and ovariectomized (ovx) C57BL/6 mice; b, Effects of AM251 on trabecular number in sham operated (sham) and ovariectomized (ovx) C57BL/6 mice. Values are expressed as percent change relative to sham operated vehicle treated control mice. c, representative photomicrograph of the tibial metaphysis 21 days after ovariectomy in a vehicle treated mouse (top panel) and an AM251 treated mouse (bottom panel). Values in the bar charts are means and standard errors. Significant differences between sham Ovx and sham Ovx + AM251 and between sham Ovx and Ovx are indicated by ** p<0.001; significant differences between Ovx and Ovx + AM251 are indicated by ## p<0.002.
Effects of CB ligands on osteoclast apoptosis and signalling pathways
We investigated the molecular mechanisms by which CB receptor blockade inhibits bone resorption, by studying the effects of CB receptor antagonists on apoptosis and key intracellular signalling pathways involved in osteoclast survival using purified rabbit osteoclasts and RANKL generated mouse osteoclasts. We found that AM251 and SR144528 stimulated apoptosis of rabbit and mouse osteoclasts by 12–17 fold above control values (Supplementary Figure 2, panel a), as detected by deoxynucleotidyl transferase–mediated dUTP nick end labelling (TUNEL) and typical changes in nuclear morphology on DAPI stained cultures (Supplementary Figure 2, panel d) Activation of caspase 3 was observed in both rabbit and mouse osteoclasts that were exposed to CB antagonists (Supplementary figure 2 panel b) and this was accompanied by a reduction in osteoclast numbers, and inhibition of bone resorption (Supplementary figure 2, panel c; data from rabbit osteoclasts shown). The only significant difference between the response of mouse and rabbit osteoclasts to CB antagonists was that maximal stimulation of apoptosis occurred 2μM in mouse osteoclasts compared with 10μM in rabbit osteoclasts, presumably because of species differences in affinity of the receptors for the ligands tested. We also investigated the effects of AM251 on activation of key survival factor in osteoclasts including extracellular signal regulated kinase (ERK), the p65 component of NFκB, NFATc1; c–jun and c–fos(14–16). These studies showed that AM251 at a concentration of 2μM abolished RANKL induced ERK phosphorylation (Figure 6, panel a) and significantly inhibited RANKL induced nuclear translocation and DNA binding of NFATc1, c–jun and c–fos (Figure 6, panels b, c and d). In contrast, AM251 did not inhibit RANKL induced NFκB (p65) activation in this assay (data not shown).
Figure 6. Cannabinoid receptor antagonists inhibit ERK, c–fos, c–jun and NFATc1 activation in mouse osteoclasts.
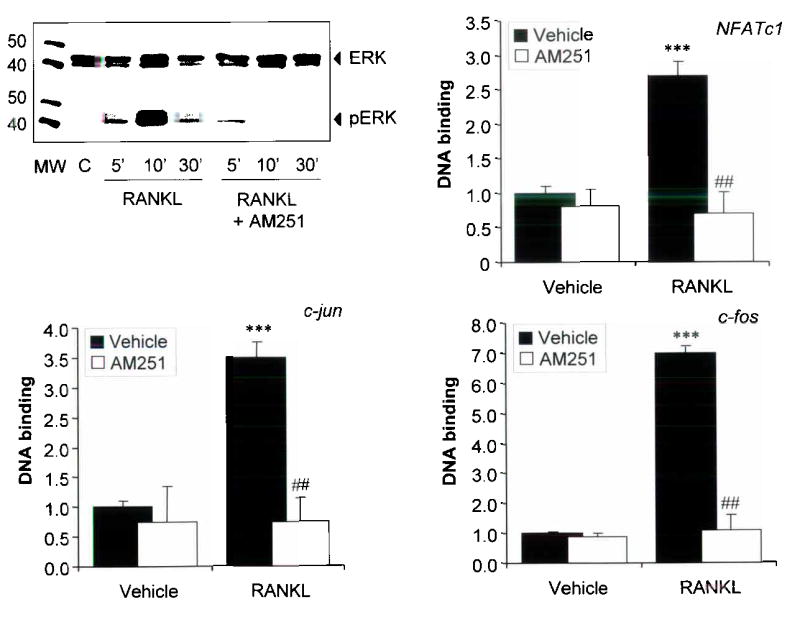
a, Effects of the CB antagonist AM251 (5μM) on ERK phosphorylation as detected by western blot in vehicle (C) and RANKL (100ng/ml) treated mouse osteoclasts. The numbers refer to the time (min) after RANKL stimulation. MW is molecular weight marker and the size of the marker bands is indicated in kDa. b, Effects of AM251 on nuclear translocation and DNA binding of phospho NFATc1 in mouse osteoclasts treated with RANKL (100ng/ml) in the presence of vehicle or AM251 (2μM). c, Effects of AM251 on nuclear translocation and DNA binding of c–jun in mouse osteoclasts treated with RANKL (100ng/ml) in the presence of vehicle or AM251 (2μM). d, Effects of AM251 on DNA binding of c–fos in mouse osteoclasts treated with RANKL (100ng/ml) in the presence of vehicle or AM251 (2μM). Values are expressed as the change relative to vehicle treated cultures. Values in the bar charts are means and standard deviations. Significant differences between vehicle and RANKL treated cultures are indicated by: *** p<0.001; and differences between AM251 and vehicle treated cultures are indicated by: ## p<0.001. The results shown are representative of three independent experiments.
Discussion
The studies presented here indicate that the CB1 receptor plays a hitherto unrecognised role in the regulation of bone mass and bone loss resulting from estrogen deficiency. Adult CB1 KO mice had significantly higher BMD than WT littermates at several skeletal sites and CB1 KO mice were completely protected against ovariectomy induced bone loss. The synthetic cannabinoid receptor antagonists AM251, SR144528 and AM630 inhibited osteoclast formation and bone resorption in vitro and both AM251 and SR144528 protected against ovariectomy induced bone loss in vivo by inhibiting osteoclastic bone resorption. Conversely, the endogenous CB agonist anadamide, and the synthetic agonist CP55940 enhanced osteoclast formation in vitro and anadamide reversed the inhibitory effects of AM251 osteoclast formation, consistent with a receptor mediated effect. Osteoclasts generated from CB1 KO mice were resistant to the inhibitory effects of AM251 compared with WT mice, consistent with the hypothesis that the osteoclast inhibition we observed with CB antagonists was mediated in part, by the CB1 receptor. We also gained evidence to show CB2 receptor mediated osteoclast inhibition can also occur in vitro, from the observation that the CB2–selective antagonist AM630 inhibited osteoclast formation equally well in cultures prepared from CB1 KO and WT mice. The inhibitory effects on osteoclast formation that we observed at high AM251 concentrations (>1000nM) in CB1 KO cultures were probably attributable to blockade of the CB2 receptor by this compound(10). The extent to which CB1 versus CB2 receptors contributed to the prevention of ovariectomy induced bone loss that we observed with CB antagonist treatment in vivo remains to be determined, as does the relative contribution of central versus peripheral CB receptors in mediating this effect. Further work will also be required to determine whether the osteoclast inhibitory effects we observed were due to antagonism of the action of endogenous CB receptor ligands or to inverse agonism, since AM630, AM251 and SR144528 have all been shown to elicit effects at CB receptors that are converse to those produced by CB agonists, even in the absence of agonist binding(17).
The mechanism of osteoclast inhibition resulting from CB receptor blockade was most likely due to osteoclast apoptosis since AM251 and SR144528 markedly enhanced apoptosis of mature rabbit osteoclasts and of RANKL generated mouse osteoclasts. Exposure of mouse osteoclasts to AM251 blocked RANKL–induced ERK phosphorylation and prevented activation of several key osteoclast stimulatory factors including NFATc1, phospho c–jun and c–fos. Interestingly however, AM251 had no inhibitory effect NFκB activation suggesting that cannabinoid receptor antagonists may cause osteoclast inhibition through the ERK – AP1 pathway, rather than the NFκB pathway.
The phenotype that we observed in CB1 KO mice indicates that the CB1 receptor plays an essential role in regulating BMD and bone loss that results from oestrogen deficiency. Furthermore, the pharmacological studies in vitro show that the CB1 and CB2 receptors may have overlapping functions in the regulation of osteoclast activity. Further studies of the skeletal phenotype in CB2 KO mice will be required to fully evaluate the role of the CB2 receptor subtype on bone metabolism and osteoclast function in vivo.
The results of our studies are important since we have shown that pharmacological antagonists of CB receptors represent a novel class of osteoclast inhibitors, whereas CB receptor agonists act as stimulators of bone resorption. This suggests that CB receptor antagonists may represent a promising new class of antiresorptive drugs for the treatment of osteoporosis and other bone diseases associated with increased osteoclast activity. Our data also raised the possibility that recreational or therapeutic use of cannabis derivatives that act as agonists at CB receptors might enhance bone loss and predispose to osteoporosis.
Materials and Methods
Cell culture
Mouse osteoclast cultures were generated by isolating mononuclear cells from the bone marrow of mouse long bones essentially as described by Takahashi(18). The cells were cultured in αMEM with 10% fetal calf serum and Macrophage colony stimulating factor (M–CSF) (100ng/ml) for 3 d. Adherent cells were collected and plated at 0.5x104 cells/well in 96–well plates in 125μl of αMEM supplemented with 10% FCS, M–CSF (25ng/ml) and RANKL (100ng/ml) for 4 d. Test substances were added on d 7 and the cultures were terminated on d 10. Rabbit osteoclast cultures were performed as described(19) and exposed to test substances for 48 h. Osteoclast formation was assessed by counting Tartrate resistant acid phosphatase positive multinucleated cells and bone resorption was assessed by measuring resorption pit area using reflected light microscopy as described(20). Apoptosis was detected by the characteristic changes in nuclear morphology following 4,6–diamidino–2–phenylinole (DAPI) staining as previously described(20), and by detection of fragmented DNA by terminal deoxynucleotidyl transferase–mediated dUTP nick end labelling (TUNEL) using the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Intergen). Cells with clearly fragmented DNA or positive ApopTag labelling were counted and expressed as a percentage of total cell number.
Immunofluorescence
Immunofluorescent staining of mouse osteoclasts for CB receptors was performed using a rabbit antibody to the CB1 receptor(21) (Caymen Chemical, catalog number 101500) and a rabbit antibody to the CB2 receptor(22) (Caymen Chemical, catalog number 101550) followed by Alexa–Fluor 488 goat–anti–rabbit IgG (Molecular Probes). Controls were performed by omitting the primary antibody. Osteoclasts were identified by dual labelling with a mouse anti–CD61 antibody directed against the vitronectin receptor (Pharmagen) followed by Alexa–Fluor 594 goat–anti–mouse IgG.
Western Blotting
Intact (p–30) and activated (cleaved, p–19) caspase–3 levels were detected by western blotting using a goat polyclonal anti–caspase–3 antibody (Santa Cruz Biotechnology, Inc), and a rabbit polyclonal anti–cleaved caspase–3 antibody (Cell Signalling Technology). Detection of native and phosphorylated ERK was by western blotting using antibodies to ERK and phospho–ERK (Cell Signalling Technology). Detection of CB1 and CB2 receptors was by western blotting using the same primary antibodies as for immunofluorescence, with an anti–rabbit secondary antibody linked to horse radish peroxidase (Caymen Chemical). The chemiluminescent signal was developed using SuperSignal (Pierce).
Molecular biology
Activation and DNA binding of NFATc1, NFκB (p65), phospho c–jun and c–fos were assessed by analysis of nuclear extracts using TRANS–AM transcription factor assay kits (Active Motif). Detection of CB1 and CB2 receptor mRNA by reverse transcription polymerase chain reaction (RT/PCR) was performed using Qiagen Quantitect custom primers for the CB1 receptor (primers 5′–CCTTGCAGATACAACCTT–3′ and 5′–TGCCATGTCTCCTTTGATA–3′; predicted product size 94bp) and the CB2 receptor (primers 5′–CATAAGCCGATCTCTCCAA–3′ and 5′–CCAAAGCTGGTGCAGGAA–3′; predicted product size 82bp). The PCR protocol consisted of an initial incubation at 95°c for 15 min., followed by 40 cycles of 94°c for 15 s., 56°c for 30 s. and 76°c for 30 s. All reactions were run with no template and no RT controls.
Drugs
All compounds were dissolved in dimethysulfoxide and added to the cultures in such that the final concentration was 0.1%. Control cultures were treated with dimethysulfoxide alone at the same concentrations. AM251 and AM630 were obtained from Tocris. SR144528 was synthesised by the coupling of 1– (4–Methylbenzyl)–5–(4–chloro–3–methylphenyl)pyrazole–3–carboxylic acid chloride and (1S)–2–endo,exo–amino–1,3,3–trimethylbicyclo[2.2.1]heptane. The identity and purity of SR144528 was verified using nuclear magnetic resonance spectroscopy.
Animals
Animal experiments were approved by the ethical review board of the University of Aberdeen and were conducted in accordance with UK Home Office regulations. The strain of CB1 KO mice and wild type littermates used in this study were as described by Pryce and colleagues(23). Experiments were also performed using C57BL/6 mice obtained from Harlan Laboratories. Ovariectomy or sham ovariectomy was performed in 9 week old adult female mice as previously described(24). Treatment with AM251 and SR144528 was commenced 2 days after ovariectomy or sham ovariectomy by intraperitoneal administration of the drug in corn oil. Controls received corn oil alone. The treatment was continued for 19 d and the experiment terminated on d 21. Eight mice were studied per group. Bone mineral density was measured by DXA at the spine and femur using a PIXIMUS scanner; and at the tibial metaphysis by pQCT using a Stratec Research M scanner or by micro computed tomography (μCT), using a Skyscan 1072 scanner. Following BMD scanning the limbs were embedded and processed for bone histomorphometry as previously described(24).
Statistics
Between group comparisons were by ANOVA with Dunnet’s post–test. IC50 values were calculated from concentration response curves using the GraphPad PRISM computer program (GraphPad Software).
Supplementary Material
Acknowledgments
This study was supported by a project grant from the arthritis research campaign to SHR, RAR and RVH; a “Proof of Concept” grant to SHR, IRG and RVH from the Scottish Enterprise; and by an NIH grant to RAR. RVH is supported by a project grant from the arthritis research campaign and DB is a Multiple Sclerosis Society senior fellow.
References
- 1.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.Helfrich MH. Osteoclast diseases. MicroscResTech. 2003;61:514–532. doi: 10.1002/jemt.10375. [DOI] [PubMed] [Google Scholar]
- 3.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S. Minireview: The OPG/RANKL/RANK System. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 5.Baldock PA, et al. Hypothalamic Y2 receptors regulate bone formation. JClinInvest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skerry TM, Genever PG. Glutamate signalling in non–neuronal tissues. Trends PharmacolSci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 7.van’t Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology. 2004;145:5068–5074. doi: 10.1210/en.2004-0205. [DOI] [PubMed] [Google Scholar]
- 8.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 10.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins LeukotEssentFatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 11.Ravinet TC, et al. Anti–obesity effect of SR141716, a CB1 receptor antagonist, in diet–induced obese mice. AmJ Physiol RegulIntegrComp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 12.Shearman LP, et al. Antidepressant–like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. BehavPharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hildebrandt AL, Kelly–Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet–induced obese mice. EurJPharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- 14.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. DevCell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 15.David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c–Jun phosphorylation–dependent and –independent mechanisms. J Cell Sci. 2002;115:4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 16.Grigoriadis AE, et al. c–Fos: a key regulator of osteoclast–macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 17.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. PharmacolRev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, N., N. Udagawa, S. Tanaka, T. Suda. Generating murine osteoclasts from bone marrow. Helfrich, M. H. and Ralston, S. H. Bone Research Protocols. 129–144 (2003). Totowa, New Jersey, Humana Press. Methods in Molecular Medicine. Walker, J. M. [DOI] [PubMed]
- 19.Coxon FP, et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI–298. JBone MinerRes. 2000;15:1467–1476. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 20.van’t Hof RJ, et al. Identification of biphenylcarboxylic acid derivatives as a novel class of bone resorption inhibitors. JBone MinerRes. 2004;19:1651–1660. doi: 10.1359/jbmr.2004.19.10.1651. [DOI] [PubMed] [Google Scholar]
- 21.Howlett AC, Song C, Berglund BA, Wilken GH, Pigg JJ. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site–directed antibodies. MolPharmacol. 1998;53:504–510. doi: 10.1124/mol.53.3.504. [DOI] [PubMed] [Google Scholar]
- 22.Casanova ML, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J ClinInvest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryce G, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- 24.Armour KE, et al. Defective bone formation and anabolic responses to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide sythase. Endocrinology. 2001;142:760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


