Abstract
Interleukin (IL)-10 is an anti-inflammatory cytokine that may play a protective role in atherosclerosis. The aim of this study was to assess the effect of IL-10 deficiency in the apolipoprotein E knockout mouse. Apolipoprotein E deficient (E−/−) and IL-10 deficient (−/−) mice were crossed to generate E−/− × IL-10−/− double knockout mice. By 16 wk, cholesterol and triglycerides were similar in double and single knockouts but the lack of IL-10 led to increased low-density lipoprotein cholesterol whereas very-low-density lipoprotein was reduced. In parallel, T-helper 1 responses and lesion size were dramatically increased in double knockout compared with E−/− controls. At 48 wk, matrix metalloproteinases and tissue factor activities were increased in lesions of double-knockout mice. Furthermore, markers of systemic coagulation were increased, and vascular thrombosis in response to i.v. thrombin occurred more frequently in E−/− × IL-10−/− than in E−/− mice. Our findings suggest that IL-10 deficiency plays a deleterious role in atherosclerosis. The early phase of lesion development was increased, and the proteolytic and procoagulant activity was elevated in advanced lesions. These data show that IL-10 may reduce atherogenesis and improve the stability of plaques.
INTRODUCTION
Activated macrophages and CD4+ T helper cells infiltrate atherosclerotic plaques at all stages of atherogenesis and synthesize cytokines that participate as autocrine or paracrine mediators in the disease process (1). Recent data suggest that the role of the immune response in atherosclerosis may be determined by the inflammatory/anti-inflammatory balance of cytokines in the microenvironment (1–6).
Under the influence of genetic and environmental factors acting at the level of antigen presentation, T helper (Th)0 cell precursors differentiate irreversibly into Th1 and Th2 polarized cells. Cytokines present in the microenvironment play a decisive role in this process. Interleukin-(IL)-12 is a key inducer of Th1 cells while IL-10 down regulates IL-12 production and facilitates Th2 differentiation (7). Th1 cells produce interferon-γ (IFN-γ) and IL-2 and mediate inflammatory responses (8), which contribute to the pathogenesis of organ-specific immune-mediated diseases (9). Th2 cells secrete IL-4 and IL-10, which promote allergic immune responses and inhibit cell-mediated, macrophage-dependent responses (8,9).
Although IL-10 positive cells (mainly macrophages and smooth muscle cells) can be found in atherosclerotic plaques (2,10), the atherosclerotic plaque is a site of Th1 activation both in humans (11,12) and in hypercholesterolemic mice (13,14). IFN-γ contributes to lesion progression (15,16) and acute clinical manifestations of coronary artery diseases (17). Recent studies suggest that the crosstalk between IL-10 and IL-12 may play a crucial role in the pathogenesis of atherosclerosis (3,4,10,11,13,14). IFN-γ may destabilize plaques by inhibiting smooth muscle proliferation, differentiation, and collagen production (18,19), stimulating macrophage production of matrix metalloproteinases (MMP) (20,21) and modulating the fibrinolytic response of endothelial cells (22,23). IL-10, on the other hand, inhibits the secretion of MMPs (24), the synthesis of tissue factor (TF), a potent initiator of the coagulation cascade (25), and the production of thrombin (26).
Fatty streak formation in fat-fed C57BL/6 mice was recently found to be accelerated in IL-10 deficient mice (2,4). However, others have shown that IL-10 may aggravate transplant arteriosclerosis (27). To study the role of IL-10 in atherosclerosis, we have analyzed the effect of IL-10 deficiency in apolipoprotein E knockout (E−/−) mice. Our data suggest that IL-10 has an important regulatory role in lipoprotein metabolism, plaque development, and remodeling.
MATERIALS AND METHODS
Animals
IL-10−/− mice (C57Bl/6-Il10tm1Cgn, Jackson Laboratories, Bar Harbor, ME, USA) were bred with E−/− mice (C57BL/6J-Apoetm1Unc). Male and female littermates from the F1 generation were bred to obtain E−/− × IL-10−/− double homozygous mice (F2). The effect of IL-10 deficiency on lesion development was evaluated at 16 and 48 wk in E−/− × IL-10−/− and E−/− × IL-10+/+ male and female siblings. Additional experiments were performed in separate sets of male E−/− × IL-10−/− and E−/− × IL-10+/+ mice at 48 wk to assess the effect of IL-10 deficiency on the advanced atherosclerotic phenotype. Mice were maintained on a standard rodent chow diet. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Sacrifice and Sampling
Mice were weighed and exsanguinated under anesthesia. Citrated blood was centrifuged at 4 °C for 5 min at 12000 g, and plasma was stored at −20 °C until analysis. Spleen, left lung, heart, and thoracic aorta were harvested after perfusion with phosphate buffered saline or 4% formaldehyde in phosphate buffered saline.
Lesion Size and Morphology
Lesion size was evaluated in 19 E−/− × IL-10−/− (9 male) and 13 E−/− × IL-10+/+ (7 male) mice at 16 wk and also in 24 E−/− × IL-10−/− (12 male) and 20 E−/− × IL-10+/+ (8 male) mice at 48 wk. Computer-assisted morphometry and immunohistochemistry were performed as described previously (6,28). Briefly, serial cryostat sections were cut from the proximal 1 mm of aortic root. The mean lesion size in each animal was determined after 4 sections, stained with hematoxylin and oil red O, were measured at every 100 μm over a 0.5-mm segment of the aortic root (6). Immunohistochemical data were obtained using 1 stained section per mouse (between 500 and 600 μm from the appearance of the aortic cusps). Visualization of sections reacted with cell-specific biotinylated monoclonals to CD4, CD8, and I-Ab (PharMingen) and avidin-biotin-peroxidase was employed for cell counting. An alkaline phosphatase–conjugated monoclonal anti-α smooth muscle (SM)–actin antibody (Sigma Chemical Co.) was used to detect smooth muscle cells. Positive cells were manually counted in 3 random sections and averaged.
Plasma Lipids and Lipoproteins
Total cholesterol and triglycerides were assayed by using commercially available kits (Roche Molecular Biochemicals, Indianapolis, IN, USA). Size fractionation of lipoproteins was performed by fast protein liquid chromatography (FPLC) using a micro-FPLC column (30 × 0.32 cm Superose6B, Amersham Pharmacia, Uppsala, Sweden) coupled to a system for online separation and subsequent detection of cholesterol as described (29). Briefly, 10 μL of plasma were injected into the FPLC column from each animal, and the cholesterol content was determined using the commercial reagent, which was continuously mixed with the separated lipoproteins at a flow rate of 40 + 40 μL/min. Absorbance was measured at 500 nm. The data were collected every 20 s.
Basal Th1/Th2 Balance
Plasma levels of IL-12 (PharMingen), IFN-γ, IL-4 (R&D Systems), and IgG subclasses (13) were measured by ELISA.
Advanced Atherosclerotic Phenotype
Effect of IL-10 deficiency on the Th1/Th2 balance in response to antigenic challenge
A separate set of 48-wk-old male mice (n = 9 E−/− × IL-10−/−and n = 9 E−/− × IL-10+/+ ) was immunized with human serum albumin (HSA) and sacrificed 2 wk later. Spleen cell suspensions were prepared and T cells were polarized in vitro toward the Th1 or Th2 phenotype (5). Mononuclear cells were cultured in the presence of HSA (0.3 μM) and a combination of either 0.1 ng/mL recombinant murine IL-12 (Pepro Tech) and 10 μg/mL anti-mouse IL-4 monoclonal antibody (mAb; PharMingen) or 20 ng/mL recombinant murine IL-4 (Pepro Tech) and 10 μg/mL anti-mouse IL-12 mAb (PharMingen). After 3 d of culture, intra-cellular cytokines were measured by flow cytometry (30) using a FACSCalibur (Becton Dickinson). Plasma levels of specific anti-HSA IgG2a and IgG1 antibodies from immunized mice were analyzed by ELISA.
TF and MMP 9 localization in atherosclerotic plaques
TF and MMP 9 were localized in 1 section/mouse from same set of aortic cryosections used for lesion quantification. MMP 9 staining was performed with purified goat–anti-mouse MMP 9 (Tebu) followed by biotinylated donkey–anti-goat IgG (Amersham Pharmacia). For TF staining, a mouse monoclonal anti-human TF type 2 (Calbiochem) was biotinylated using a commercial kit (Tebu) and revealed by avidin-horseradish peroxidase (Vector Lab) and Fast Red substrate (Dako).
TF and MMP activities
Procoagulant activity (PCA) was quantified (n = 10 E−/− × IL-10−/− and n = 8 E−/− × IL-10+/+ mice) on plasma, aortas, and spleen mononuclear cells (107 cells per mouse) from a separate set of 48-wk-old mice. After removal of the adventitia under direct magnified vision, thoracic aortas were cryohomogenized in liquid nitrogen. Dry aorta homogenates and spleen cell pellets were treated with 500 μL 16 mM octyl-β-d-glycopyranoside (10 min at 37 °C). After centrifugation (14000 g, 4 °C, 30 min), the supernatants were collected and their protein content was measured (Bio-Rad). PCA was assessed on the supernatants and in plasma using a 1-step plasma recalcification time. Then 100 μL of 25 mM CaCl2 was added to 100 μL citrated normal mouse platelet-poor plasma (Sigma), mixed with 100 μL supernatant or 200 μL plasma sample, and the clotting time was recorded. Measured PCA was identified as TF by preincubation of the sample with 100 nM of inactivated human factor VIIa (Novo Nordisk, Copenhagen, Denmark). This preincubation induced a lengthening of the clotting time (approximately 72% inhibition of PCA). Clotting times were compared with those of serial dilutions of a standard TF preparation (Biomérieux). PCA was expressed as mU/mg protein.
MMP gelatinolytic activity was measured (n = 10 E−/− × IL-10−/−and n = 8 E−/− × IL-10+/+, group) on the supernatant of spleen mononuclear cells (107 cells per mouse) prepared as above and aortas from a separate set of mice. Thoracic aortas were cut into approximately 1-mm rings and incubated in 250 μL serum-free Dulbecco’s Modified Eagle’s medium. After 24 h, conditioned media were collected, centrifuged (14000 g, 10 min, 4 °C), and tested for protein content (Bio-Rad). Pro-MMP 9, MMP 9, pro-MMP 2, and MMP 2 gelatinase activities were measured by SDS-PAGE zymography on 10% acrylamide gels containing 0.1% gelatin. After electrophoresis, gels were soaked with 2.5% Triton X-100 solution (2 × 30 min, 20 °C) and incubated at 37 °C for 19 h in 50 mmol/L Tris HCl, pH 7.8, 10 mmol/L CaCl2. Gelatinolysis in the gels was visualized by staining with 0.005% Coomassie Brilliant Blue R-250 in 5% acetic acid, 10% ethanol solution. Gelatinolytic bands were quantified by densitometry. Results are expressed as 103 AU/107 cells (spleen mononuclear cells) or AU/mg protein (aortas).
In vivo thrombosis
Plasma levels of prothrombin fragments 1 + 2 (F1+2) were measured by ELISA (Behring; n = 10, E−/− × IL-10−/−, and n = 8, E−/− × IL-10+/+ 48-wk-old mice). In order to assess the procoagulant state, separate sets of anesthetized mice (n = 10, E−/− × IL-10−/−and n = 10, E−/− × IL-10+/+ ) were injected intravenously with sublethal doses of human thrombin (1 U/g body weight). Mice surviving ≥ 15 min were euthanized. The trachea was perfused with 4% formaldehyde prior to harvesting the heart and the left lung. The heart was cut into 4 portions: base (including the origin of the coronary arteries), middle-high (from the left and right atria to the middle of the left ventricle), middle-low (about 3 mm from the middle of the left ventricle to the apex), and apex. Heart portions and lungs were embedded in paraffin blocks and 4 consecutive 5-μm–thick sections (50-μm interval) were stained with Masson’s trichrome. All artery profiles in each section were identified and the percentage of thrombosed arteries was determined under the microscope.
Statistical Analysis
Results are expressed as mean ± SEM. Differences between groups were compared by the Mann-Whitney U test or chi-square test and were considered statistically significant when P < 0.05.
RESULTS
E−/− × IL-10−/− double knockout and E−/− × IL-10+/+ mice were selected after genotyping the offspring derived from the original crossbreeding pairs. Body weight was similar in the 2 groups throughout the study as were plasma protein levels (Tables 1 and 2). Therefore, E−/− × IL-10−/− mice did not show signs of malabsorption despite the tendency of IL-10−/− animals to develop enterocolitis (31).
Table 1.
Metabolic and immunological characteristics of 16-wk-old mice
| E−/− × IL-10+/+ |
E−/− × IL-10−/− |
|||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Male | n | Female | n | Male | n | Female | n |
| Plasma proteins and lipids | ||||||||
| Plasma protein (g/dL) | 4.91 ± 0.18 | 7 | 4.89 ± 0.09 | 4 | 4.85 ± 0.12 | 9 | 4.90 ± 0.11 | 5 |
| Total cholesterol (mM) | 12.6 ± 1.0 | 7 | 11.1 ± 1.5 | 4 | 11.1 ± 0.7 | 9 | 8.7 ± 0.7 | 4 |
| Triglycerides (mg/dL) | 1.5 ± 0.1 | 7 | 1.3 ± 0.1 | 4 | 1.4 ± 0.1 | 9 | 1.1 ± 0.1 | 4 |
| Immune system status at baseline | ||||||||
| Total IgG2a (AU) | 0.985 ± 0.062 | 7 | 0.879 ± 0.102 | 4 | 0.965 ± 0.114 | 9 | 1.149 ± 0.038a | 5 |
| Total IgG1 (AU) | 0.515 ± 0.021 | 7 | 0.501 ± 0.035 | 4 | 0.520 ± 0.048 | 9 | 0.546 ± 0.007 | 5 |
| IL-12 (pg/mL) | 60 ± 14 | 7 | 74 ± 16 | 4 | 258 ± 147b | 9 | 109 ± 31 | 5 |
| IFNγ (pg/mL) | 253 ± 27 | 7 | 209 ± 96 | 4 | 320 ± 82b | 9 | 444 ± 31a | 5 |
P < 0.05.
P < 0.001 compared with sex matched E−/− × IL-10+/+ mice.
Table 2.
Metabolic, immunological, hemostatic, and proteolytic characteristics of 48-wk-old male mice
| Analyte | E−/− × IL-10+/+ | n | E−/− × IL-10−/− | n |
|---|---|---|---|---|
| Plasma proteins and lipids | ||||
| Plasma protein (g/dL) | 5.14 ± 0.12 | 12 | 4.79 ± 0.17 | 12 |
| Total cholesterol (mM) | 12.3 ± 0.5 | 7 | 7.9 ± 1.5a | 9 |
| Triglycerides (mg/dL) | 1.4 ± 0.1 | 10 | 1.4 ± 0.1 | 10 |
| Immune system status at baseline | ||||
| Total IgG2a (AU) | 0.810 ± 0.070 | 6 | 1.208 ± 0.046c | 6 |
| Total IgG1 (AU) | 0.530 ± 0.023 | 6 | 0.556 ± 0.051 | 6 |
| IL-12 (pg/mL) | 65 ± 11 | 6 | 1826 ± 1654c | 6 |
| IFNγ (pg/mL) | 223 ± 10 | 6 | 272 ± 12a | 6 |
| Immune response to antigenic challenge (HSA) | ||||
| Anti-HSA IgG2a (AU) | 3.71 ± 0.34 | 9 | 4.86 ± 0.19c | 9 |
| Anti-HSA IgG1(AU) | 4.76 ± 0.25 | 9 | 3.28 ± 0.34b | 9 |
| CD4+ IFNγ+ (%) | 5 ± 2 | 9 | 17 ± 4a | 9 |
| CD4+ IL-4+ (%) | 10 ± 2 | 9 | 9 ± 3 | 9 |
| Coagulation factors | ||||
| Plasma prothrombin F1+2 (nM) | 0.13 ± 0.01 | 8 | 0.30 ± 0.08b | 10 |
| Plasma tissue factor (mU/mg protein) | 3.99 ± 1.40 | 8 | 2.75 ± 1.75 | 10 |
| Aortic tissue factor (U/mg protein) | 3.1 ± 0.4 | 8 | 6.0 ± 1.0a | 10 |
| Spleen MNC tissue factor (mU/mg protein) | 2.78 ± 0.36 | 8 | 2.19 ± 0.22 | 10 |
| Thrombosed coronary arteries (%) | ||||
| Left ventricle portion (n° vessels) | ||||
| Base (2.7 ± 0.3) | 20.0 ± 8.1 | 10 | 70.0 ± 8.2b | 10 |
| Middle-high (6.8 ± 0.4) | 13.3 ± 4.5 | 10 | 54.2 ± 6.3b | 10 |
| Middle-low (11.2 ± 0.6) | 28.9 ± 3.1 | 10 | 54.2 ± 6.1b | 10 |
| Apex (7.8 ± 0.5) | 5.3 ± 3.3 | 10 | 35.5 ± 5.9b | 10 |
| Proteolysis | ||||
| MMP9 distribution (shoulder/total plaque) | 0.53 ± 0.06 | 8 | 0.76 ± 0.07b | 10 |
| Aortic pro-MMP9 activity (AU/mg protein) | 1708 ± 466 | 8 | 4374 ± 1141a | 10 |
| Spleen MNC pro-MMP9 activity (103 AU/107 cells) | 141 ± 13 | 8 | 1097 ± 97a | 10 |
| Aortic pro-MMP2 activity (AU/mg protein) | 7331 ± 516 | 8 | 7142 ± 442 | 10 |
| Aortic MMP2 activity (AU/mg protein) | 1937 ± 193 | 8 | 1688 ± 178 | 10 |
P < 0.05.
P < 0.01.
P < 0.001.
MNC, mononuclear cells.
Lack of IL-10 Accelerates Atherogenesis in 16-wk-old Mice
Early lesion development was increased substantially in mice lacking IL-10 (74706 ± 12385 compared with 26739 ± 3522 μm2 in E−/− × IL-10+/+ mice, P < 0.01). The difference in lesion size in female mice accounted for the statistical difference between the 2 genotypes; in male mice, lesions tended to be larger in IL-10 deficient mice but the difference did not reach statistical significance (Figure 1). Immunohistochemical staining for α SM–actin, CD4 (Th lymphocytes), CD8 (Tc lymphocytes), and I-Ab (MHC-II positive cells including macrophages) did not show any differences between E−/− × IL-10−/− and E−/− × IL-10+/+ mice (data not shown).
Figure 1.
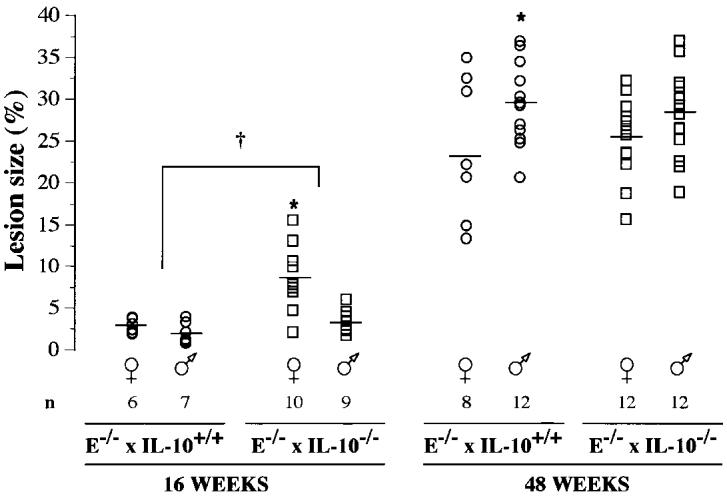
Computer-assisted morphometry was applied to aortic cryosections, stained with oil red O, harvested at the level of the aortic cusps. Data show lesion size (surface area of lesions/surface area of vessel, %) and were averaged from 4 sections per mouse (400, 500, 600, and 700 μm from the appearance of the 1st cusp). —, mean; *P < 0.05, female compared with male mice; †P < 0.05, E−/− × IL-10−/− compared with E−/− × IL-10+/+ mice.
IL-10 Deficiency Modifies Lipoprotein Profiles Independently from the Effect on Lesion Size in 16-wk-old Mice
Sixteen-week-old E−/− × IL-10−/− mice had cholesterol and triglyceride levels similar to those of E−/− × IL-10+/+ mice (see Table 1) but the lack of IL-10 resulted in significant effects on lipoprotein profiles. FPLC analysis revealed a dramatic shift from large very-low-density lipoprotein (VLDL) to smaller low-density-lipoprotein (LDL) particles (Figure 2). These lipoprotein modifications were evident in both male and female E−/− × IL-10−/− mice but did not correlate with lesion size.
Figure 2.
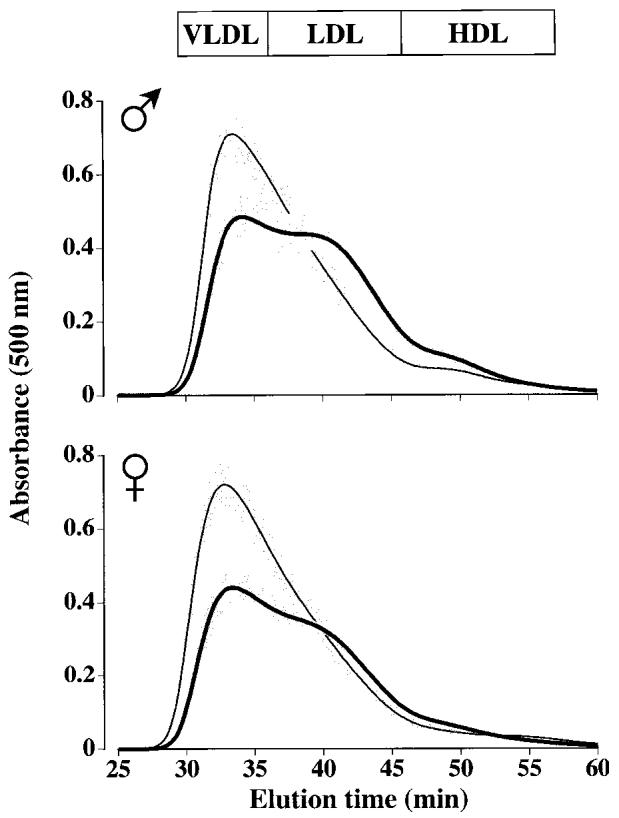
Plasma lipoprotein patterns of 16-wk-old male (A) and female (B) mice following separation by FPLC. Lines represent means and gray sections represent SEM. A: Thin line, 7 male E−/− × IL-10+/+ mice; thick line, 9 male E−/− × IL-10−/− mice. B: Thin line, 4 female E−/− × IL-10+/+ mice; thick line, 4 female E−/− × IL-10−/− mice. In both panels, light gray areas show the results of 2 different human samples, after 4 repetitive injections (before, after, and between mouse samples) to monitor assay drift over time and to show elution profile for human.
Lack of IL-10 Induces a Th1 Shift in 16-wk-old E−/− Female Mice
In parallel with lesion increase, there was a clear Th1 cytokine shift at 16 wk, as detected by increased plasma levels of IL-12 and IFN-γ in E−/− × IL-10−/− compared with E−/− × IL-10+/+ mice (see Table 1). As expected for mice on a C57BL/6 background, IL-4 (Th2) levels were below the ELISA detection limit of 31 pg/mL in all mice. The impact of Th1 circulating cytokines on the IgG Th1 isotype (IgG2a) was observed only in female E−/− × IL-10−/− mice at this stage of the disease (see Table 1).
No Detectable Effect of IL-10 Deficiency on Lesion Size at 48 wk
By 48 wk, aortic lesions in E−/− × IL-10+/+ mice were approximately 10 times larger than at 16 wk (see Figure 1). However, lesions size no longer differed significantly between E−/− × IL-10−/− and E−/− × IL-10+/+ groups (303204 ± 25586 μm2 and 349065 ± 28183 μm2, not significant; see Figure 1). Furthermore, no difference was found in the content of smooth muscle cells or inflammatory cells in the different groups, as assessed by immunohistochemistry (data not shown). At this age, independently of the genotype, male mice tended to have larger lesions than female mice (see Figure 1). Thus, to evaluate the phenotype of advanced lesions, we used male mice.
At 48 wk, a Th1 Shift Is Evident in Male E−/− × IL-10−/−Mice
IL-12 and IFN-γ plasma levels remained elevated in old male double knockout mice compared with control mice (see Table 2). While basal plasma levels of total IgG1 (Th2) were similar in the 2 groups at all time points, the basal Th1 shift (IgG2a switch), which was detected in female mice by the age of 16 wk (see Table 1), became evident in male mice only by the age of 48 wk (see Table 2).
Although basal Th2 responses did not appear to be affected by IL-10 deficiency, they could have been affected upon antigenic stimulation. Thus, we analyzed the polarization of immune responses by intracellular staining of CD4+ T cells in draining lymph nodes after immunization with HSA. The percentage of Th1 polarized IFN-γ+ CD4+ T cells upon antigenic stimulation was increased threefold in IL-10 deficient apoE−/− mice, while the proportion of IL-4+ CD4+ Th2 cells was similar in the 2 groups (see Table 2). Accordingly, this Th1 bias influenced the humoral response: plasma levels of anti-HSA IgG2a (Th1) antibodies were higher in E−/− × IL-10−/− whereas levels of anti-HSA IgG1 (Th2) antibodies were lower in E−/− × IL-10−/− mice than in E−/− × IL-10+/+ mice (see Table 2).
IL-10 Deficiency Is Associated with MMP 9 Redistribution and Increased Activity
Immunohistology of aortic plaques showed that MMP 9 antigen was more abundant in E−/− × IL-10−/− than in E−/− × IL-10+/+ 48-wk-old mice; the increase is particularly striking in the macrophages infiltrating the shoulder region (Figure 3).
Figure 3.
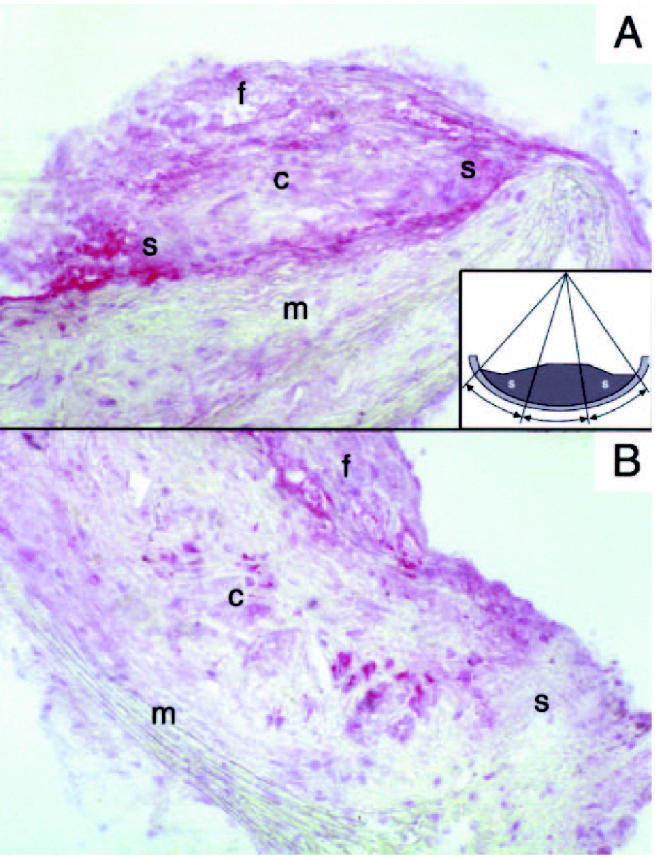
MMP 9 localization in plaques from E−/− × IL-10−/− (A) and E−/− × IL-10+/+ (B) mice. Original magnification ×200; c, core; f, fibrous cap; m, media; s, shoulder region. Note MMP 9 accumulates in the shoulder regions in E−/− × IL-10−/− and not in E−/− × IL-10+/+ mice. Quantification was performed by computer-assisted morphometry. Plaques were divided into 3 portions as indicated in the insert (s, shoulder). The optical density within shoulder regions and the optical density within the total surface area of the plaque were computed.
The ratio of MMP 9 expression in the shoulder regions to total MMP 9 in the plaque, assessed by computer-assisted morphometry, was significantly higher in advanced lesions of E−/− × IL-10−/− mice (see Table 2). Furthermore, gelatinolytic pro-MMP 9 activity was greater in the aortas of E−/− × IL-10−/− mice compared with E−/− mice (see Table 2, Figure 4). Pro-MMP 9 activity also was significantly increased in spleen mononuclear cells of E−/− × IL-10−/−mice (Figure 4). In contrast, aortic gelatinolytic activity of pro-MMP 2 and MMP 2 were similar in the 2 groups (see Table 2).
Figure 4.
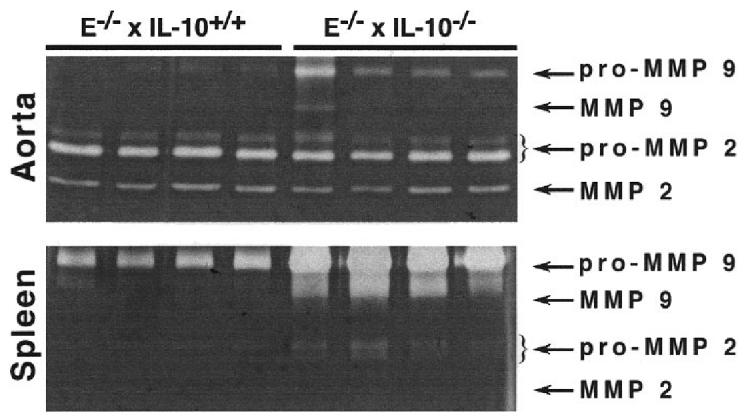
Gelatinolytic activities (zymography) of aortas and spleen mononuclear cells from 4 E−/− × IL-10+/+ and 4 E−/− × IL-10−/− mice. Samples were prepared as described in Materials and Methods. Gels were submitted to scanning densitometry analysis (see Table 2).
Lack of IL-10 Promotes a Procoagulant State in 48-wk-old E−/− Mice
In vivo thrombin formation (as assessed by thrombin F1+2 plasma levels [32]) was increased in E−/− × IL-10−/− compared with E−/− × IL-10+/+ mice (see Table 2). Similarly, protein extracts from the aortas of 48-wk-old E−/− × IL-10−/− mice contained significantly higher levels of TF activity compared with those from E−/− × IL-10+/+ mice (see Table 2). In contrast, the TF content in plasma and in spleen mononuclear cells was similar in E−/− × IL-10−/−and E−/− × IL-10+/+ mice. Immunohistology of aortic plaques revealed the presence of TF antigen, with similar spatial distribution, in the 2 strains (data not shown).
Thrombin-induced Thrombosis Is Precipitated in E−/− × IL-10−/− Mice
The thrombotic response to intravenous thrombin injection was dramatically enhanced by the lack of IL-10. Within 5 min of injection, 8 of 10 E−/− × IL-10−/− mice died of pulmonary embolism compared with only 2 of 10 E−/− × IL-10+/+ mice (P < 0.01, chi-square test). Independent from the presence of atherosclerotic plaques, thrombosis was evident in coronary arteries, where it typically obstructed main coronaries and their epicardial and intramyocardial branches (Figure 5). Thrombi were composed mainly of platelets (light gray) and erythrocytes (bright red, see Figure 5). The total number of coronary artery sections was recorded, and the percentage of coronary sections occupied by platelet thrombi was calculated for each portion of the heart. The results showed that intracoronary platelet thrombi occurred significantly more frequently in the hearts of E−/− × IL-10−/− than in those of IL-10 expressing E−/− mice (see Table 2).
Figure 5.
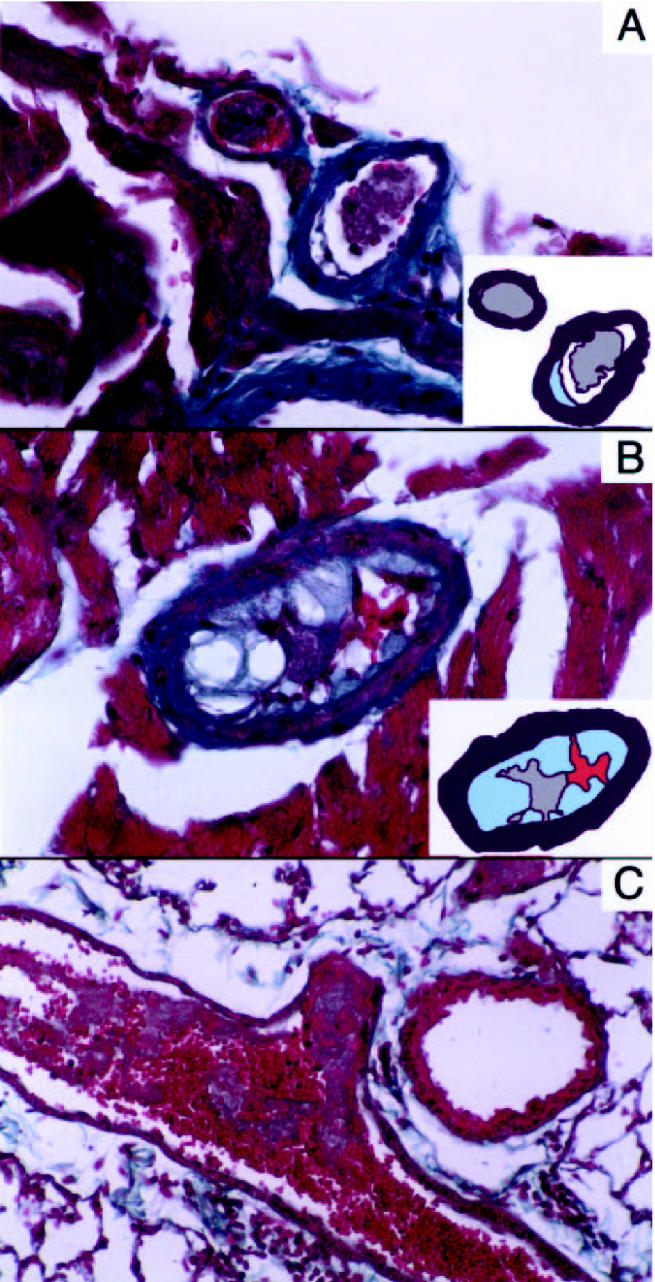
Platelet arterial thrombi in thrombin-injected E−/− × IL-10−/−mice. Masson’s trichrome, magnification ×200 (A,B) and ×100 (C). A: Platelet thrombi occupy the lumen of 2 small epicardial branches of the left anterior descending coronary artery. An atherosclerotic plaque is evident in 1 of 2 thrombosed arteries. Insert: A model depicts the thrombus (gray) and the plaque (light blue). B: A thrombus occluding the lumen of an atherosclerotic intramyocardial coronary branch. Insert: A model depicts the platelet-rich part of the thrombus (gray), the erythrocytes (red), and the plaque (light blue). C: An occlusive red clot in a pulmonary artery of a mouse that died within 5 min of intravenous thrombin injection.
DISCUSSION
We have previously suggested that proinflammatory Th1 immunity is proatherogenic while the counterbalancing Th2 activity may be antiatherogenic (1,5,12,13). Recent studies support this notion by showing enhanced fatty streak development in IL-10 deficient C57BL/6 mice (2,4), reduced carotid collar-induced stenosis in LDL receptor knockout mice after gene transfer of IL-10 (33), and reduced atherosclerosis after transfer of IL-10 overexpressing T cells into LDL receptor knockout mice (3). Our present data confirm and extend this concept by showing that IL-10 deficient E−/− mice exhibit larger lesions in the early phase of disease and increased proteolytic and procoagulant activities in advanced atherosclerosis.
We already have reported that young female E−/− mice are more susceptible to atherosclerosis than males (34). In the present work, IL-10 deficiency led to increased lesion size in the early phase of disease development exclusively in females. Furthermore, a recent report shows that IFN-γ inhibition affect atherosclerotic lesion development only in male mice (35). Interestingly, other immunoinflammatory disorders show clear-cut sex dependence (36–40). Altogether, these findings raise the question whether the effect of sex on atherosclerosis relates to differences in the sexes’ reactivity to cytokines. Indeed, we found that in spite of a similar increase in Th1 cytokines in male and female double knockout mice, the accompanying Th1 IgG switch was detectable earlier in female than male mice. This suggests that regulatory properties of Th1 cytokines may differ according to sex. Sex hormones may actually modulate cytokine production in vivo, which in turn could contribute to sex-related differences in normal and pathological immune responses (41).
It is not unexpected that IL-10 deficiency increased atherosclerosis susceptibility at the onset of lesion development since the early phases of atherosclerosis are dominated by macrophages, which are exquisitely sensitive to Th1 and Th2 cytokines. In mice with advanced lesions, no significant difference in lesion size was observed between IL-10 deficient and IL-10 competent mice. This could imply that IL-10 does not affect the further growth of atheroma at this stage. However, old IL-10 deficient E−/− mice presented a significant reduction in total plasma cholesterol levels (see Table 2), which may counterbalance any lesion promoting activities at this stage.
Our data identify plasma lipoprotein metabolism as an important target for IL-10. By 16 wk, gel filtration analysis showed that E−/− mice had high VLDL levels and only a small LDL notch. IL-10 deficiency led to a reduced VLDL peak with a shift to LDL, which appeared as a distinctive peak in the plasma of compound E−/− × IL-10−/− mice. Since there was a shift from VLDL to LDL rather than a total increase in cholesterol-rich lipoproteins, no significant changes were observed in total cholesterol or triglyceride levels. The precise mechanism causing this shift remains unclear. One would expect that a loss of IL-10 should lead to an increased secretion of proinflammatory cytokines including tumor necrosis factor-α, which is known to inhibit lipoprotein lipase (42). However, this should result in increased rather than decreased VLDL and is associated with hypertriglyceridemia. Further studies will be required to characterize the effect of IL-10 on lipoproteins.
Small, dense LDL is proatherogenic in man, probably because it penetrates and is trapped in the subendothelial intima to a greater extent than larger VLDL particles (43). An increase in the smaller LDL particles correlates with accelerated atherosclerosis in murine models (44) and partly may explain the increase in early atherosclerosis in the E−/− × IL-10−/− mice. However, the switch to LDL was more pronounced in male mice, which also tended to have higher cholesterol levels but smaller lesions than females. Therefore mechanisms other than lipid profile modifications are likely playing a role in lesion increase in E−/− × IL-10−/− mice.
Recent studies have suggested that an enhanced Th1 response may be responsible for clinical instability (17). Interestingly, patients with rheumatoid arthritis, a disease considered to be Th1-driven, exhibit greater prevalence of atherosclerosis (45) and an increase in proatherogenic, small dense LDL particles (46). Experimental studies show that E−/− mice have a Th1-biased phenotype (13) and pharmacological Th1 blockade by pentoxifylline reduces the progression of atherosclerosis in E−/− mice (5). Therefore, it was interesting to evaluate whether IL-10 deficiency could enhance the Th1-biased phenotype of E−/− mice and whether this in turn could influence plaque growth and stability. We assessed the Th balance both in basal conditions and upon antigenic stimulation. Indeed, an imbalance of Th1 and Th2 cytokines was detected by increased plasma levels of IL-12 and IFN-γ and increased antigen-elicited IgG2a antibodies and IFN-γ+ T cells in double knockout mice. While this Th1-bias was accompanied by a higher susceptibility to atherosclerosis at the onset of the disease, this was not evident at a more advanced stage, indicating either that the lack of IL-10 was counterbalanced during the disease process by overexpression of other cytokines or that the Th balance only influences plaque growth at the early stages.
Clinical instability of atherosclerosis is related to activation of local inflammatory and immune cells with increased expression of MMPs (20) and TF (47) in the culprit plaque and increased systemic production of MMPs (48) and thrombin (32). Based on the reported inhibitory effects of IL-10 on MMPs and TF (24,25), we speculated that IL-10 deficiency may destabilize plaques. Indeed, plaques of E−/− × IL-10−/− mice were richer in pro-MMP 9 and TF activities, suggesting a protective role for IL-10 in plaque stability. MMP 9 was more abundant in the shoulder regions of lesions found in the aortic cusps in E−/− × IL-10−/− mice. It is possible that such plaques are more prone to rupture. Although no overt ruptures were detected in this study, others have found evidence for plaque rupture in arteries of E−/− mice (49,50). In parallel, TF activity in atherosclerotic aortas and plasma levels of prothrombin F1+2 were increased in E−/− × IL-10−/− mice. This indicates that IL-10 deficiency resulted in an enhanced procoagulant state, which is a hallmark of acute atherosclerotic events (51). Since IL-10−/− mice are known to be susceptible to venous thrombosis (52), it was interesting to evaluate whether IL-10 also could affect arterial thrombosis in atherosclerotic hearts. Thrombin-injected E−/− × IL-10−/− mice died almost immediately of pulmonary thromboembolism. Interestingly, in addition to pulmonary emboli, increased coronary artery thrombosis was observed in 48-wk-old E−/− × IL-10−/− compared with E−/−mice of the same age. It is unlikely that intravascular coagulation was a post mortem event since we prevented the formation of post-mortal clots by perfusion-fixation at sacrifice. Since thrombi also occurred at sites not affected by atherosclerosis, it is possible that the propensity for thrombosis was independent of atherosclerosis. These issues notwithstanding, our data point to a protective role for IL-10 in atherosclerosis and thrombosis. Certain effects of IL-10 deficiency depended on sex and the stage of lesions, indicating that cytokines likely are playing an exquisitely time- and sex-regulated role in atherogenesis. It is tempting to speculate that cytokine treatment may be a novel therapeutic approach for atherosclerosis in the future. As a 1st step, it will be important to identify the therapeutic window and the molecular targets for IL-10 in lipoprotein metabolism as well as vascular inflammation.
Acknowledgments
We thank Inger Bodin, Liliane Louedec, Chantal Mandet, Mirre Mikkola, Ingrid Törnberg, and Kristina Edwardsson for excellent technical assistance. GC is the recipient of a Marie Curie TMR grant (BMH4-CT98-5108). This work was in part supported by an INSERM-MFR (France-Sweden) collaboration grant and by the Swedish Medical Research Council (6816, 13571, 14053), Heart & Lung Foundation, the Karolinska Institute and Söderberg Foundation.
REFERENCES
- 1.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 2.Mallat Z, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 3.Pinderski LJ, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–71. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 4.Pinderski Oslund LJ, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 5.Laurat E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 6.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–53. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 10.Mallat Z, et al. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 19:611–6. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- 11.Uyemura K, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–25. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–42. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, et al. IFN-γ potentiates atherosclerosis in ApoE knockout mice. J Clin Invest. 1997;99:2752–61. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–24. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liuzzo G, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon γ inhibits both proliferation and expression of differentiation-specific α-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989;170:1595–608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscl Thromb. 1991;11:1223–30. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 20.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–50. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 21.Saren P, Welgus HG, Kovanen PT. TNF-γ and IL-1β selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–65. [PubMed] [Google Scholar]
- 22.Arnman V, Stemme S, Rymo L, Risberg B. Interferon-γ modulates the fibrinolytic response in cultured human endothelial cells. Thromb Res. 1995;77:431–40. doi: 10.1016/0049-3848(95)93879-5. [DOI] [PubMed] [Google Scholar]
- 23.Gallicchio M, Hufnagl P, Wojta J, Tipping P. IFN-γ inhibits thrombin- and endotoxin-induced plasminogen activator inhibitor type 1 in human endothelial cells. J Immunol. 1996;157:2610–7. [PubMed] [Google Scholar]
- 24.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5:189–96. [PubMed] [Google Scholar]
- 25.Ramani M, et al. Interleukin-10 inhibits endotoxin-induced tissue factor mRNA production by human monocytes. FEBS Lett. 1993;334:114–6. doi: 10.1016/0014-5793(93)81693-t. [DOI] [PubMed] [Google Scholar]
- 26.Pajkrt D, et al. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89:2701–5. [PubMed] [Google Scholar]
- 27.Furukawa Y, Becker G, Stinn JL, Shimizu K, Libby P, Mitchell RN. Interleukin-10 (IL-10) augments allograft arterial disease: paradoxical effects of IL-10 in vivo. Am J Pathol. 1999;155:1929–39. doi: 10.1016/S0002-9440(10)65512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest. 1998;102:910–8. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gullberg H, Rudling M, Forrest D, Angelin B, Vennstrom B. Thyroid hormone receptor β-deficient mice show complete loss of the normal cholesterol 7α-hydroxylase (CYP7A) response to thyroid hormone but display enhanced resistance to dietary cholesterol. Mol Endocrinol. 2000;14:1739–49. doi: 10.1210/mend.14.11.0548. [DOI] [PubMed] [Google Scholar]
- 30.Openshaw P, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 32.Biasucci LM, et al. Temporal relation between ischemic episodes and activation of the coagulation system in unstable angina. Circulation. 1996;93:2121–7. doi: 10.1161/01.cir.93.12.2121. [DOI] [PubMed] [Google Scholar]
- 33.Von Der Thusen JH, et al. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLR−/− mice. FASEB J. 2001;15:2730–2. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 34.Caligiuri G, Nicoletti A, Zhou X, Tornberg I, Hansson GK. Effects of sex and age on atherosclerosis and autoimmunity in apoE deficient mice. Atherosclerosis. 1999;145:301–8. doi: 10.1016/s0021-9150(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 35.Whitman SC, Ravisankar P, Daugherty A. IFN-γ deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–70. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 36.Carlsten H, Tarkowski A, Holmdahl R, Nilsson LA. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr/lpr mice. Clin Exp Immunol. 1990;80:467–73. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlsten H, Nilsson N, Jonsson R, Tarkowski A. Differential effects of oestrogen in murine lupus: acceleration of glomerulonephritis and amelioration of T cell-mediated lesions. J Autoimmun. 1991;4:845–56. doi: 10.1016/0896-8411(91)90048-h. [DOI] [PubMed] [Google Scholar]
- 38.Carlsten H, et al. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol. 1992;144:190–202. doi: 10.1016/0008-8749(92)90236-i. [DOI] [PubMed] [Google Scholar]
- 39.Carlsten H, Tarkowski A. Histocompatibility complex gene products and exposure to oestrogen: two independent disease accelerating factors in murine lupus. Scand J Immunol. 1993;38:341–7. doi: 10.1111/j.1365-3083.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 40.Holmdahl R, Carlsten H, Jansson L, Larsson P. Oestrogen is a potent immunomodulator of murine experimental rheumatoid disease. Br J Rheumatol. 1989;28:54–8. doi: 10.1093/rheumatology/xxviii.suppl_1.54. [DOI] [PubMed] [Google Scholar]
- 41.Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. 2000;100:384–90. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tracey KJ, Cerami A. Metabolic responses to cachectin/TNF. A brief review. Ann NY Acad Sci. 1990;587:325–31. doi: 10.1111/j.1749-6632.1990.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 43.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–56. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 44.Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in ApoE(−/−) and LDLR(−/−) mice. Arterioscler Thromb Vasc Biol. 2001;21:1567–70. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson SW, et al. Increased prevalence of atherosclerosis in patients with medium term rheumatoid arthritis. J Rheumatol. 2001;28:2597–602. [PubMed] [Google Scholar]
- 46.Hurt-Camejo E, et al. Elevated levels of small, low-density lipoprotein with high affinity for arterial matrix components in patients with rheumatoid arthritis: possible contribution of phospholipase A2 to this atherogenic profile. Arthritis Rheum. 2001;44:2761–7. doi: 10.1002/1529-0131(200112)44:12<2761::aid-art463>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Ardissino D, et al. Tissue-factor antigen and activity in human coronary atherosclerotic plaques. Lancet. 1997;349:769–71. doi: 10.1016/S0140-6736(96)11189-2. [DOI] [PubMed] [Google Scholar]
- 48.Kai H, et al. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–72. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld ME, et al. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–92. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 51.Merlini PA, et al. Heightened thrombin formation but normal plasma levels of activated factor VII in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1995;15:1675–9. doi: 10.1161/01.atv.15.10.1675. [DOI] [PubMed] [Google Scholar]
- 52.Downing LJ, et al. IL-10 regulates thrombus-induced vein wall inflammation and thrombosis. J Immunol. 1998;161:1471–6. [PubMed] [Google Scholar]


