Abstract
The early secretory antigenic target (ESAT)-6 purified protein and peptides from Mycobacterium tuberculosis were evaluated as antigens for the immunodiagnosis of tuberculosis (TB). Because the control of TB requires improved diagnostic procedures, efforts have increased to identify Mycobacterium tuberculosis–specific epitopes for the immunodiagnosis of active TB and to discriminate between active and latent states of infection. Two multiepitopic peptides from ESAT-6 protein were selected by computational analysis. Patients with active TB (7 HIV+ and 20 HIV−) and control patients (17 HIV+ and 28 HIV−) were enrolled. Enzyme-linked immunospot assay analysis for interferon-γ expression by peripheral blood mononuclear cells was quantified after stimulation with selected ESAT-6 peptides, purified protein derivative, or the intact ESAT-6 protein. During active TB, 20 of 27 patients responded in vitro to ESAT-6 peptides and 23 of 27 patients to purified protein derivative. None of the controls without active TB, including individuals with latent TB infection, recognized ESAT-6 peptides. By contrast, latently infected individuals did respond in vitro to both intact ESAT-6 protein and purified protein derivative. Thus, high T-cell response frequencies to ESAT-6 peptides are present only during active TB and can be used to discriminate between active and latent forms of infection.
INTRODUCTION
Tuberculosis (TB) is a global health problem with one-third of the world’s population latently infected with Mycobacterium tuberculosis (MTB) and approximately 8 million cases of active disease occurring each year (1). The magnitude of the TB problem has increased in the past decade because of the spread of HIV, which increases the risk of developing active TB (2). In general, infection by MTB is controlled initially by host defenses, and the infection remains latent. However, latent TB infection has the potential to develop into active TB at any time. Because active TB is infectious and leads to the spread of MTB, rapid diagnosis and effective treatment of individuals with active TB are the most important component of TB control programs. Moreover, identification and treatment of persons with latent MTB infection who are at high risk of progressing to active disease, also may contribute to TB control (3). Therefore, an improvement of the methods used to diagnose and discriminate between active and latent TB infection may have a positive effect on TB control, in addition to benefiting individual patients. Currently, microbiological diagnosis of active TB relies on acid fast bacilli (AFB) staining and on culture tests with a sensitivity of 50% to 70% and 80% to 85%, respectively (4). The molecular diagnostic techniques available are reliable for AFB-staining positive samples (95% sensitivity) but not for AFB-staining negative specimens (48% to 53% sensitivity) (4,5).
MTB evokes a strong cell-mediated immune response with a high production of interferon (IFN)-γ, such that the detection of MTB-specific IFN-γ T cells may prove a useful diagnostic strategy. Until very recently, the tuberculin skin test has been the only medical tool used to evaluate immune response against MTB in both active and latent TB. However, this test has significant limitations because the purified protein derivative (PPD) used for tuberculin skin test is a crude precipitate of filtered MTB that contains more than 200 antigens widely shared among environmental mycobacteria (6,7). Furthermore, in patients with active TB, the tuberculin skin test is 75% to 90% sensitive, but among those with disseminated disease, this sensitivity falls to 50% (7) and is even lower in HIV+ patients with only mild degrees of immunosuppression (7,8). Moreover, a positive tuberculin skin test does not discriminate between latent and active TB. Further clinical workup is needed to obtain a correct diagnosis in subjects with a positive test (4).
In an effort to develop more accurate diagnostic tools, recent studies have led to the identification of the genomic segment RD1, present in MTB but absent from all strains of Mycobacterium bovis bacillus Calmette and Guerin (BCG) as well as almost all environmental mycobacteria (9,10,11). Therefore, RD1 gene products offer the potential for the development of new diagnostic tests that may differentiate MTB infection from BCG vaccination as well as exposure to environmental mycobacteria. Early secretory antigenic target (ESAT)-6 is a secreted protein encoded by RD1 and has been shown to be a dominant target for cell-mediated immunity in animal models (12,13), patients with TB (14,15), and household contacts of TB patients who develop TB within 2 y (16). ESAT-6–specific IFN-γ secreting CD4+ T cells were identified as accurate markers of TB infection in both HIV− and HIV+ individuals using an ex vivo enzyme-linked immunospot (ELISPOT) assay (17–22). However, in subjects from countries with high rates of exposure to TB, the assay did not allow for the discrimination between latent and active TB, a distinction that is required for better global control of TB (18,19,23).
In this study, we employed a quantitative implemented peptide binding motif analysis (24–27) to identify promiscuous human leukocyte antigen (HLA) class II epitopes encoded in the ESAT-6 protein. We then designed 2 multiepitopic ESAT-6 peptides that were predicted to elicit cellular immune responses in peripheral blood mononuclear cells from individuals with active TB. The diagnostic utility of these peptides was evaluated by analyzing patient samples with IFN-γ–based ELISPOT and enzyme-linked immunosorbent assay (ELISA). Of particular interest was the ability of these peptides to differentially elicit responses between patients with active and latent TB infection.
MATERIALS AND METHODS
Study Population
All subjects were prospectively recruited at the National Institute for Infectious Diseases (INMI) “L. Spallanzani” in Rome and at the Division of Infectious Diseases in Belcolle Hospital in Viterbo, Italy. We enrolled 27 patients with clinical and radiographic features consistent with TB and with positive MTB cultures from 1 or more clinical specimens. Among these TB patients, those with pulmonary TB were AFB positive.
As HIV+/AIDS controls, 8 patients without opportunistic infections and 9 AIDS patients with opportunistic infections different from TB were enrolled. In particular, the opportunistic infections included neurotoxoplasmosis, intestinal criptosporidiosis, cytomegalovirus retinitis, and recurrent pneumonia (1, 1, 2, and 5 subjects, respectively). As HIV− controls we included 28 individuals with the following pathologies: bacterial pneumonia, pleuritis, bacterial bone infection, tonsillar infection, acute hepatitis B, cirrhosis, bacterial cellulitis, lymphoma, orchitis, and brain abscess (5, 1, 1, 1, 4, 3, 1, 1, 1, and 1 subjects, respectively); 9 individuals were healthy controls.
Information regarding mean age, sex, ethnicity, BCG vaccination, TB localization, HIV-RNA viral load (detected by the Quantiplex HIV-1 RNA version 3.0 assay [bDNA v3.0; Bayer Diagnostics, Emeryville, CA, USA]), CD4 cell counts and number of persons on highly active antiretroviral therapy are shown in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Status | TB+HIV+ | TB+HIV− | AIDS/HIV+ | HIV− Controls |
|---|---|---|---|---|
| Number of subjects analyzed | 7 | 20 | 17 | 28 |
| Mean age in y ± SE | 38 ± 2 | 32 ± 3 | 39 ± 5 | 43 ± 4 |
| Sex | 6 males, 1 female | 14 males, 6 females | 12 males, 5 females | 21 males, 7 females |
| Ethnicity | ||||
| North Africans | 1 | 2 | 2 | 2 |
| Caucasians Western Europe | 5 | 12 | 11 | 21 |
| Caucasians Eastern Europe | — | 2 | 1 | 3 |
| Asian | — | 1 | — | 2 |
| South Americans | 1 | 3 | 3 | — |
| BCG vaccination | 1 | 5 | 7 | 9 |
| Localization of TB | ||||
| Pulmonary | 5 | 12 | — | — |
| Extrapulmonary | 1 | 5 | — | — |
| Pulmonary and extrapulmonary | 1 | 3 | — | — |
| HIV-RNA (copies/mL) ± SE | 46330 ± 17260 | — | 33390 ± 11120 | — |
| Mean CD4 cell number/μL ± SE | 238 ± 86 | 713 ± 94 | 321 ± 56 | 1121 ± 101 |
| HAART | 5 | — | 5 | — |
HAART, highly active antiretroviral therapy.
After informed consent was obtained, an EDTA blood sample was drawn from enrolled individuals. For TB and AIDS patients, blood samples were obtained within 3 wk of beginning therapy for TB or opportunistic infection. Control subjects underwent tuberculin skin test administered by the Mantoux method using 0.1 mL (5TU) of Biocinetest-PPD tuberculin (Chiron SpA, Italy) injected intradermally in the volar surface of the forearm. Palpable induration at 48 to 72 h was measured and recorded. Individuals with an induration ≥5 mm if HIV+ or ≥10 mm if HIV− were classified as positive and underwent chest radiograph and clinical examination to rule out active TB according to the American Thoracic Society Guidelines (4). Latent TB-infected subjects were defined as individuals with a positive tuberculin skin test but without clinical and radiological signs of active TB (4).
Epitope Prediction
Sequences of the ESAT-6 MTB-specific antigen were obtained by the Swiss-Prot database. The protein sequences then were analyzed by quantitative implemented HLA peptide-binding motifs databases SYFPEITHI (24), EpiPredict (25), and ProPred (26, 27) for the identification and scoring of each putative epitope for all the available HLA–class II alleles in the databases. Each selected peptide (Table 2) contained 1 or more epitopes able to bind at least 4 different HLA-DR, 2 different HLA-DP, and 2 different HLA-DQ specificities with a putative binding ability of 80% maximum binding for any allele belonging to an HLA–class II serological specificity. Together, the designed peptide epitopes were able to cover more than 90% of the HLA–class II haplotypes present in different human populations (data not shown). Sequence homology searches of all known protein sequences confirmed that the sequences of the selected peptides were uniquely restricted to MTB complex proteins.
Table 2.
CD4+ T-cell epitopes in ESAT-6 protein selected to be broadly recognized in patients with active TB
| Position | Sequence | HLA–class II serological specificities covereda |
|---|---|---|
| 6–28 | WNFAGIEAAASAIQGNVTSIHSL | HLA-DR: 1, 3, 4, 8, 11(5), 13(6), 52, 53
HLA-DP: 1, 2, 4, 9 HLA-DQ: 1, 2, 5, 6, 8 |
| 66–79 | NALQNLARTISEA | HLA-DR: 3, 8, 11(5), 13(6), 15(2), 52
HLA-DP: 4, 9 HLA-DQ: 1, 6, 7 |
Listed are the HLA–class II serological specificities to which the alleles putatively able to bind to the selected peptides belong.
Peptides
Peptides were synthesized with free amino acid termini using an Applied Biosystem SYNERGY peptide synthesizer and Fmoc chemistry. All synthetic peptides were purified by reverse-phase chromatography to > 90% purity. Sequence and purity were confirmed by mass spectrometry and analytical reverse-phase chromatography. Lyophilized peptides were diluted in dimethyl sulfoxide (DMSO) at stock concentrations of 10 mg/mL for each peptide and stored at −80 °C.
Cell Isolation and Cultures
Peripheral blood mononuclear cells were separated by standard means and resuspended in a complete medium (RPMI, 10% heat-inactivated fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, and 10 U/mL penicillin-streptomycin, all from Euroclone Ltd, United Kingdom). Cell cultures with 2 × 105 peripheral blood mononuclear cells were performed in duplicates and seeded in 96-well plates to perform ELISPOT (see Ex Vivo ELISPOT Assay for Single-cell IFN–γ Release) or to harvest supernatants to evaluate cytokine production by ELISA (see IFN-γ ELISA). In the HIV+ patients with CD4+ T-cell counts < 100/μL, CD8+ T cell depletion was performed (see Immunomagnetic Cell Depletions) to enrich the samples in CD4+ T cells. In all cases, both cell cultures were treated with the following stimuli: single peptides at 10 and 50 μg/mL or with a pool of the 2 ESAT-6 peptides at 1 μg or 10 μg each peptide, PPD (batch RT47; Statens Serum Institut, Copenhagen, Denmark) at 10μg/mL, phytohemagglutinin (PHA) (Sigma, St Louis, MO, USA) at 5 μg/mL, ESAT-6 protein (Lionex, Braunschweig, Germany) at 10 μg/mL. Negative controls included peripheral blood mononuclear cells, either unstimulated or treated with DMSO at the same concentration present in the peptide solutions, and peripheral blood mononuclear cells treated with an irrelevant peptide (MHC class II associated invariant chain derived peptide (CLIP), AA 105-117, with the sequence NH2-SKMRMATPLLMQA-COOH) diluted in complete medium containing the same DMSO concentration used for the ESAT-6 peptides.
Immunomagnetic Cell Depletions
CD8+ T cell–depleted peripheral blood mononuclear cells were obtained using Dynabeads M-450 (Dynal, Oslo, Norway) and following manufacturer’s instructions. These beads reliably deplete 99% of the target cell population, as evaluated after each experiment by fluorescence activated cell sorter (FACS Calibur) analysis (data not shown).
Ex Vivo ELISPOT Assay for Single-cell IFN-γ Release (Enumeration of Circulating Peptide- or Stimulus-specific T Cells from Peripheral Blood)
Polyvinylidene difluoride–backed plates with 96 wells (MAIPS45; Millipore, Sunnyvale, CA, USA) were precoated with capture antibody (Ab) (IFN-γ coating monoclonal, M-700A, Pierce-Endogen Inc, Rockford, IL, USA), dissolved in phosphate-buffered saline at a concentration of 5 μg/mL, and kept at 4 °C overnight. Ab-coated plates were then washed 4 times with 200 μL/well sterile PBS. Plates were blocked to prevent nonspecific protein binding with 200 μL/well sterile PBS, containing 10% fetal calf serum for 2 h at room temperature. Peripheral blood mononuclear cells stimulated as described above were incubated for 40 h at 37 °C in 5% CO2. Cells then were removed by 4 rinses with 200 μL/well of PBS followed by 4 additional rinses with 200 μL/well of PBS/ 0.05% Tween®20 (Sigma). Incubation for 100 min with secondary anti–IFN-γ biotin-labeled Ab (Ab M-701B, Pierce-Endogen) diluted in PBS/4% bovine serum albumin (fraction V, Sigma) at a concentration of 1 μg/mL, was followed by streptavidin-HRP (Pierce-Endogen) for 30 min at room temperature in the dark. AEC substrate (AEC staining kit, AEC101, Sigma) was added, and plates were observed for spot development for up to 20 min at room temperature in the dark. Plates were then washed with distilled water to stop the reaction and allowed to dry overnight. Spots then were counted by an automated ELISA-spot assay video analysis system (A-EL-VIS, Hannover, Germany). Evaluated spots had a size > 15 U (1 U = 500 μm2).
When the average number of spot-forming cells in duplicate wells of stimulated cultures (with PPD, PHA, ESAT-6 protein, or ESAT-6 peptides) exceeded that in controls by 5 spot-forming cells and were at least 2-fold higher, the responses were scored as positive (17–22). To obtain the absolute value, the number of spot-forming cells in the negative controls was subtracted from the number of spot-forming cells in the stimulated cultures. In particular, negative controls for the ESAT-6 peptide-stimulated peripheral blood mononuclear cells included the average number of spots obtained in peripheral blood mononuclear cells treated with the same DMSO concentration used to dilute the peptides and the average number of spots obtained in peripheral blood mononuclear cells treated with CLIP. As negative controls for blood mononuclear cells stimulated with PPD, PHA, or ESAT-6 protein, the average number of spots produced by unstimulated peripheral blood mononuclear cells was used.
IFN-γ ELISA
Half of the volume of the supernatants, removed on day 7 from the cell cultures seeded in 96-well round-bottom plates (Costar, Corning Inc, NY, USA), was stored at –80 °C to evaluate cytokine production. An IFN-γ commercial kit was employed according to the manufacturer (R&D Systems, Minneapolis, MN, USA). Results were expressed as pg/mL per million peripheral blood mononuclear cells. Responses in stimulated peripheral blood mononuclear cells (with PPD, PHA, ESAT-6 protein, or ESAT-6 peptides) were scored as positive if the IFN-γ production in test wells was 3-fold higher than in negative controls (15). To obtain the absolute value, the number of IFN-γ production in negative controls was subtracted from the value obtained in the stimulated cultures. Negative controls for the ESAT-6 peptide-peripheral blood mononuclear cells included peripheral blood mononuclear cells treated with the same DMSO concentration used to dilute the peptides and peripheral blood mononuclear cells treated with CLIP. Unstimulated peripheral blood mononuclear cells were used as negative controls for peripheral blood mononuclear cells stimulated with PPD, PHA, or ESAT-6 protein.
Statistical Analysis
Differences between groups were evaluated by the Mann-Whitney U test, by Fisher test, and Pearson r. P values ≤ 0.05 were considered significant. To define a positive criterion that would maximize the discrimination ability of the assay and minimize the number of erroneous diagnoses of active TB, a receiver operator characteristic curve was constructed for ELISA and ELISPOT data using LABROC-1 software.
RESULTS
Selection of MTB Promiscuous Epitopes
To overcome the restriction imposed by the patients’ HLA-haplotypes in the analysis of MTB-specific T-helper response, we selected 2 areas of ESAT-6 by quantitative implemented peptide binding motif analysis. These areas contained promiscuous epitopes (ESAT-66–28 and ESAT-666–79) (see Table 2) that were able to bind more than 90% of the HLA–class II haplotypes present in different human populations (28). ESAT-6 induces a strong cell-mediated immune response (29) and is actively secreted by dividing mycobacteria (30,31). Both ESAT-6-derived peptides were multiepitopic as they contained differently recognized T-cell epitopes relevant to natural processing pathways (17).
ESAT-6 Peptide-specific IFN-γ –secreting T Cells in TB Patients With or Without HIV Infection at the Time of TB Diagnosis
At the time of TB diagnosis, the number of ESAT-6 peptide–specific IFN-γ –secreting T cells in TB patients with or without HIV infection was determined. All of the TB patients included in the study had positive MTB cultures, and all of the pulmonary TB patients were AFB positive. To score the results as positive, either the response ratio of stimulated compared with unstimulated peripheral blood mononuclear cells was calculated or in the case of ESAT-6 peptides, a receiver operator characteristic curve was constructed to calculate a cutoff value of 32 spot-forming cells and 76 pg/mL IFN-γ per million peripheral blood mononuclear cells for ELISPOT and ELISA, respectively. Both approaches yielded identical results in terms of scoring the samples as positive or negative.
A comparison between the data obtained by the ELISPOT and ELISA assays was performed in 13 individuals with active TB (Table 3). The ELISPOT assay was shown to be more sensitive compared with ELISA in 4 of 13 TB patients studied (see Table 3, patient code: 1, 3, 9, 10), as previously indicated with different experimental approaches in the literature (32,33). Given the higher sensitivity of the ELISPOT, further analyses were restricted to this assay system.
Table 3.
In vitro ESAT-6 peptides pool response in terms of IFN-γ production evaluated simultaneously by ELISA and ELISPOT assays in HIV+ and HIV− individuals with active TB
| Patients Code | HIV Status | ELISAa (pg/mL per million PBMC) | ELISAa (score) | ELISPOTb (SFCs per million PBMC) | ELISPOTb (score) |
|---|---|---|---|---|---|
| 1 | + | 70 | − | 55 | + |
| 2 | + | 215 | + | 265 | + |
| 3 | + | 63 | − | 100 | + |
| 4 | + | 0 | − | 30 | − |
| 5 | − | 215 | + | 145 | + |
| 6 | − | 750 | + | 60 | + |
| 7 | − | 163 | + | 215 | + |
| 8 | − | 1450 | + | 110 | + |
| 9 | − | 63 | − | 55 | + |
| 10 | − | 0 | − | 85 | + |
| 11 | − | 0 | − | 15 | − |
| 12 | − | 3275 | + | 155 | + |
| 13 | − | 0 | − | 10 | − |
For the ELISA, the positive response cutoff was 76 pg/mL per million PBMC above negative controls (see Materials and Methods).
For the ELISPOT assay, the positive response cutoff was 32 spot-forming cells per million PBMC above negative controls (see Materials and Methods). PBMC, peripheral blood mononuclear cells; SFC, spot-forming cells.
As shown in Figure 1, a significant difference in the number of IFN-γ spot-forming cells was observed between the TB+HIV+ group and the control HIV+ group (P = 0.0001), and between the TB+ HIV− and the control HIV− groups (P = 0.0001). No statistical correlation was observed between the response magnitude to ESAT-6 peptide pool and either CD4+ T-cell count (Pearson r = 0.4, P > 0.05) or plasma HIV-viremia (Pearson r = 0.25, P > 0.05).
Figure 1.
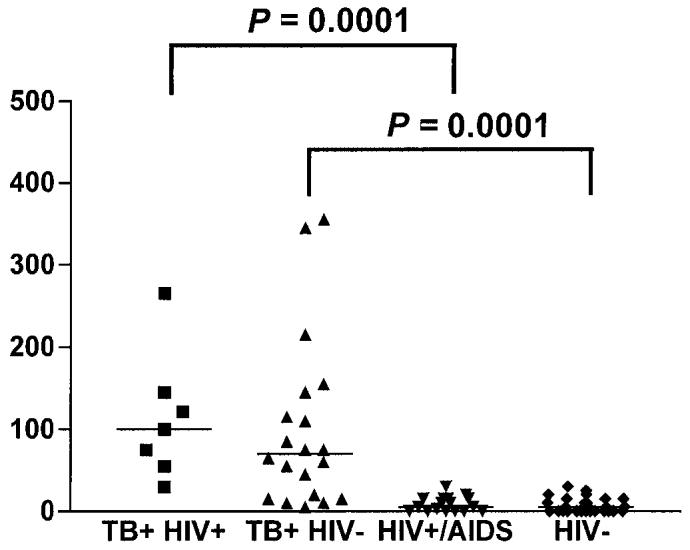
IFN-γ response to ESAT-6 peptides in patients with active TB and controls. Detection of IFN-γ was performed by ELISPOT assay in HIV− and HIV+ patients with or without active TB. The highest number of ESAT-6–peptide specific IFN-γ–secreting T cells (spot-forming cells) is reported for each individual. Horizontal bars represent the mean of the spot-forming cells for each group of patients. The P values denote the difference between the responders in each group of patients.
A summary of the data obtained from in vitro responses to individual ESAT-6 peptides, pooled peptides, and PPD is shown in Table 4. Of note, among all patients with active TB, either HIV+ or HIV−, a positive response to the pool was observed in 20 of 27 (P < 0.0001) compared with 0 of 45 controls, whereas a positive response to PPD was found in 23 of 27 (P < 0.01) compared with 26 of 45 controls. Thus, among controls without active TB, regardless of HIV status, none were scored positive to the pooled peptides although 58% responded in vitro to PPD. The sensitivity of the assay among the whole population studied was 74% (20 of 27) and the specificity was 100%.
Table 4.
In vitro ESAT-6 peptide pool and PPD responses in terms of IFN-γ production evaluated by ELISPOT assay in HIV+ and HIV− individuals with or without active TB disease
| In vitro ESAT-6 peptide pool responses
|
In vitro PPD responses
|
|||
|---|---|---|---|---|
| TB | No TB | TB | No TB | |
| Positive responses/total cases | 20/27a | 0/45 | 23/27b | 26/45 |
| Percentage | 74 | 0 | 85 | 58 |
P < 0.0001 compared with no TB cases.
P < 0.01 compared with no TB cases.
Association of the In Vitro Response to ESAT-6 Peptides with Active TB
It has been reported previously that subjects with latent TB infection may respond to ESAT-6 peptides and to the whole ESAT-6 protein (17–21). To evaluate whether peptides selected by computational analysis would allow for the reliable discrimination between active and latent TB, peripheral blood mononuclear cells from 12 patients with active TB that responded in vitro to the ESAT-6 peptide pool, and 19 subjects with latent TB infection (defined by a positive tuberculin skin test with no clinical or radiological signs of active TB) were tested (4).
As shown in Figure 2, of the patients with active TB, 92% (11 of 12) recognized the ESAT-66–28 peptide, 33% (4 of 12) recognized the ESAT-666–79 peptide, and all recognized either the whole ESAT-6 protein or PPD (12 of 12). By contrast, no response to ESAT-6 peptides, either pooled or separate, was observed in peripheral blood mononuclear cells from individuals with latent TB infection, whereas a response to the whole ESAT-6 protein or PPD was present in 73% (14 of 19) and 100% (19 of 19) of the individuals studied, respectively. Thus, ESAT-6 selected peptides, rather than the intact ESAT-6 protein or PPD, allow for the rapid detection of active TB, and discriminate between active and latent disease.
Figure 2.
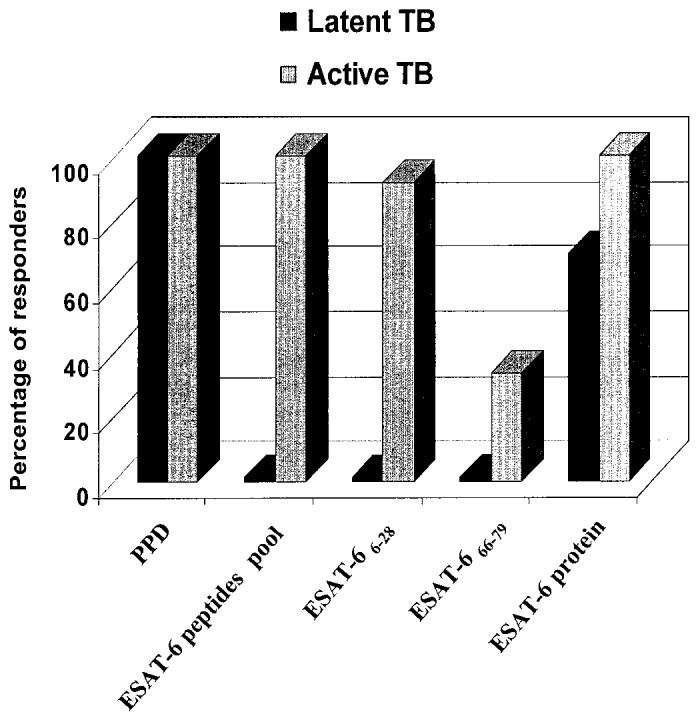
Association of the in vitro response to ESAT-6 peptides to active TB. The in vitro response to PPD, to the pool of ESAT-6 peptides, and to the whole ESAT-6 protein are shown in patients with active TB compared with those with latent TB infection. Mapping was defined by peptide-specific IFN-γ ELISPOT assay responses for 12 of the patients with active TB responsive to ESAT-6 peptide pool. The figure shows the percentage of subjects responding to the pool of ESAT-6 peptides and, among these, the percentage of responders to each single peptide.
DISCUSSION
In this pilot study, we established an immune assay for the diagnosis of active in contrast to latent TB. To this end, we designed 2 multiepitopic peptides based on ESAT-6, a protein highly specific to MTB, that triggers IFN-γ production in peripheral blood mononuclear cells in an antigen-specific manner. We found that responses to these ESAT-6 peptides, as measured by ELISPOT, were strongly associated with active TB. In contrast, peripheral blood mononuclear cells from patients with latent TB infection failed to respond to either of the 2 ESAT-6 peptides, although they responded to the whole ESAT-6 protein. Of note, we were able to detect these ESAT-6 peptide specific responses in both HIV+ and HIV− patients with active TB. The overall sensitivity of our immune diagnostic assay in patients with active TB, based on data obtained by ELISPOT assay, was 74% with a specificity of 100%.
ESAT-6 was chosen because it is a dominant target for cell-mediated immunity in animal models (12,13) and in patients with TB (14,15,17). Further, ESAT-6 peptide-specific IFN-γ–secreting CD4+ T cells have been recently identified as accurate markers of MTB infection in patients with culture-confirmed active disease (18). In our study, ESAT-6 selected peptides were identified by using quantitative implemented peptide-binding motif analysis to define epitopes that cover all the patients’ HLA haplotype. Our results demonstrate the utility of the selected ESAT-66–28 peptide as a tool for the diagnosis of active TB in both HIV+ and HIV− patients. Of note, this peptide is highly recognized by a genetically heterogeneous population, and despite the limitation inherent in the use of a small population, we found that 92% of the subjects that responded to the pooled peptides mounted a positive response to the ESAT-66–28 peptide alone. This response rate is substantially greater than that previously reported for a single ESAT-6 peptide, ESAT-61–15, for which the maximum response rate was 57% (18). The higher frequency of the response obtained using ESAT-66–28 is probably related to the potential of this multiepitopic peptide (see Table 2) to enter into an increased number of natural processing pathways (16), thus allowing a better presentation in the context of MHC class II alleles and T-cell recognition. In this context, it seems likely that the responses obtained with our ESAT-66–28 peptide is the sum of the responses obtained with single peptides of ESAT-6 (ESAT-61–15, ESAT-66–20, ESAT-611–25, and ESAT-616–30) that have previously been evaluated (17,18,20,21). The response to the pool of ESAT-6 peptides was shown to be useful because all TB patients responding in vitro to single peptides also responded to the peptides pool.
The in vitro ESAT-6 peptide-based immunological assay described in this study presents a sensitivity of 74% and suggests that the absence of a response in some patients with active TB may be due to their particular state of immune suppression. In particular, 1 of the nonresponder patients was under corticosteroid therapy and 6 subjects had severe multiorgan TB. The lack of a response in these patients is in agreement with previously published observations reporting the absence of a response to ESAT-6 in peripheral blood mononuclear cells from patients with radiographically determined severe TB disease (15). The sensitivity for TB diagnosis reported in the literature using unselected ESAT-6 peptides is higher compared with what we described (16,18,19); however, in those studies it was not possible to discriminate between active and latent TB. In our study, in persons with latent TB infection characterized by a positive tuberculin skin test, we demonstrated an in vitro response to PPD (19 of 19) and to the whole ESAT-6 protein (14 of 19), but not to the related selected peptides (0 of 19). These data demonstrate a strong association between the ESAT-6–peptides elicited responses and active TB. This suggests that T-cells with specificity to these peptides are present at high frequency during active disease but not during latent infection. In addition, note that in our study, 13 of 45 control subjects had recently immigrated (less than 2 y) from countries with a high burden of TB (see Table 1); however none of them responded to the ESAT-6 peptides despite the fact that they had positive tuberculin skin tests. If confirmed with a larger number of patients, these results indicate that ESAT-6 peptides could be used to reliably distinguish between active and latent infection.
Regarding the patients with latent TB infection, we note that the in vitro positive response to ESAT-6 protein could be related to infection with mycobacteria different from MTB, such as Mycobacterium kansasii, szulgai, flavescens, and marinum because the ESAT-6 gene also is present in these atypical mycobacteria (34). Moreover an ESAT-6 homologue has recently been identified in Mycobacterium leprae and some MTB-infected individuals showed cross-reactivity with this antigen (35). Although none of the individuals in this study presented clinical signs or a previous history of leprosy or of a disease associated with the mycobacteria mentioned above, we cannot rule out the possibility that individuals were exposed to them. In addition, to confirm the specificity of our assay, it will be important to include in future studies patients with mycobacterial diseases other than TB.
Diagnosis of TB in HIV+ patients is difficult (36,37), and this is a significant global problem. HIV disease occurs throughout the world especially in countries with limited resources (1). Thus, it is important to have reliable diagnostic tests that identify active TB in HIV+ patients. Within the HIV+ group studied here, the sensitivity of the assay was 86% (6 of 7) and was comparable with the sensitivity reported in the HIV− group (70%, 14 of 20). Surprisingly, the magnitude of the response to ESAT-6 peptide pool did not correlate with either CD4+ T-cell counts or with plasma HIV-RNA levels. Certainly, further studies on a larger number of patients are necessary to better define the relationship between plasma viremia, CD4+ T-cell counts, and responsiveness to our ESAT-6 peptides. A recent paper by Chapman and others (19) describes the detection of TB infection in HIV+ individuals using the ELISPOT assay for IFN-γ performed with unselected peptides from ESAT-6 and culture filtrate protein-10. Whereas this report is important because it clearly demonstrates the utility of such an assay in diagnosing TB in immunocompromised patients, the assay employed in their study cannot discriminate between active and latent TB.
In conclusion, we have demonstrated that multiepitopic peptides derived from ESAT-6 using computational analysis can be used to diagnose active TB and, more importantly, to discriminate between active TB and latent TB. By employing the ELISPOT method to detect IFN-γ–producing cells, an accurate diagnosis of active TB can be obtained in 2 d compared with the 15 to 40 d required for a diagnosis that relies on bacterial cultures (5,6), thus allowing prompt and correct therapy. While we could not evaluate the predictive value of the assay because of the method used for patient enrollment (that is, patients were deliberately chosen with active TB without performing a prospective enrollment of individuals with suspected TB), the negative and positive predictive values of our immunological assay, if applied to a population of patients with suspected TB in an industrial country with a prevalence of active diseases of 6% (38), would be 98.4% and 100%, respectively. Within a population with a higher prevalence of TB, that is, 40%, the negative predictive value would drop to 85.2%. Although the sensitivity of this assay requires further improvement, its capacity to aid in the identification of active infections may prove useful in suppressing the transmission of TB.
Acknowledgments
The authors are grateful to all patients and the nursing staff who took part in this study. We thank V Colizzi and F Poccia for the helpful discussions, M Brescia for technical assistance, R Urso, M De Marco, R Maddaluno, and A Govoni for helping in the recruitment of patients, Jim Arthos, Zana Mariano, and Carla Nisii for editing the manuscript, and Massimo Carrara for helping with the graphics. This work was supported by Current Research Project of the Italian Ministry of Health grants.
REFERENCES
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country WHO global surveillance and monitoring project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Di Perri G, et al. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet. 1989;2:1502–4. [PubMed] [Google Scholar]
- 3.Jasmer RM, Nahid P, Hopewell PC. Latent tuberculosis infection. New Eng J Med. 2002;347:1860–6. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 5.Forbes BA. Critical assessment of gene amplification approaches on the diagnosis of tuberculosis. Immunol Invest. 1997;26:105–16. doi: 10.3109/08820139709048919. [DOI] [PubMed] [Google Scholar]
- 6.Fine PE, et al. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–75. [PubMed] [Google Scholar]
- 7.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 8.Shafer RW, Edlin BR. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin Infect Dis. 1996;22:683–704. doi: 10.1093/clinids/22.4.683. [DOI] [PubMed] [Google Scholar]
- 9.Behr MA, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 10.Arend SM, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797–807. doi: 10.1086/345760. [DOI] [PubMed] [Google Scholar]
- 11.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen P, Andersen AB, Sorensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–72. [PubMed] [Google Scholar]
- 13.Pollock JM, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–92. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrichs T, et al. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–58. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Ravn P, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–45. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 16.Doherty TM, et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol. 2002;40:704–6. doi: 10.1128/JCM.40.2.704-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalvani A, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 18.Lalvani A, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AL, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 20.Lalvani A, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–8. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 21.Pathan AA, et al. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: association with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 22.Elhay MJ, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–6. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso FL, et al. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect Immun. 2002;70:6707–14. doi: 10.1128/IAI.70.12.6707-6714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–9. doi: 10.1007/s002510050595. Refer to http://www.syfpeithi.de. [DOI] [PubMed] [Google Scholar]
- 25.Fleckenstein B, Jung G, Wiesmüller KH. Quantitative analysis of peptide-MHC class II interaction. Semin. Immunol. 1999;11:405–16. doi: 10.1006/smim.1999.0198. Refer to http://www.epipredict.de. [DOI] [PubMed] [Google Scholar]
- 26.Sturniolo T, et al. Generation of tissue-specific and promiscuous HLA ligand database using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 1999;17:555–61. doi: 10.1038/9858. Refer to http://www.imtech.res.in/raghava/propred/page2.html. [DOI] [PubMed] [Google Scholar]
- 27.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–7. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 28.Proceedings of the 12th IHWC. (1997) HLA genetic diversity of HLA functional and medical implication. Charron D. (ed) EDK Medical and Scientific International Publisher, Paris.
- 29.Andersen AB, Brennan P. (1994) Proteins and antigens of Mycobacterium tuberculosis In: Tuberculosis Bloom B. (eds.) ASM Press, Washington DC. pp. 307–32.
- 30.Orme IM. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–2. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen P, Heron I. Specificity of protective memory immune response against Mycobaterium tuberculosis. Infect Immun. 1993;61:844–51. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanguay S, Killion JJ. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994;13:259–63. [PubMed] [Google Scholar]
- 33.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204:57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 34.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geluk A, et al. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect Immun. 2002;70:2544–8. doi: 10.1128/IAI.70.5.2544-2548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafer RW, Kim DS, Weiss JP, Quale JM. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine. 1991;70:384–97. doi: 10.1097/00005792-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Chaisson RE, et al. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy and survival. Am Rev Respir Dis. 1987;136:570–4. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 38.Divinagracia RM, Timothy JH, Stanley B, Schluger NW. Screening by specialists to reduce unnecessary test ordering in patients evaluated for tuberculosis. Chest. 1998;114:681–4. doi: 10.1378/chest.114.3.681. [DOI] [PubMed] [Google Scholar]


