Abstract
The aim of the present study was to compare aldehyde levels resulting from lipid peroxidation in exhaled breath condensate (EBC) and induced sputum (IS) supernatant of subjects with asthma and chronic obstructive pulmonary disease (COPD).
Aldehydes (malondialdehyde (MDA), acrolein, n-hexanal (C6), n-heptanal (C7), n-nonanal (C9), 4-hydroxynonenal (HNE) and 4-hydroxyhexenal (HHE)) in both biological fluids were measured by liquid chromatography-tandem mass spectrometry.
MDA concentrations in sputum were 132.5 nM (82.5–268.8) and 23.7 nM (9–53.7) in EBC. Similarly, C6, C7 and C9 concentrations in IS were 1.5–4.7-fold higher than in EBC. Acrolein levels were 131.1 nM (55.6–264.6) in IS and 45.3 nM (14.4–127.1) in EBC. The concentrations of HNE and HHE in IS were not significantly different from the levels in EBC. Aldehyde levels in EBC did not show any correlation with aldehyde levels in IS or with differential sputum cellular count. In COPD, MDA in EBC, but not its IS counterpart, was negatively correlated with the severity of disease.
In conclusion, the data presented here show that aldehydes can be detected in both exhaled breath condensate and supernatant of induced sputum, but that their relative concentrations are different and not correlated with each other. Therefore, with regard to lipid peroxidation products, exhaled breath condensate and induced sputum must be considered as independent techniques.
Keywords: Aldehydes, exhaled breath condensate, induced sputum
Oxidative stress is part of inflammatory processes common to many lung diseases associated with chronic airway inflammation, such as asthma and chronic obstructive pulmonary disease (COPD) [1]. The increased oxidative stress in patients with asthma and COPD derives from the burden of inhaled oxidants, and reactive oxygen species generated by several inflammatory, immune and structural cells of the airways [2].
Oxidative stress can be assessed and monitored through the determination of the levels of biomarkers, such as hydrogen peroxide [3], lipid peroxidation-derived products, namely aldehydes and isoprostanes [4, 5], and protein carbonyl groups [6]. The oxidative biomarkers may be found in different biological samples, such as serum [7], bronchoalveolar lavage (BAL) [8], sputum [9] and exhaled breath condensate (EBC) [10].
The current authors recently set up a robust analytical method for the simultaneous determination of different aldehydes, namely malondialdehyde (MDA), acrolein, n-hexanal (C6), n-heptanal (C7), n-nonanal (C9), 4-hydroxynonenal (HNE) and 4-hydroxyhexenal (HHE), in EBC using liquid chromatography-tandem mass spectrometry (LC-MS/MS) [11]. The selected aldehydes are by-products of lipid peroxides, which are thought to reflect oxidant-induced damage on unsaturated lipids in cell membranes [12]. Using this analytical method, an increased level of aldehydes (mainly MDA) has been demonstrated in EBC of asthmatic children and adults with COPD when compared to control groups [13, 14]. These data support the use of this safe and simple method for the assessment of oxidative stress in lung diseases as a biomarker of chronic airway inflammation.
It must be noted, however, that the EBC technique is still in its infancy and, before it can become accepted as a routine investigation, further work is required. In addition to the ongoing refinements of the techniques applied to detect and analyse different biomarkers in EBC, there is also a lack of information on the relative proportions of those biomarkers in other relevant biological fluids [15]. Although in some studies biomarker levels in EBC have been compared with inflammatory indices obtained by other well-validated means, such as sputum [16-18], there are no studies where the same biomarkers have been measured in both biological fluids. The comparison of biomarker levels in two different biological fluids could also shed light on whether the sampling site of the two methods within the respiratory tract is similar or different. In addition, there are no studies applying LC-MS/MS in the analysis of sputum supernatants for the assessment of oxidative stress in diseases such as asthma or COPD.
The aim of the present study was to compare aldehyde levels in EBC and in the sol phase of induced sputum (IS) of patients with chronic airway inflammation, by means of a new analytical method based on LC-MS/MS.
Materials and methods
Subjects
The study included 10 subjects with mild-to-moderate asthma, 11 subjects with mild-to-moderate and severe COPD, and nine healthy nonsmoking controls. Cases and controls were recruited among inpatients and healthy volunteers attending the Allergology and Immunology Unit and the Rehabilitative Pneumology Unit of Maugeri Foundation (Pavia, Italy). Characteristics of the study subjects are shown in table 1.
Table 1.
Characteristics of study subjects
| Asthmatics | COPD | Controls | |
|---|---|---|---|
| Age yrs | 38.5 (24–67) | 74 (34–83) | 32 (25–50) |
| Sex F/M | 5/5 | 1/10 | 8/1 |
| Steroid treatment n | 3 | 3 | 0 |
| FEV1 L | 2.8 (0.9–4.7) | 1.5 (0.8–3.1)* | 3.2 (2.08–5.78) |
| FVC L | 3.9 (2.9–5.4) | 2.9 (1.5–4.2) | 3.55 (2.54–6.82) |
| FEV1 % pred | 93.5 (54–125) | 66.5 (45–82)* | 115 (90–136) |
| FVC % pred | 105 (89–115) | 86 (64–164) | 110 (93–134) |
Data are presented as median (range), unless otherwise stated. COPD: chronic obstructive pulmonary disease; F: female; M: male; FEV1: forced expiratory volume in one second; FVC: forced vital capacity.
p<0.05 asthmatics versus COPD patients.
All the patients with COPD met the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for the diagnosis of COPD [19]. Patients with COPD had a history of cough and sputum production for >2 consecutive yrs and for most days in a consecutive 3-month period, and fixed airflow obstruction, which was defined as post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity <70% and a post-bronchodilator reversibility of FEV1<12%, measured at baseline and after inhalation of a β2-agonist (salbutamol 400 μg, via metered-dose inhaler). All COPD patients had a history of cigarette smoking (two were current smokers, 50±11.8 pack-yrs) and the remaining patients were ex-smokers. Three COPD patients were being treated with inhaled corticosteroid therapy.
Asthma was diagnosed, according to the National Institutes of Health guidelines [20], as consisting of increase in FEV1 in response to a bronchodilator. Three asthmatics were current smokers (8.8±4.4 pack-yrs) and the remaining asthmatics were never-smokers. Three asthmatics were being treated with inhaled corticosteroid therapy.
None of the COPD and asthma patients was taking oral corticosteroids and none reported any worsening of their symptoms (exacerbation) for at least 2 weeks prior to collection of EBC and IS.
This study conformed to the declaration of Helsinki and was approved by the Internal Review Board of the Maugeri Foundation. Informed consent was obtained from all patients.
Study design
This was an observational study and subjects were requested to collect EBC first, followed by induction of sputum. EBC and IS samples were stored under the same conditions (−80°C) for the same period of time ( months).
Exhaled breath condensate collection. EBC samples were collected in a condensing device, composed of two glass chambers (Incofar Srl, Modena, Italy), as described previously [21]. Briefly, the subjects, without a nose clip, were instructed to tidally breathe through their mouths into a two-way nonrebreathing valve (Mallinckrodt Medical Srl, Modena, Italy) for 20 min. To minimise salivary contamination, the two-way valve served as a saliva trap, with a 12-cm banded tube vertically positioned between the mouthpiece and the condensing device, while the volunteer's mouth remained in a position lower than the device's inlet. In addition, subjects were asked to swallow their saliva periodically. The contamination of saliva in EBC samples was excluded by measuring salivary α-amylase. EBC samples were then immediately stored in sterile polypropylene test tubes at −80°C. Larstad et al. [22] previously reported that EBC samples could be stored for 3 months at −80°C without losses. This observation was further extended in the current study, showing that EBC aldehyde levels did not statistically change when stored for up to 8 months at −80°C (data not shown).
The long-term reproducibility of aldehyde measurements in EBC was assessed in a group of normal smokers (n=15), who were evaluated on five consecutive separate occasions, collected over a 6-week period.
Sputum induction and collection. Sputum was induced and processed at the Allergology and Immunology Unit of Maugeri Foundation. IS was obtained according to international guidelines [23]. Briefly, FEV1 was measured before and 10 min after inhalation of salbutamol (200 μg). Ultrasonically nebulised (De Vilbiss 65; De Vilbiss Co., Somerset, PA, USA) hypertonic (4.5%) saline was inhaled for 1, 2, 4, 6 and 16 min. FEV1 was measured 1 min after each inhalation period. Subjects were instructed to adequately rinse their mouth with water, and to cough and produce sputum after each inhalation period.
IS was conserved at 4°C before processing was initiated within 2 h. IS processing was performed according to the literature [24]. Briefly, sputum plugs were selected from saliva, and were weighed and treated with a volume (in μL) of 0.1% dithiothreitol (DTT) (sputolysin 10%; Calbiochem, La Jolla, CA, USA), diluted in 3% bovine serum albumin (BSA), equal to four times (in μL) the weight (in mg) of sputum plugs. IS samples were mixed and incubated at 37°C for 15 min. Subsequently, an equal volume of phosphate buffered saline (PBS) was added to the mixture and filtered with a 70-μm cell strainer (Falcon; Becton Dickinson, Franklin Lakes, NJ, USA). Cells were separated from supernatant by centrifugation at 300×g for 5 min and diluted in a volume of 1% BSA in PBS equal to the volume of DTT first added. Cell count and viability by Trypan blue exclusion were determined with optical microscopy. Cytospins were stained with Diff-Quick (Dade Diagnostika GmbH, Unterscheiheim, Germany) and analysed for differential cell count. Sputum samples with <30% squamous cells were considered acceptable. Sputum supernatant was stored at −80°C.
In preliminary experiments, it was verified that the addition of 0.1% DTT to aqueous standard mixtures of aldehydes, as well as to naïve EBC samples, and their subsequent incubation did not affect the concentration of any aldehydes, nor when a 10-fold-more concentrated solution (1% DTT) was used, nor when incubation lasted up to 60 min (table 2).
Table 2.
Effect of dithiothreitol (DTT) on biomarker levels in exhaled breath condensate (EBC)
| EBC samples treated with DTT | Naïve EBC samples | p-value# | |
|---|---|---|---|
| MDA | 8.1 (5.5–11.9) | 8.3 (5.6–16.6) | 0.92 |
| Acrolein | 10.0 (7.7–29.2) | 10.2 (7.1–16.3) | 0.76 |
| C6 | 21.4 (14.7–25.8) | 21.1 (13–30.7) | 0.41 |
| C7 | 16.8 (10.4–32.2) | 16.5 (9.85–28.5) | 0.38 |
| C9 | 27.5 (16.6–49.9) | 28.3 (18.2–48.7) | 0.53 |
Data are presented as nM median (range). MDA: malondialdehyde; C6: n-hexanal; C7: n-heptanal; C9: n-nonanal.
Wilcoxon matched-paired test.
Aldehyde measurements. Aldehyde analyses in EBC and IS were performed at the Laboratory of Industrial Toxicology of the University of Parma (Parma, Italy). Aldehydes in EBC, namely MDA, acrolein, HHE, HNE, C6, C7 and C9, were measured after derivatisation with 2,4-dinitrophenylhydrazine (DNPH) by LC-MS/MS (API 365; Perkin Elmer Sciex, Thornhill, Canada), as previously described [11]. Briefly, ionisation of the analytes was obtained by atmospheric pressure chemical ionisation, in positive-ion mode for MDA and in negative-ion mode for all other aldehydes. DNPH derivatives were separated on a Supelcosil C18 DB column (75×4.6 mm inside diameter, 3 μm; Supelco; Bellefonte, PA, USA), using variable proportions of 20-mM of aqueous acetic acid and methanol.
The sample preparation procedure was adapted for sputum samples. IS samples were added with the derivatising agent (DNPH) dissolved in acetonitrile, vortexed and centrifuged at 10,000×g for 4 min, in order to obtain precipitation of proteins. Subsequently, 20 μL of the supernatant were injected into the LC-MS/MS system and analysed.
Due to the small sample volume available, it was impossible to apply the standard addition method to every sample. Calibration was then performed by spiking a pooled IS sample with standard mixtures, in order to obtain concentration increases in the range 20–200 nM for MDA, acrolein, C6, C7 and C9, and in the range 1–20 nM for HHE and HNE (r2>0.997). The intra-session variability of aldehyde measurements in IS was within 2–8% for all analytes.
Statistical analysis
Data are presented as median (range). Comparisons between groups were evaluated by the Mann-Whitney U-test and by the Wilcoxon matched-paired test, when appropriate. Correlations between variables were evaluated by the Spearman's rank test. A p-value <0.05 indicated a statistical significance.
Results
Clinical and safety
IS and EBC collection was not associated with significant adverse effects.
Aldehyde levels in induced sputum and exhaled breath condensate
MDA, C6, C7 and C9 were detectable in all samples of both biological specimens. Acrolein was not detectable in any IS control specimens, but was detectable in EBC. HHE was detectable in 19 and 21 samples of IS and EBC, respectively. HNE was detectable in 22 and 23 samples of IS and EBC, respectively.
The mean coefficient of variation (%) of aldehyde measurements in EBC was: 18.5±7.8 for MDA; 12.6±10.1 for acrolein; 34.7±12.7 for HHE; 25.2±13.0 for HNE; 17.0±7.9 for C6; 20.5±8.9 for C7; and 17.7±8.0 for C9.
A chromatogram of the different classes of aldehydes measured in a sample of IS is shown in figure 1. This example demonstrates the feasibility of the identification of the studied aldehydes in the supernatants from IS by means of LC-MS/MS.
Fig. 1.
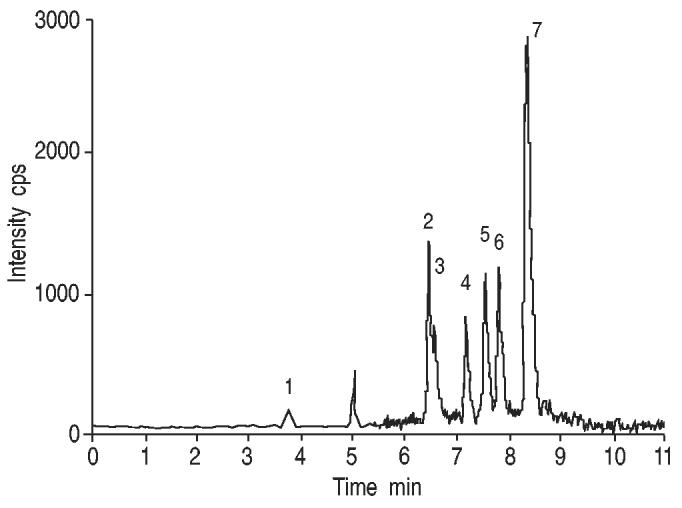
Chromatogram of the different classes of aldehydes measured in an induced sputum sample. Peak identification and analyte concentration: 1) malondialdehyde, 33.7 nM; 2) 4-hydroxyhexenal, 7.3 nM; 3) acrolein, 15.8 nM; 4) 4-hydroxynonenal, 7.9 nM; 5) n-hexanal, 14.9 nM; 6) n-heptanal, 15.6 nM; and 7) n-nonanal, 64.7 nM.
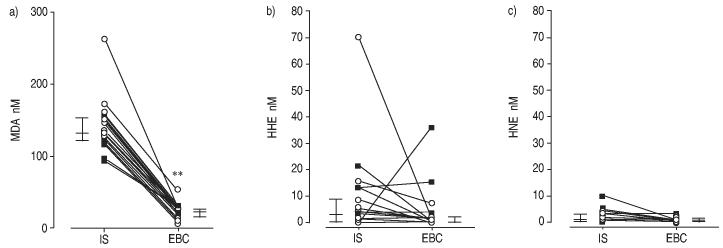
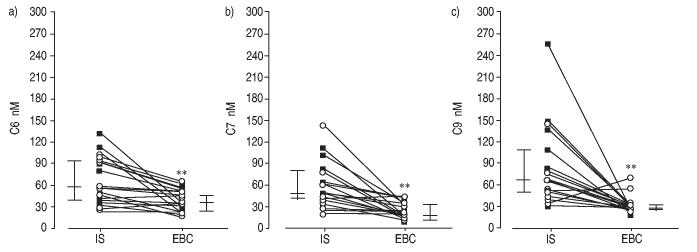
Considering all cases, IS levels of different aldehydes were higher than EBC levels (figs 2 and 3, and table 3). In this context, MDA concentrations were 132.5 nM (2.5–268.8) in IS and 23.7 nM (9–53.7) in EBC (p<0.01) (fig. 2). Similarly, C6, C7 and C9 concentrations were 1.5–4.7-fold higher in IS than in EBC (fig. 3). Acrolein levels were 131.1 nM (55.6–264.6) in IS and 45.3 nM (14.4–127.1) in EBC (p<0.001). In contrast, the concentrations of hydroxylated aldehydes (HHE and HNE) in IS were not significantly different from the concentrations in EBC (fig. 2).
Fig. 2.
a) Malondialdehyde (MDA), b) 4-hydroxynonenal (HHE) and c) 4-hydroxyhexenal (HNE) paired levels in exhaled breath condensate (EBC) and induced sputum (IS) of subjects with asthma (○) and chronic obstructive pulmonary disease (■). Vertical bars represent median and 25–75 percentiles. **: p<0.01.
Fig. 3.
a) n-Hexanal (C6), b) n-heptanal (C7) and c) n-nonanal (C9) paired levels in exhaled breath condensate (EBC) and induced sputum (IS) of subjects with asthma (○) and chronic obstructive pulmonary disease (■). Vertical bars represent median and 25–75 percentiles. **: p<0.01.
Table 3.
Aldehydes in exhaled breath condensate (EBC) and induced sputum (IS) from asthmatics, subjects with chronic obstructive pulmonary disease (COPD) and healthy nonsmoking controls
| LOD | Asthma |
COPD |
Control |
||||
|---|---|---|---|---|---|---|---|
| EBC | IS | EBC | IS | EBC | IS | ||
| MDA | 1.0 | 21.1 (9–53.7)¶ | 134.3 (97.1–262.8)*,¶ | 24 (11.8–32.6)¶ | 128.2 (92.5–158.1)#,¶ | 9.8 (9.2–10.6) | 87.4 (64.5–108.5)+ |
| Acrolein | 1.0 | 48.3 (18.1–127.1)¶ | 116.8 (0.5–197.7)*,¶ | 55.6 (14.4–94.1)¶ | 147.0 (55.6–264.6)#,¶ | 9.4 (6.9–20.3)+ | ND |
| C6 | 1.0 | 40.2 (17.2–63.7)¶ | 58.4 (24.8–102.9)*,¶ | 32 (20.8–61.2)¶ | 49 (32–132.5)#,¶ | 22.2 (15.3–38.1) | 29 (20.5–50.5)+ |
| C7 | 1.0 | 18.7 (8.7–43.6) | 42 (20.1–143.6)* | 17.7 (7.5–42.1) | 60.6 (32.2–111.6)# | 15.2 (7.7–33.8) | 51.7 (29.7–80.38)+ |
| C9 | 1.0 | 31.1 (22.8–70.1) | 51.6 (35.1–145.9)* | 26.7 (16.5–34.4) | 84.2 (31.9–256.6)#,¶ | 27.2 (14.9–47.5) | 39.7 (18–102.9)+ |
| 4-HHE | 0.3 | ND (ND–7.1) | 2.98 (ND–70) | ND (ND–35.9) | 3.1 (ND–21.4) | 0.7 (ND–3.3) | ND (ND–5.4) |
| 4-HNE | 0.6 | 0.9 (ND–2.3) | 0.5 (ND–3.8) | 0.9 (ND–2.9) | 1.1 (ND–10) | 1.3 (0.6-6.1) | 0.9 (ND–4.9) |
LOD: limit of detection; MDA: malondialdehyde; C6: n-hexanal; C7: n-heptanal; C9: n-nonanal; 4-HHE: 4-hydroxynonenal; 4-HNE: 4-hydroxyhexenal; ND: not detectable.
p<0.05 EBC versus IS in asthma
p<0.05 EBC versus sputum in COPD
p<0.05 versus control values
EBC versus sputum in controls.
There were no differences in aldehyde concentrations both in IS and in EBC between patients with asthma and those with COPD (table 3). No differences in aldehydes levels were observed in both IS and EBC between patients who were taking inhaled corticosteroid treatment and those who were not.
In controls, MDA, C6, C7 and C9 concentrations were higher in IS than in EBC samples. On the contrary, acrolein levels were detectable only in EBC but not in IS samples. MDA, acrolein and C6 levels were higher in cases than in controls, in both specimens (table 3).
Correlations
Aldehyde levels in IS did not show any correlation with aldehyde levels in EBC, in respect to either cases or controls. In contrast with EBC, some aldehydes in IS were significantly correlated, considering all subjects: MDA versus C6 (r=0.7, p=0.0004), C6 versus C9 (r=0.6, p=0.004), C7 versus C9 (r=0.6, p=0.003).
IS and EBC aldehyde levels did not correlate with differential sputum cellular counts, neither in asthma nor in COPD. In COPD, MDA levels in EBC negatively correlated with FEV1 % predicted (r=−0.70, p=0.01) (fig. 4), whereas no correlation was observed between aldehyde levels in EBC and spirometric values in asthma.
Fig. 4.
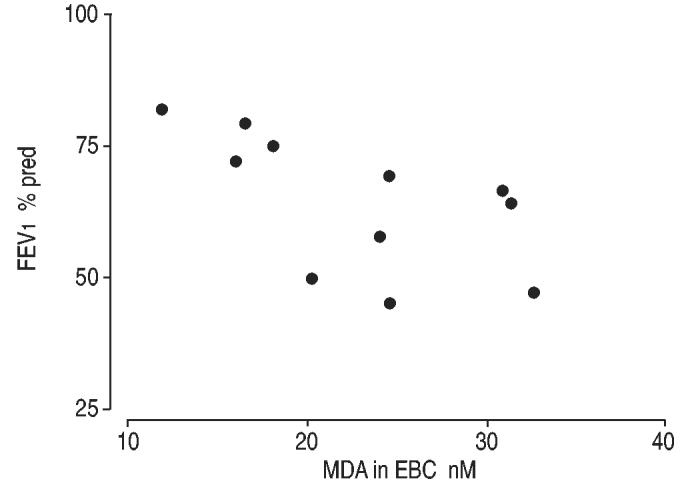
Correlation between malondialdehyde (MDA) in exhaled breath condensate (EBC) and forced expiratory volume in one second (FEV1) in chronic obstructive pulmonary disease patients.
Discussion
By determining aldehyde concentrations through LC-MS/MS, and using them as biomarkers of oxidative stress and lipid peroxidation in IS and EBC of patients with chronic airway inflammation, this study demonstrates that: 1) it is feasible to measure aldehyde concentrations using this technique in IS supernatants as well as in EBC; 2) most aldehydes had higher concentrations in IS than in EBC, and aldehyde levels in IS and EBC did not correlate; 3) aldehydes in IS and EBC did not correlate with cellularity of IS; 4) the concentrations of some aldehydes in IS were significantly correlated with each other; and 5) in COPD patients, the concentrations of MDA in EBC, but not in IS, were inversely correlated with FEV1 values.
To the best of the authors' knowledge, this is the first report demonstrating that biomarkers of lipid peroxidation (aldehydes) can be measured by LC-MS/MS in IS as well as in EBC of subjects with chronic airway inflammation. Thus, this presented the opportunity to compare the concentrations of some biomarkers of lipid peroxidation in different biological fluids obtained by noninvasive techniques of sampling lung secretions. Higher levels of all but hydroxylated aldehydes (HHE and HNE) were observed in IS as compared to EBC; EBC aldehyde levels did not show any correlation with their respective concentrations in IS.
Several possible explanations might account for such results. 1) Different dilution factors. IS is likely to be less diluted with water than EBC; in fact, it is known that EBC consists of aerosolised particles diluted with a very great amount of water vapour [25]. In this regard, it is acknowledged that a proper analytical comparison between compounds in different biological fluids would require the knowledge of dilution factors of each fluid, in order to compare the absolute number of moles rather than concentrations. However, for both IS and EBC, at this stage of the research, information regarding a gold standard factor of dilution is not yet available. Furthermore, it would be of interest having a comparison marker (such as total lipids or a lipid unaltered by oxidation), to compare the oxidation products with. This would not be a dilution marker, but rather a compound that could serve as an internal monitor for oxidative imbalance. 2) Different composition of IS and EBC. Sputum can be considered as a bio gel, with a high concentration of different types of cells and enzymes [26, 27], while EBC can be considered an aqueous solution, with a low concentration of salts, lipids and proteins. Therefore, the degradation and/or biotransformation of aldehydes in the medium could be different. 3) Different site of sampling within the lung. It can be speculated that the technique of IS samples predominantly the proximal airways [28], where most of the airway secretions are likely to be more concentrated, whereas EBC is thought to more closely reflect the composition of alveolar lining fluid, because of the much greater surface area from which generation of aerosols may occur [29].
Among different biomarkers, only MDA, acrolein and C6 clearly distinguished between cases and controls, in both EBC and IS; thus, these biomarkers can be proposed as useful indicators to investigate lung diseases. However, further studies with age-sex matched controls are needed to further validate the cross-sectional study.
Interestingly, acrolein was not detectable in any IS from controls, but measurable levels were present in the respective EBC specimens. A possible explanation of the data could be related to the high reactivity of acrolein, which may lead to acrolein adducts; probably, the number of potential targets is much lower in EBC than in IS. On the contrary, acrolein levels were always detectable in IS of cases, probably because of their very high levels compared to those in controls.
A practical conclusion coming from these data is that, with regard to lipid peroxidation products, EBC and IS must be considered as independent matrices.
For the evaluation of oxidative stress, BAL, IS and EBC have been employed. However, there is no gold standard for noninvasive assessment of lipid peroxidation. Thus, it is unclear which technique is “better” or “more accurate”.
Whereas no correlations were found among different aldehydes in EBC, some aldehydes in IS were significant correlated with each other. An ongoing in vitro experiment in the present authors' laboratory, which is aimed at assessing whether the volatility can affect the recovery of various aldehydes in EBC, may, in part, explain the data; in fact, it was verified that the more volatile aldehydes, namely acrolein and saturated aldehydes (but not MDA which is less volatile), possibly due to their higher vapour pressures at physiological temperatures, are more difficult to capture whatever condenser is used and are probably lost, in part, with the gaseous and vapour phase of exhaled air. This may account for the lack of correlation that was observed among aldehydes in EBC. Conversely, IS concentrations seemed to be less affected by the volatility of each substance and, therefore, it may be considered as a more homogeneous way of sampling airway secretions. However, infiltrating cells and their debris in IS might represent a source of aldehydes. Thus, the latter might represent less relevant biomarkers of oxidative stress and lipoperoxidation of epithelial cell membranes.
Aldehyde levels in IS did not correlate with lung function. In keeping with the results of Paredi et al. [30], who showed that exhaled ethane, another biomarker of lipid peroxidation, is correlated with airway obstruction in COPD, a negative correlation was found between MDA in EBC and disease severity, as assessed by FEV1 in this study. The correlation between MDA in EBC and FEV1 was not observed in asthmatics, probably because most of them had normal lung function tests. Acrolein and saturated aldehyde values in EBC did not correlate with lung function; therefore, compared to other aldehydes, MDA seems to be a better biomarker to assess COPD severity. However, mainly due to the low number of subjects recruited, further studies are needed to ascertain whether exhaled MDA may be of some use to evaluate COPD severity. In addition, the negative relationship between MDA (relatively volatile substance) and FEV1 could also be accounted for by a differential dilution of the same MDA production rate in variable values of exhaled air.
The concentration of aldehydes in EBC and IS did not distinguish between patients with asthma and COPD, and this could be related to the fact that lipid peroxidation is not a disease-specific process. However, it is recognised that the patients' characteristics are quite heterogeneous, as some were current smokers whereas others were using steroids. These differences and the limited number of subjects should not affect the comparisons between EBC and IS (main aim of the study), although they may mean that further studies are needed to better compare asthmatics and COPD patients for these parameters.
The data presented here are in keeping with the publications of Paredi and co-workers [30, 31], who found similar levels of exhaled ethane in asthma (2.06 parts per billion (ppb)) and COPD (2.77 ppb). On the contrary, the differential cellular count in IS clearly distinguished between asthma and COPD. The latter data are entirely consistent with previous studies [32, 33] and support the validity of IS for describing cellular inflammatory processes in the airways and discriminating between respiratory diseases [34].
The measurement of MDA as marker of oxidative stress has been criticised by some. However, this was mainly related to the fact that MDA was usually quantified by the thiobarbituric acid (TBA)-reacting substances assay, which is a generic assay of lipid peroxidation in biological samples (MDA represents only a small fraction of the detected oxidation products, and TBA-reactive materials are formed during heating of the sample) [35]. To increase the specificity of the MDA determination, previous authors have successfully measured aldehydes (MDA and HNE) by HPLC [22, 36]. To further improve the quality of the measurements, aldehydes in IS and EBC were measured by LC-MS/MS in this study, which is now considered the reference technique for analytical analysis [37].
In the present study, the concentrations of MDA, C6 and C7 in EBC of COPD patients were lower than previously reported [14], thus stressing the need of guidelines defining standardised procedures, and to limit differences due to condensing devices and sampling techniques. This will never eliminate differences between studies due to the characteristics of subjects being examined. In addition, the evaluation of long-term reproducibility of selected biomarkers in EBC showed that a certain degree of variation exists in the results. Possibly, the normalisation of EBC data with selected variables, such as the amount of condensed exhaled air or an inner standard, could reduce this variability.
In conclusion, the data presented in this study show that most aldehydes had higher concentrations in induced sputum than in exhaled breath condensate, and that aldehyde levels did not correlate between sampling methods. Therefore, with regard to lipid peroxidation products, exhaled breath condensate and induced sputum must be considered as independent techniques.
Footnotes
This study was supported in part by grant 1R01 HL72323-01 from the National Heart, Blood and Lung Institute (NHLBI; Bethesda, USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or National Institute of Health.
References
- 1.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 2.Rahman I. Oxidative stress, chromatin remodelling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 3.Lases EC, Duurkens VA, Gerritsen WB, Haas FJ. Oxidative stress after lung resection therapy: a pilot study. Chest. 2000;117:999–1003. doi: 10.1378/chest.117.4.999. [DOI] [PubMed] [Google Scholar]
- 4.Pryor WA, Bermudez E, Cueto R, Squadrito GL. Detection of aldehydes in bronchoalveolar lavage of rats exposed to ozone. Fundam Appl Toxicol. 1996;34:148–156. doi: 10.1006/faat.1996.0185. [DOI] [PubMed] [Google Scholar]
- 5.Montuschi P, Nightingale JA, Kharitonov SA, Barnes PJ. Ozone-induced increase in exhaled 8-isoprostane in healthy subjects is resistant to inhaled budesonide. Free Radic Biol Med. 2002;33:1403–1408. doi: 10.1016/s0891-5849(02)01084-5. [DOI] [PubMed] [Google Scholar]
- 6.Winterbourn CC, Buss IH, Chan TP, Plank LD, Clark MA, Windsor JA. Protein carbonyl measurements show evidence of early oxidative stress in critically ill patients. Crit Care Med. 2000;28:143–149. doi: 10.1097/00003246-200001000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Duflo F, Debon R, Goudable J, Chassard D, Allaouchiche B. Alveolar and serum oxidative stress in ventilator-associated pneumonia. Br J Anaesth. 2002;89:231–236. doi: 10.1093/bja/aef169. [DOI] [PubMed] [Google Scholar]
- 8.Schock BC, Young IS, Brown V, Fitch PS, Shields MD, Ennis M. Antioxidants and oxidative stress in BAL fluid of atopic asthmatic children. Pediatr Res. 2003;53:375–381. doi: 10.1203/01.PDR.0000049625.51462.D1. [DOI] [PubMed] [Google Scholar]
- 9.Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax. 2001;56:13–18. doi: 10.1136/thorax.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath I, MacNee W, Kelly FJ, et al. “Haemoxygenase-1 induction and exhaled markers of oxidative stress in lung diseases”, summary of the ERS Research Seminar in Budapest, Hungary, September, 1999. Eur Respir J. 2001;18:420–430. doi: 10.1183/09031936.01.00231201. [DOI] [PubMed] [Google Scholar]
- 11.Andreoli R, Manini P, Corradi M, Mutti A, Niessen WM. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:637–645. doi: 10.1002/rcm.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pryor WA, Godber SS. Noninvasive measures of oxidative stress status in humans. Free Radic Biol Med. 1991;10:177–184. doi: 10.1016/0891-5849(91)90073-c. [DOI] [PubMed] [Google Scholar]
- 13.Corradi M, Folesani G, Andreoli R, et al. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 14.Corradi M, Rubinstein I, Andreoli R, et al. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1380–1386. doi: 10.1164/rccm.200210-1253OC. [DOI] [PubMed] [Google Scholar]
- 15.Zurek MA, Sterk PJ, Djukanovic R. Non invasive assessment of inflammation. In: Gibson GJ, editor. Respiratory Medicine. Saunders Press; London: 2003. pp. 402–414. [Google Scholar]
- 16.Gessner C, Hammerschmidt S, Kuhn H, et al. Exhaled breath condensate nitrite and its relation to tidal volume in acute lung injury. Chest. 2003;124:1046–1052. doi: 10.1378/chest.124.3.1046. [DOI] [PubMed] [Google Scholar]
- 17.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 18.Hunt JF, Erwin E, Palmer L, et al. Expression and activity of pH-regulatory glutaminase in the human airway epithelium. Am J Respir Crit Care Med. 2002;165:101–107. doi: 10.1164/ajrccm.165.1.2104131. [DOI] [PubMed] [Google Scholar]
- 19.NHLBI/WHO . National Institutes of Health; National Heart, Lung, and Blood Institute; 2001. Workshop report: Global initiative for chronic obstructive lung disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. (Publication no. 2701). [Google Scholar]
- 20.NHLBI/WHO . National Institutes of Health; National Heart, Lung, and Blood Institute; 2001. Workshop Report: Global Strategy for Asthma Management and Prevention. (Publication no. 02–3659). [Google Scholar]
- 21.Horvath I, Donnelly LE, Kiss A, et al. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158:1042–1046. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- 22.Larstad M, Ljungkvist G, Olin AC, Toren K. Determination of malondialdehyde in breath condensate by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;5:107–114. doi: 10.1016/s0378-4347(01)00437-6. [DOI] [PubMed] [Google Scholar]
- 23.Djukanovic R, Sterk P, Fahy JV, Hargreave FE. Standardized methodology of sputum induction and processing. Eur Resp J. 2002;20:1s–55s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 24.Pignatti P, Delmastro M, Perfetti L, et al. Is dithiothreitol affecting cells and soluble mediators during sputum processing? A modified methodology to process sputum. J Allergy Clin Immunol. 2002;110:667–668. doi: 10.1067/mai.2002.128279. [DOI] [PubMed] [Google Scholar]
- 25.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165:663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 26.Sanders NN, De Smedt SC, Demeester J. Mobility and stability of gene complexes in biogels. J Control Release. 2003;87:117–129. doi: 10.1016/s0168-3659(02)00355-3. [DOI] [PubMed] [Google Scholar]
- 27.Deneuville E, Perrot-Minot C, Pennaforte F, et al. Revisited physicochemical and transport properties of respiratory mucus in genotyped cystic fibrosis patients. Am J Respir Crit Care Med. 1997;156:166–172. doi: 10.1164/ajrccm.156.1.9606123. [DOI] [PubMed] [Google Scholar]
- 28.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164:1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 29.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110:28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 30.Paredi P, Kharitonov SA, Leak D, Ward S, Cramer D, Barnes PJ. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:369–373. doi: 10.1164/ajrccm.162.2.9909025. [DOI] [PubMed] [Google Scholar]
- 31.Paredi P, Kharitonov SA, Barnes PJ. Elevation of exhaled ethane concentration in asthma. Am J Respir Crit Care Med. 2000;162:1450–1454. doi: 10.1164/ajrccm.162.4.2003064. [DOI] [PubMed] [Google Scholar]
- 32.Keatings VM, Evans DJ, O'Connor BJ, Barnes PJ. Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluid. Thorax. 1997;52:372–374. doi: 10.1136/thx.52.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maestrelli P, Saetta M, Di Stefano A, et al. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995;152:1926–1931. doi: 10.1164/ajrccm.152.6.8520757. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 35.Gutteridge JMC, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 36.Peters R, Hellenbrand J, Mengerink Y, Van der Wal S. On-line determination of carboxylic acids, aldehydes and ketones by high-performance liquid chromatography-diode array detection-atmospheric pressure chemical ionisation mass spectrometry after derivatization with 2-nitrophenylhydrazine. J Chromatogr A. 2004;1031:35–50. doi: 10.1016/j.chroma.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 37.Shen TL, Noon KR. Liquid chromatography-mass spectrometry and tandem mass spectrometry of peptides and proteins. Methods Mol Biol. 2004;251:111–140. doi: 10.1385/1-59259-742-4:111. [DOI] [PubMed] [Google Scholar]