Abstract
The pathogenicity and immunogenicity induced in BALB/c mice by intranasal (i.n.) inoculation of enterotoxigenic Escherichia coli (ETEC) strains H10407 (O78:H11:CFA/I:LT+:ST+) and B7A (O148:H28:CS6:LT+:ST+) (two ETEC strains previously used in human challenge trials) were studied. The i.n. inoculation of BALB/c mice with large doses of ETEC strains H10407 and B7A caused illness and death. The H10407 strain was found to be consistently more virulent than the B7A strain. Following i.n. challenge with nonlethal doses of H10407 and B7A, the bacteria were cleared from the lungs of the mice at a steady rate over a 2-week period. Macrophages and neutrophils were observed in the alveoli and bronchioles, and lymphocytes were observed in the septa, around vessels, and in the pleura of the lungs in mice challenged with H10407 and B7A. In mice i.n. challenged with H10407, serum immunoglobulin G (IgG) and IgM antibodies were measured at high titers to the CFA/I and O78 lipopolysaccharide (LPS) antigens. In mice i.n. challenged with B7A, low serum IgG antibody titers were detected against CS6, and low serum IgG and IgM antibody titers were detected against O148 LPS. The serum IgG and IgM antibody titers against the heat-labile enterotoxin were equivalent in the H10407- and B7A-challenged mice. The CFA/I and O78 LPS antigens gave mixed T-helper cell 1-T-helper cell 2 (Th1-Th2) responses in which the Th2 response was greater than the Th1 response (i.e., stimulated primarily an antibody response). These studies indicate that the i.n. challenge of BALB/c mice with ETEC strains may provide a useful animal model to better understand the immunogenicity and pathogenicity of ETEC and its virulence determinants. This model may also be useful in providing selection criteria for vaccine candidates for use in primate and human trials.
Enterotoxigenic Escherichia coli (ETEC) is one of the most common causes of diarrhea in children in developing countries as well as in travelers to these areas (6). It is estimated that worldwide there are 650 million cases of diarrhea annually with 800,000 deaths in children under the age of 5 (21). Nearly half of all travelers to developing countries experience at least one episode of diarrhea during their stay, with ETEC being responsible for 20 to 50% of all cases (48). The illness caused by ETEC ranges from a mild diarrhea with little to no dehydration to a very severe and potentially fatal cholera-like disease (45).
ETEC organisms are noninvasive bacteria that colonize the small intestine. They do so by initially attaching to mucosal surfaces by means of colonization factors (CF) (21). Subsequent elaboration of enterotoxins, a heat-labile enterotoxin (LT) and/or a heat-stable enterotoxin (ST), results in diarrheal disease (8). There are three primary CF antigens (CFA), CFA/I, CFA/II, and CFA/IV, which have been found on 50 to 75% of ETEC bacteria isolated from humans with diarrhea in various geographic locations worldwide (5, 23). CFA/I consists of a single fimbrial antigen that is homogeneous, whereas CFA/II and CFA/IV are heterogeneous antigens. CFA/II is composed of coli surface-associated subcomponents CS1, CS2, and CS3, and CFA/IV is comprised of CS4, CS5, and CS6 antigens (8, 45).
Fimbrial vaccines have been administered to pregnant cattle, sheep, and swine in order to protect the suckling neonates against ETEC colibacillosis (34, 38, 39). These vaccines induced antifimbrial antibody responses detected in the milk and colostrum of lactating farm animals. The suckling neonates were then passively protected from intestinal colonization by ETEC. Chinese Meishan and European Large White pigs have also been used in the study of E. coli expressing CF (13). Problems are encountered with large animals, such as housing, treatment facilities, expense, and difficulty in carrying out procedures (12). Also, the number of large animals available for screening can be a limiting factor in vaccine studies.
Human ETEC challenge trials have been conducted. Levine and coworkers demonstrated with volunteers that a prior episode of diarrhea as a result of either ETEC strain H10407 (32) or strain B7A (33) conferred significant protective immunity against a subsequent homologous challenge. Previous studies (33) have indicated that immunity against somatic antigens present on the bacteria is more important than immunity against the LT and/or ST toxins for prolonged protection. Several field studies (9, 51) have found that multiple episodes of diarrhea induced by LT-positive ETEC strains are common. This indicates that immunity to the LT alone is unable to provide significant protection against subsequent ETEC infection. Freedman and coworkers (20) demonstrated protection against challenge with ETEC strain H10407 following the oral administration of milk-derived anti-CFA/I antibodies. They concluded that antibodies against CFA/I alone are sufficient for protection. Levine and coworkers (30) also have demonstrated that protective immunity against ETEC challenge can be induced by immune responses to CFs alone. Volunteers administered a nontoxigenic CS1-CS3-positive strain showed significant protection when challenged with a toxigenic CS1-CS3-positive strain.
Lack of an ETEC animal model has hampered the study of the pathogenesis and immune response of this bacterial infection. Studies involving ETEC have utilized mice (12, 14, 15), rats (28), guinea pigs (16), and rabbits (17, 19, 24). Potential problems arising in the application of these animal models may include the inability of ETEC to elicit an immune response in the animal, inability to adhere to and colonize the animal gut, inability of ETEC to cause symptoms consistent with diarrhea, and the resistance of the animal to ETEC with age. The removable intestinal tie-adult rabbit diarrhea model has been used previously in the study of ETEC-induced acute diarrheal diseases (37, 43, 44). However, the removable intestinal tie-adult rabbit diarrhea model is a very invasive surgical procedure and stressful to the animal. The infant mouse model may aid in helping to further understand the mechanisms of the pathogenesis of ETEC diarrhea due to the immaturity of the bacterial flora in the intestines of the newborn and the susceptibility of the infant mouse to the STa enterotoxin (12, 22).
The design of a safe and effective vaccine that could reduce morbidity and mortality caused by ETEC would be of importance in public health (45). There is at present no licensed vaccine against ETEC for use in at-risk individuals. Likewise, there is no completely suitable small animal model to study the immunogenicity and efficacy of potential ETEC vaccines prior to testing in primates or volunteers. Our present aim is to develop a small animal model by which the antigenicity, immunogenicity, and efficacy of experimental ETEC vaccines can be studied. By the use of a small animal model, such as an intranasal (i.n.) adult mouse model, comparisons of potential vaccine formulations and schedules and effects of adjuvants can be better determined prior to use in primates and humans, thereby saving expense as well as time. Thus, better planning of future volunteer studies and more rational vaccine design may be possible.
In this study, the clearance of ETEC from the lungs and the histopathological response in the alveoli and bronchioles as well as the septa and/or vessels and pleura of the lungs were measured following i.n. challenge of BALB/c mice with viable ETEC strains H10407 and B7A (two strains previously utilized in volunteer studies). The serum antibody isotype and immunoglobulin G (IgG) subclass titers were also measured following ETEC challenge, and the relationship of T-helper cell responses (Th1-Th2) was examined.
MATERIALS AND METHODS
Bacteria and inoculum preparation.
The ETEC strains used were H10407 (O78:H11:CFA/I:LT+:ST+) and B7A (O148:H28:CS6:LT+:ST+). The strains were maintained in Trypticase soy broth (Difco Laboratories, Detroit, Mich.)-20% glycerol and stored in stock cultures at −80°C. Stock cultures of ETEC strains H10407 and B7A were cultivated on CFA agar plates (1% Casamino Acids, 0.15% yeast extract, 2% agar, 0.04 mM MnCl2, and 0.4 mM MgSO4) at 37°C for 12 to 18 h. The bacteria were likewise cultivated on MacConkey agar (Difco) to ascertain the presence of only lactose-fermenting colonies, and Gram staining was performed on random colonies to verify the presence of a single morphological cell type indicative of a pure culture of ETEC. Bacteria were scraped off the surface of the CFA agar plates and suspended in sterile 50 mM phosphate-buffered saline (PBS), pH 7.2, by gentle agitation and rocking for 30 min at room temperature. Dilutions were made using PBS until an optical density at 600 nm of 1.0 (corresponding to 1010 CFU/ml) was obtained. Appropriate dilutions needed for animal trials were made from this standardized bacterial suspension. The actual number of viable bacterial cells that were administered to the animals was determined by a CFU procedure (i.e., a 10-fold serial dilution and plating onto Trypticase soy agar plates).
Mice.
Female BALB/c mice (8 to 10 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, Maine). The mice were housed in microisolator cages and provided with food and water ad libitum. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996 edition. All procedures were reviewed and approved by the Walter Reed Army Institute of Research Animal Care and Use Committee and performed in the same facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
i.n. inoculation of mice.
Mice were lightly anesthetized with Metofane (methoxyfluorane) (Schering-Plough Animal Health Co., Union, N.J.) in a glass desiccator and challenged with ETEC strains H10407 and B7A at doses of 1 × 108, 5 × 108, 1 × 109, and 5 × 109 bacteria in order to measure morbidity and mortality. Fifty microliters of each preparation was administered dropwise to the external nares of each mouse with a P200 Pipetman pipette (Ranin Instrument, Inc., Woburn, Mass.). Control mice were administered 50 μl of the PBS diluent.
Measure of clearance of bacterial cells from lungs.
Anesthetized mice were challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria). Three mice from each group, H10407 and B7A challenged, were euthanized, and the lungs were aseptically removed and placed in 1 ml of sterile PBS. The lungs were then completely homogenized by using Potter-Elvehjem glass tissue grinders (Fisher Scientific, Hanover Park, Ill.) to free bacteria into suspension, and a CFU procedure was performed to determine the number of bacteria present in the lungs at a given time postchallenge. CFA plates were used to determine the total number of bacterial cells present in the lungs, and MacConkey plates were used to ascertain that only lactose-fermentation-positive bacteria indicative of ETEC were present in the lungs of the mice.
Histopathology.
Anesthetized mice were i.n. challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria), and the lungs of the mice were examined microscopically. Three mice from H10407- and B7A-challenged and PBS control groups of mice were euthanized, and the lungs were infused with 10% neutral buffered formalin and removed for further immersion fixation. The fixed lung tissue was then processed, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin (H&E). The lungs were examined microscopically, and the number of inflammatory cells was noted. “Moderate-to-severe” indicates that the section of lung affected was from 11 to >41% with high numbers of inflammatory cells (neutrophils, macrophages, lymphocytes, and plasma cells), “minimal-to-mild” indicates that the section of lung affected was from 1 to 10% with low numbers of inflammatory cells, and “none” indicates that no histopathological changes were observed.
Isolation and purification of antigens. (i) CFA/I.
The CFA/I fimbriae were purified from ETEC strain 1933D (O71:H−:CFA/I:LT−:ST+). Bacteria were grown on CFA agar plates overnight at 37°C. The cells were suspended in PBS and heated to 60°C for 30 min. The bacterial suspension was then centrifuged at 9,000 × g for 20 min. The supernatant containing CFA/I was raised to 20% ammonium sulfate saturation, and the precipitate was recovered by centrifugation at 37,000 × g for 20 min. The precipitate was dialyzed extensively against PBS and stored at −80°C. A modified Lowry protein assay (42) was used to determine the protein content of CFA/I and then adjusted to 1 mg/ml. The purity of CFA/I was determined by Coomassie blue-stained sodium dodecyl sulfate (SDS)-16% polyacrylamide gel electrophoresis followed by densitometric scanning to be greater than 96%.
(ii) CS6.
The complete four-gene operon for CS6 (approximately 5 kb) was cloned into HB101 on a plasmid containing the gene for kanamycin resistance. The clone produced CS6 under fermentation conditions at a reduced temperature of 30°C under the control of the native CS6 promoter. The fermentation broth was harvested at 9,000 × g for 20 min with the bacteria being removed by membrane filtration, and the medium was separated from the CS6 by tangential flow ultrafiltration. The CS6 was further purified by precipitation at 25% ammonium sulfate saturation, and the CS6-ammonium sulfate solution was buffer exchanged with PBS. The purified CS6 was stored at −80°C. A modified Lowry protein assay (42) was used to determine the protein content of CS6 to be 1.3 mg/ml. The purity of CS6 was determined by Coomassie blue-stained SDS-16% polyacrylamide gel electrophoresis followed by densitometric scanning to be greater than 99%.
(iii) LPS.
The Darveau and Hancock (10) procedure was used to isolate and purify lipopolysaccharide (LPS) from ETEC strains H10407 (O78) and B7A (O148). Disrupted ETEC bacterial cells were treated with DNase (Sigma Chemical Co, St. Louis, Mo.), RNase (Sigma), proteinase (Sigma), and SDS, and the LPS extract was subjected to ethanol-magnesium chloride precipitation and high-speed centrifugation. The amount of protein present in the LPS sample was determined with the bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) to be less than 0.01% (wt/wt).
(iv) LT.
The LT was obtained from Sigma.
ELISA.
Antibodies raised against the CFA/I, CS6, LPS, and LT were measured by the use of a previously described enzyme-linked immunosorbent assay (ELISA) procedure (35). Blood for serological studies was collected from the mice 10, 14, 21, 28, 35, and 42 days following challenge. Blood was collected from the mice following decapitation, allowed to clot overnight at 4°C, and centrifuged at 1,000 × g for 20 min, and the serum was collected and stored individually at −80°C. Twofold serially diluted immune serum was added to antigen-coated wells followed by goat anti-mouse IgG(γ), IgM(μ), and IgA(α) isotype (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) antibody- or goat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 subclass (Southern Biotechnology, Birmingham, Ala.) antibody-alkaline phosphate conjugate. Absorbance was read at 405 nm on an ELISA reader (MR5000; Dynex Technologies, Inc., Chantilly, Va.). Endpoint titers were expressed as the reciprocal of the highest serial dilution of immune serum at which the A405 was at least twice that of the nonimmune control serum (from mice administered PBS) and the A405 of the immune serum was at least 0.2.
Statistical analysis.
Antibody titers were transformed logarithmically, and the standard deviations were calculated by using GraphPad Prism version 3.0a for Macintosh (GraphPad Software, San Diego, Calif.). The Student t test was used to compare the mean serum antibody titer values of different groups of mice with those of the PBS control mice, where differences in P values of <0.05 were considered to be significant.
RESULTS
i.n. challenge with viable ETEC strains H10407 and B7A.
Mice were challenged with various doses of H10407 and B7A strains to measure morbidity and mortality. Strain H10407 was more virulent than strain B7A, causing death earlier following challenge and higher mortality at doses of 5 × 108 and 1 × 109 bacteria (Table 1). Clinical signs following challenge with strains H10407 and B7A were lethargy and/or prostration, slow and/or labored breathing, hunched posture, hypothermia, and hair follicle erection. The percentage of weight loss in the mice was also used to measure the degree of illness following challenge with the H10407 and B7A strains. Mice given H10407 had maximum percentage weight losses (expressed as the maximal mean weight loss of the group of mice challenged with a particular bacterial dose) as follows: 5 × 107 bacteria, 12.3%; 1 × 108 bacteria, 14.8%; and 5 × 108 bacteria, 20.8%. Mice given B7A had maximum percentage weight losses as follows: 5 × 107 bacteria, 10.9%; 1 × 108 bacteria, 13.6%; 5 × 108 bacteria, 18.1%; and 1 × 109 bacteria, 22.5%.
TABLE 1.
| Strain and no. of organisms | No. of dead mice at day postchallenge:
|
Totalb | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| H10407 | ||||||||
| 0c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/12 (0) |
| 1 × 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/12 (0) |
| 5 × 108 | 0 | 3 | 1 | 2 | 1 | 0 | 0 | 7/12 (58) |
| 1 × 109 | 0 | 8 | 4 | 12/12 (100) | ||||
| 5 × 109 | 0 | 6 | 6/6 (100) | |||||
| B7A | ||||||||
| 0c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/12 (0) |
| 1 × 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/12 (0) |
| 5 × 108 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 3/12 (25) |
| 1 × 109 | 0 | 0 | 5 | 4 | 0 | 0 | 1 | 10/12 (83) |
| 5 × 109 | 0 | 1 | 5 | 6/6 (100) | ||||
Mice were challenged i.n. with the bacterial dose in 50 μl of PBS diluent administered dropwise to the external nares of each mouse. The data recorded are a combination of two independent trials, except for the 5 × 109 bacterial dose.
Number of dead mice/total number of mice (percent mortality).
Control mice were administered 50 μl of PBS i.n.
Kinetics of clearance and histopathology following i.n. challenge with viable ETEC strains H10407 and B7A.
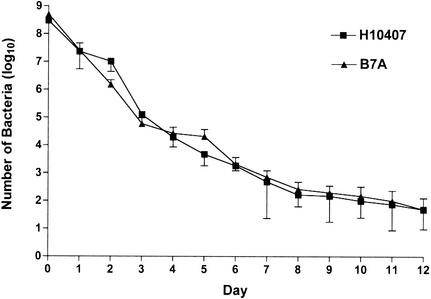
Mice were i.n. challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria), and the kinetics of clearance of the bacteria from the lungs of the mice were measured. The bacteria were cleared at a steady rate over a 14-day period, after which time no bacteria were able to be cultured from the lungs of the infected mice (Fig. 1). There was no difference in the rates of clearance of the H10407 and B7A strains from the lungs of the mice.
FIG. 1.
Kinetics of clearance following i.n. challenge with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria). Three mice from each group, H10407 and B7A challenged, were euthanized each 24-h period postchallenge, and the lungs were aseptically removed. A CFU procedure was performed to determine the number of bacteria present in the lungs at a given time postchallenge. The points indicate the means of the CFU from three mice for each of the ETEC strains. The error bars represent ±1 standard deviation of the mean response.
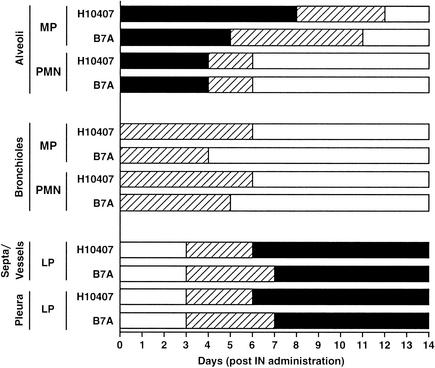
The histopathological changes that occurred in the lungs of the mice that were i.n. challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria) were measured over a 6-week period (Fig. 2 and 3). Multifocal bronchopneumonia was the main pathological feature upon microscopic evaluation (Fig. 3B), with no evidence of systemic spread outside the lungs. By 24 h, increased numbers of macrophages were observed in the alveoli; in the alveoli of the mice challenged with B7A this response continued up to day 5, and in the alveoli of the mice challenged with H10407 this response continued to day 8. High numbers of neutrophils were noted in the alveoli of the mice challenged with H10407 and B7A at 24 h, remained at these high numbers up to day 4, and were no longer detected by day 7. In the bronchioles of mice challenged with H10407 and B7A, there was only a slight increase in mononuclear cells and neutrophils present from 24 h until 4 to 6 days. The lymphocytes were noted in the septa and around vessels as well as in the pleura starting at day 3 for H10407- and B7A-challenged mice; by 6 to 7 days the lymphocytes had reached their highest numbers and remained at high numbers at least until 6 weeks. No histopathological changes were detected in the nasal cavity (upper respiratory tract) of the mice following challenge with H10407 and B7A at any time postchallenge. The mice administered only PBS had no histopathological changes observable in the lungs (Fig. 3A).
FIG. 2.
Histopathology of lungs of mice following i.n. challenge with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria). Three mice from H10407- and B7A-challenged and PBS control groups of mice were euthanized each 24-h period postchallenge, and the lungs were removed, fixed, and stained with H&E. The lungs were examined microscopically, and the number of inflammatory cells was noted. Moderate-to-severe (solid black bars) indicates that the section of lung affected was from 11 to >41% with high numbers of inflammatory cells (neutrophils, macrophages, lymphocytes, and plasma cells); minimal-to-mild (hatched bars) indicates that the section of lung affected was from 1 to 10% with low numbers of inflammatory cells; and none (solid white bars) indicates that no histopathological changes were observed. No histopathological changes were observed in the lungs of the mice administered only PBS. MP, macrophages or histiocytes; PMN, polymorphonuclear leukocytes (neutrophils); LP, lymphoid proliferation (lymphocytes and plasma cells).
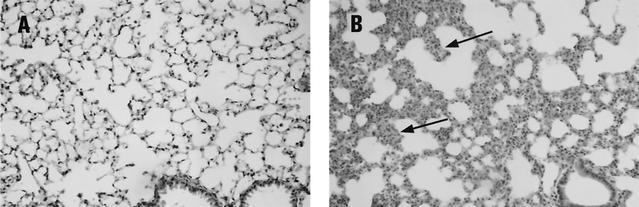
FIG. 3.
Mouse lung histopathology. (A) Control mouse i.n. administered PBS at day 1 has normal alveoli and bronchioles and fine, delicate septa. (B) Mouse challenged with H10407 (2 × 108 bacteria) at day 1 has pneumonia with increased macrophages and neutrophils that obscure alveoli and thicken the septa (arrows). Many macrophages contain intracellular bacilli (data not shown). H&E was used for staining. Magnification, ×200.
Serum antibody response to CFA/I, CS6, LPS, and LT following i.n. challenge with viable ETEC strains H10407 and B7A.
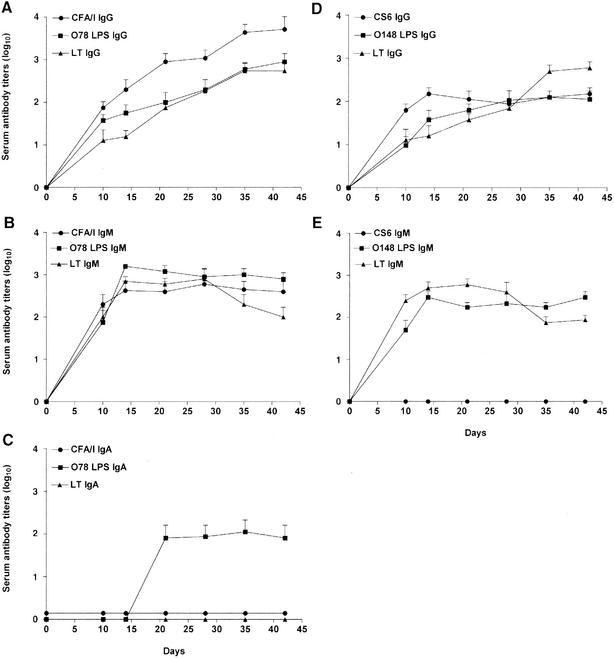
Mice were challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria), and serum was collected at days 10, 14, 21, 28, 35, and 42 (Fig. 4). Following challenge with strain H10407, the serum IgG (Fig. 4A) and IgM (Fig. 4B) antibody titers against CFA/I, O78 LPS, and LT were detected by day 10. The IgG titers increased over the course of the trial out to week 6, whereas the IgM titers to the somatic antigens (CFA/I and O78 LPS) remained essentially constant and the IgM titers to the LT decreased. Following challenge with H10407, IgA responses were detected to O78 LPS (Fig. 4C). In contrast, no serum IgA response was measured against any antigen following challenge with B7A. Following challenge with strain B7A, the serum IgG antibody titers to the somatic antigens (CS6 and O148 LPS) remained essentially constant, whereas the serum IgG antibody titers to the LT increased over the course of the trial up to week 6 (Fig. 4D). The serum IgG antibody responses to the somatic antigens (CS6 and O148 LPS) were measured at lower titers than those of the H10407-challenged mice; however, the IgG response to the LT was nearly identical to that of the H10407-challenged mice. No serum IgM titers were detected against CS6, and the IgM response to the O148 LPS was similar to that seen with the O78 LPS in that the titers remained essentially constant up to week 6, albeit lower (Fig. 4E). The serum IgM response to the LT was similar to the IgM response seen for the H10407-challenged mice, with the titers decreasing over time.
FIG. 4.
Mice were challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria), and serum was collected at days 10, 14, 21, 28, 35, and 42. Serum IgG (A), IgM (B), and IgA (C) antibody responses were measured against CFA/I, O78 LPS, and LT following i.n. challenge with H10407, and serum IgG (D) and IgM (E) antibody responses were measured against CS6, O148 LPS, and LT following i.n. challenge with B7A. No serum IgA antibody response was measured in mice challenged with B7A (data not shown). Endpoint ELISA titers were expressed as the reciprocal of the highest dilution of immune serum at which the A405 was at least twice that of the nonimmune control serum (mice administered PBS) and the A405 of the immune serum was at least 0.2. Data are presented as the mean titers (of four mice) of the IgG, IgM, and IgA antibodies. The error bars represent ±1 standard deviation of the mean response. At all titers P was <0.05 compared with the PBS control mice, except on day 10 for O148 LPS IgG (D) and days 10 and 14 for LT IgG for both H10407 (A)- and B7A (D)-challenged mice.
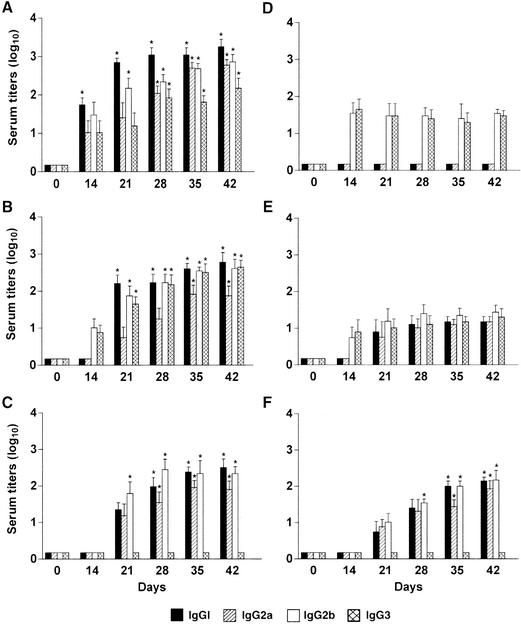
Serum IgG subclass antibody response to CFA/I, CS6, LPS, and LT following i.n. challenge with viable ETEC strains H10407 and B7A.
Mice were i.n. challenged with H10407 (2 × 108 bacteria) and B7A (4 × 108 bacteria), and serum was collected at days 14, 21, 28, 35, and 42 (Fig. 5). The CFA/I of H10407 induced a response by all four IgG subclasses (Fig. 5A), whereas only IgG2b and IgG3 subclasses were detected against the CS6 of B7A (Fig. 5D). The major serum IgG subclass responses to CFA/I were IgG1 followed by IgG2b and IgG2a. The i.n. administration of strains H10407 and B7A to mice induced all four IgG subclasses against the LPS (Fig. 5B and E); however, the H10407 strain induced anti-LPS IgG subclass titers a log or more greater than those induced by the B7A strain. The predominant serum IgG subclasses to the O78 LPS were IgG1 followed by IgG2b and IgG3 (Fig. 5B), whereas, to the O148 LPS, IgG2b was the predominant serum subclass measured (Fig. 5E). The major serum IgG subclass responses to the LT of both H10407- and B7A-challenged mice were IgG1 and IgG2b, with no IgG3 subclass measured (Fig. 5C and F). The LT IgG subclass titers were first noted at week 3, whereas the somatic antigen (CF and LPS) IgG subclass titers were first detected at week 2 for both H10407- and B7A-challenged mice.
FIG. 5.
Serum IgG subclass antibody responses measured to CFA/I, CS6, LPS, and LT. Serum IgG subclass antibody responses were measured against CFA/I (A), O78 LPS (B), and LT (C) following i.n. challenge with H10407, and the serum IgG subclass antibody responses were measured against CS6 (D), O148 LPS (E), and LT (F) following i.n. challenge with B7A. Endpoint ELISA titers were expressed as the reciprocal of the highest dilution of immune serum at which the A405 was at least twice that of the nonimmune control serum (mice administered PBS) and the A405 of the immune serum was at least 0.2. Data are presented as the mean titers (of four mice) of the IgG subclasses. The error bars represent ±1 standard deviation of the mean response. *, P < 0.05 compared to the PBS control mice.
The IgG subclasses to CFA/I and O78 LPS gave mixed Th1-Th2 responses with a consistently IgG1 > IgG2a (Th2 > Th1) profile, indicating predominant Th2 responses. The IgG subclass responses to the LT of both the H10407- and B7A-challenged mice gave mixed Th1-Th2 responses with predominant Th2-like profiles.
DISCUSSION
At present, there is no licensed vaccine against ETEC, nor is there a completely suitable small animal model in which the immune response to ETEC can be studied. We administered ETEC strains H10407 and B7A i.n. to mice and noted pathological and immunological responses. The results allow us to better understand the applicability of using an i.n. mouse model in the study of potential ETEC vaccines prior to their administration to primates and humans.
The i.n. challenge of mice as a model for studying pathogenicity and immune response has been performed with other enteric bacterial pathogens. The i.n. administration of Campylobacter jejuni to mice has been used to study infection and vaccination-acquired immunity (3). The i.n. administration of Shigella spp. to mice has been useful in assessing potential vaccine candidates (49) and the extent of illness and pathology compared to that observed for volunteers (50).
Strains H10407 and B7A when administered i.n. to mice at nonlethal doses were begun to be cleared from the lungs of the mice quickly and within 24 h were reduced in number by at least 1 log. The bacteria were cleared at a steady rate from the lungs of the mice; however, they remained at detectable levels at least up to 14 days postchallenge. The length of time in which the ETEC bacterial cells remain in the lungs of the mice allows an immune response to be initiated and subsequently measured. When lethal doses were administered i.n. to the mice, the number of bacteria in the lungs of the mice increased by at least 1 log prior to the death of the mice by 48 h (data not shown).
Both ETEC strains induced severe inflammation in the lungs of the mice, with the H10407 strain causing a slightly more pronounced inflammatory response than that seen following administration of the B7A strain. Both induced high levels of pulmonary macrophages and neutrophils in the alveoli within 24 h of administration, with the macrophages remaining 5 to 6 days longer than the neutrophils. Bronchioles also had an influx of macrophages and neutrophils that was less severe than that observed in the alveolar spaces. These leukocytes gave way to lymphoid proliferation in the septa, around vessels, and in the pleura. The main pathological feature noted in the lungs of the mice following i.n. challenge with H10407 and B7A was multifocal bronchopneumonia. No evidence of systemic spread was detected in the mice in this study.
The H10407 strain given i.n. to mice caused deaths earlier postchallenge and resulted in higher mortality than did the B7A strain at the same doses. The H10407 strain induced high serum antibody titers against the CFA/I and O78 LPS somatic antigens, whereas the B7A strain induced low antibody titers to the CS6 and O148 LPS somatic antigens. The serum antibody titers to the LT in the H10407- and B7A-challenged mice were nearly equivalent.
Results of earlier studies (16, 33, 46) from different human ETEC challenge trials are similar to those seen in our i.n. mouse studies, with the H10407 strain being consistently more virulent than the B7A strain. The H10407 strain induces illness and diarrhea in a greater number of volunteers than does the B7A strain at similar doses. Also, challenge with the H10407 strain induces a greater serum immune response than does the B7A strain, inducing antibody titers in a higher number of volunteers than does the B7A strain at similar doses. In trials conducted by Levine and coworkers (31), H10407 when given orally at a dose of 5 × 108 bacteria caused diarrhea in all seven volunteers; however, the B7A strain when given orally at a dose 20 times greater (1 × 1010 bacteria) caused diarrhea in only four of six volunteers (67%).
Following i.n. challenge of mice with the H10407 strain, CFA/I induced a predominantly IgG1 subclass response. This is in agreement with that measured by Alves and coworkers (2), in which intramuscular administration of CFA/I to mice led to a predominantly IgG1 response.
High titers of antigen-specific IgG2b were measured following challenge with the H10407 and B7A strains. Possible roles of IgG2b in the immune response have been demonstrated by several investigators. It has been shown that the complement-fixing antibodies of the IgG2a and IgG2b subclasses are often more important in mice for protection against infectious organisms than are the IgG1 and IgG3 subclasses (1, 27). Results from the work of Isaka and coworkers (25, 26) indicate that IgG1 and IgG2b appear to act in the neutralization of exotoxins produced by Clostridium diphtheriae and Clostridium tetani. Kremer and coworkers (29) have reported that IgG1 and/or IgG2b may be an important neutralizing isotype produced in mice following mucosal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin.
High antibody titers of the IgG3 subclass were measured against the O78 LPS in mice challenged with H10407. Bacterial cell wall antigens such as LPS induce a T-cell-independent immune response in mice that is primarily IgG3 (41). In the murine system, it has been shown previously that naive IgM-IgD B cells can be activated by LPS to initiate switching to IgG3 (18). Murray and coworkers (36) demonstrated that LPS-stimulated B cells secreted primarily IgG3 and IgM antibodies. IgG3 has been shown elsewhere to have a good complement-fixing ability and to be protective against bacteria (18, 40).
No significant IgG3 response was measured against the LT antigen in mice challenged with H10407 and B7A. This is in agreement with results seen in a study by Takahashi and coworkers (47) in which the IgG subclass response following oral administration of the B subunit of LT to mice was IgG1, IgG2a, and IgG2b. The IgG subclass response to LT in the H10407- and B7A-challenged mice was in agreement with others (7, 11, 47) in that the LT generated a mixed Th1-Th2-like profile. The coexistence of IgG1, IgG2a, and IgG2b subclasses in sera without IgG3 is indicative of a mixed Th1-Th2 response (4).
A major concern for consideration with the i.n. mouse model is the use of the lungs as the target organ for an enteropathogenic organism. The absence of the normal target cells of ETEC in the lungs of mice, and thus the likely lack of the normal receptors to which the CFs and enterotoxins adhere, along with the absence of the natural flora present in the intestines of humans, may affect the overall outcome of an infection. Other possible physiological conditions (e.g., acidic pH, proteolytic enzymes, and peristalsis) that are absent or altered in the lungs of the mice may also affect the course of an infection. Another consideration is that ETEC bacteria may not release the enterotoxins in the lungs of the mice to the extent that they do in the human intestines due to the lack of certain environmental stimuli (e.g., natural target receptors, acidic pH, and bile salts). Even though there are major differences between the human intestinal tract and the mouse respiratory tract, the i.n. murine model appears to allow the study of antisomatic and antitoxin immune responses following challenge with ETEC strains. However, the obvious limitations of using a murine pulmonary model with enteropathogenic bacteria need to be taken into account.
By the i.n. administration of ETEC strains H10407 and B7A to mice, we have been able to demonstrate pathological and immunological responses in the mice against the whole bacteria and to specific virulence determinants of these ETEC strains. In our research, histopathological responses in the alveoli and bronchioles as well as the septa and/or vessels and pleura of the lungs were measured following i.n. challenge with viable ETEC strains H10407 and B7A. The serum antibody isotype and IgG subclass titers were also measured following the ETEC challenge, and the relationship of T-helper cells was noted. The research indicates that the murine i.n. challenge model may be useful in better understanding the immunogenicity and pathogenicity of ETEC strains and in screening potential vaccine candidates against ETEC prior to volunteer trials.
Acknowledgments
ETEC strains H10407 and B7A were kindly provided by Myron Levine, Center for Vaccine Development, University of Maryland, Baltimore. We thank Craig Morrissette for assistance with statistical analysis of the data and Lee Collins for assistance with the graphics.
Funding for this study was provided by the Military Infectious Disease Research Program, U.S. Army Medical Research and Materiel Command, Fort Detrick, Md., and the National Vaccine Program Office, Centers for Disease Control, Atlanta, Ga.
Editor: V. J. DiRita
REFERENCES
- 1.Allison, A. C., and N. E. Byars. 1986. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J. Immunol. Methods 95:157-168. [DOI] [PubMed] [Google Scholar]
- 2.Alves, A. M. B., M. O. Lasaro, D. F. Almeida, and L. C. S. Ferreira. 1998. Immunoglobulin G subclass responses in mice immunized with plasmid DNA encoding the CFA/I fimbria of enterotoxigenic Escherichia coli. Immunol. Lett. 62:145-149. [DOI] [PubMed] [Google Scholar]
- 3.Baqar, S., A. L. Bourgeois, L. A. Applebee, A. S. Mourad, M. T. Kleinosky, Z. Mohran, and J. R. Murphy. 1996. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect. Immun. 64:4933-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baras, B., M.-A. Benoit, L. Dupre, O. Poulain-Godefroy, A.-M. Schacht, A. Capron, J. Gillard, and G. Riveau. 1999. Single-dose mucosal immunization with biodegradable microparticles containing a Schistosoma mansoni antigen. Infect. Immun. 67:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binsztein, N., M. J. Jouve, G. I. Viboud, L. L. Moral, M. Rivas, I. Orskov, C. Ahren, and A.-M. Svennerholm. 1991. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J. Clin. Microbiol. 29:1893-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, R. E. 1986. The epidemiology of cholera and enterotoxigenic Escherichia coli diarrheal disease, p. 23-32. In J. Holmgren, A. Lindberg, and R. Mollby (ed.), Development of vaccines and drugs against diarrhea. Studentlitteratur, Lund, Sweden.
- 7.Boyaka, P. N., M. Marinaro, J. L. Vancott, I. Takahashi, K. Fujihashi, M. Yamamoto, F. W. Van Ginkel, R. J. Jackson, H. Kiyono, and J. R. McGhee. 1999. Strategies for mucosal vaccine development. Am. J. Trop. Med. Hyg. 60:35-45. [DOI] [PubMed] [Google Scholar]
- 8.Cassels, F. J., and M. K. Wolf. 1995. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J. Ind. Microbiol. 15:214-226. [DOI] [PubMed] [Google Scholar]
- 9.Cravioto, A., R. E. Reyes, F. Trujillo, F. Uribe, A. Navarro, J. M. de la Roca, J. M. Hernandez, G. Perez, and V. Vazquez. 1990. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am. J. Epidemiol. 131:886-904. [DOI] [PubMed] [Google Scholar]
- 10.Darveau, R. P., and R. E. W. Hancock. 1983. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 155:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce, G., V. Giannelli, M. Pizza, D. Lewis, P. Everest, R. Rappuoli, and G. Dougan. 1999. Genetically detoxified mutants of heat-labile toxin from Escherichia coli are able to act as oral adjuvants. Infect. Immun. 67:4400-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchet-Suchaux, M. 1999. Suckling mouse model of enterotoxigenic Escherichia coli infection, p. 241-253. In O. Zak and M. A. Sands (ed.), Handbook of animal models of infection. Academic Press, San Diego, Calif.
- 13.Duchet-Suchaux, M. F., A. M. Bertin, and P. S. Menanteau. 1991. Susceptibility of Chinese Meishan and European Large White pigs to enterotoxigenic Escherichia coli strains bearing colonization factor K88, 987P, K99, or F41. Am. J. Vet. Res. 52:40-44. [PubMed] [Google Scholar]
- 14.Duchet-Suchaux, M., C. Le Maitre, and A. Bertin. 1990. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J. Med. Microbiol. 31:185-190. [DOI] [PubMed] [Google Scholar]
- 15.Duchet-Suchaux, M. 1988. Protective antigens against enterotoxigenic Escherichia coli O101:K99,F41 in the infant mouse diarrhea model. Infect. Immun. 56:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont, H. L., S. B. Formal, R. B. Hornick, M. J. Snyder, J. P. Libonati, D. G. Sheahan, E. H. LaBrec, and J. P. Kalas. 1971. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 285:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Edelman, R., R. G. Russell, G. Losonsky, B. D. Tall, C. O. Tacket, M. M. Levine, and D. H. Lewis. 1993. Immunization of rabbits with enterotoxigenic E. coli colonization factor antigen (CFA/I) encapsulated in biodegradable microspheres of poly(lactide-co-glycolide). Vaccine 11:155-158. [DOI] [PubMed] [Google Scholar]
- 18.Esser, C., and A. Radbruch. 1990. Immunoglobulin class switching: molecular and cellular analysis. Annu. Rev. Immunol. 8:717-735. [DOI] [PubMed] [Google Scholar]
- 19.Evans, D. G., R. P. Silver, D. J. Evans, Jr., D. G. Chase, and S. L. Gorbach. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12:656-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman, D. J., C. O. Tacket, A. Delehanty, D. R. Maneval, J. Nataro, and J. H. Crabb. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177:662-667. [DOI] [PubMed] [Google Scholar]
- 21.Gaastra, W., and A.-M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 22.Giannella, R. A. 1976. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect. Immun. 14:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gothefors, L., C. Ahren, B. Stoll, D. K. Barua, F. Orskov, M. A. Salek, and A.-M. Svennerholm. 1985. Presence of colonization factor antigens on fresh isolates of fecal Escherichia coli: a prospective study. J. Infect. Dis. 152:1128-1133. [DOI] [PubMed] [Google Scholar]
- 24.Honda, T., M. Arita, and T. Miwatani. 1984. Characterization of new hydrophobic pili of human enterotoxigenic Escherichia coli: a possible new colonization factor. Infect. Immun. 43:959-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaka, M., Y. Yasuda, S. Kozuka, T. Taniguchi, K. Matano, J.-I. Maeyama, T. Komiya, K. Ohkuma, N. Goto, and K. Tochikubo. 2000. Induction of systemic and mucosal antibody responses in mice immunized intranasally with aluminium-non-adsorbed diphtheria toxoid together with recombinant cholera toxin B subunit as an adjuvant. Vaccine 18:743-751. [DOI] [PubMed] [Google Scholar]
- 26.Isaka, M., Y. Yasuda, S. Kozuka, Y. Miura, T. Taniguchi, K. Matano, N. Goto, and K. Tochikubo. 1998. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine 16:1620-1626. [DOI] [PubMed] [Google Scholar]
- 27.Katz, D., S. Lehrer, O. Galan, B.-E. Lachmi, and S. Cohen. 1991. Adjuvant effects of dimethyl dioctadecyl ammonium bromide, complete Freund's adjuvant and aluminium hydroxide on neutralizing antibody, antibody-isotype and delayed-type hypersensitivity responses to Semliki Forest virus in mice. FEMS Microbiol. Immunol. 76:305-320. [DOI] [PubMed] [Google Scholar]
- 28.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1982. Arousal of mucosal secretory immunoglobulin A antitoxin in rats immunized with Escherichia coli heat-labile enterotoxin. Infect. Immun. 37:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer, L., G. Riveru, A. Baulard, A. Capron, and C. Locht. 1996. Neutralizing antibody responses elicited in mice immunized with recombinant bacillus Calmette-Guérin producing the Schistosoma mansoni glutathione S-transferase. J. Immunol. 156:4309-4317. [PubMed] [Google Scholar]
- 30.Levine, M., J. G. Morris, G. Losonsky, E. Boedeker, and B. Rowe. 1986. Fimbriae (pili) adhesins as vaccines, p. 143-145. In D. Lark, S. Normak, and E. Brent-Uhlin (ed.), Protein-carbohydrate interactions in biological systems. Academic Press, London, United Kingdom.
- 31.Levine, M. M., R. E. Black, C. C. Brinton, Jr., M. L. Clements, P. Fusco, T. P. Hughes, S. O'Donnell, R. Robins-Browne, S. Woods, and C. R. Young. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand. J. Infect. Dis. Suppl. 33:83-95. [PubMed] [Google Scholar]
- 32.Levine, M. M., M. B. Rennels, L. Cisneros, T. P. Hughes, D. R. Nalin, and C. R. Young. 1980. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am. J. Epidemiol. 111:347-355. [DOI] [PubMed] [Google Scholar]
- 33.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon, H. W., and T. O. Bunn. 1993. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine 11:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris, D. D., and R. H. Whitlock. 1987. Therapy of suspected septicemia in neonatal foals using plasma-containing antibodies to core lipopolysaccharide (LPS). J. Vet. Intern. Med. 1:175-182. [DOI] [PubMed] [Google Scholar]
- 36.Murray, P. D., D. T. McKenzie, S. L. Swain, and M. F. Kagnoff. 1987. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J. Immunol. 139:2669-2674. [PubMed] [Google Scholar]
- 37.Mynott, T. L., D. S. Chandler, and R. K. J. Luke. 1991. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect. Immun. 59:3708-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy, B. 1980. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxigenic colibacillosis. Infect. Immun. 27:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy, B., H. W. Moon, R. E. Isaacson, C.-C. To, and C. C. Brinton. 1978. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect. Immun. 21:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuberger, M. S., and K. Rajewsky. 1981. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 11:1012-1016. [DOI] [PubMed] [Google Scholar]
- 41.Perlmutter, R. M., D. Hansburg, D. E. Briles, R. A. Nicolotti, and J. M. Davie. 1978. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 121:566-572. [PubMed] [Google Scholar]
- 42.Peterson, G. L. 1983. Determination of total protein. Methods Enzymol. 91:95-119. [DOI] [PubMed] [Google Scholar]
- 43.Spira, W. M., R. B. Sack, and J. L. Froehlich. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect. Immun. 32:739-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svennerholm, A.-M., M. M. McConnell, and G. Wiklund. 1992. Roles of different putative colonization factor antigens in colonization of human enterotoxigenic Escherichia coli in rabbits. Microb. Pathog. 13:381-389. [DOI] [PubMed] [Google Scholar]
- 45.Svennerholm, A.-M., J. Holmgren, and D. A. Sack. 1989. Development of oral vaccines against enterotoxigenic Escherichia coli diarrhoea. Vaccine 7:196-198. [DOI] [PubMed] [Google Scholar]
- 46.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 318:1240-1243. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi, I., M. Marinaro, H. Kiyono, R. J. Jackson, I. Nakagawa, K. Fujihashi, S. Hamada, J. D. Clements, K. L. Bost, and J. R. McGhee. 1996. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 173:627-635. [DOI] [PubMed] [Google Scholar]
- 48.Taylor, D. N., and P. Echeverria. Etiology and epidemiology of travelers' diarrhea in Asia. Rev. Infect. Dis. 8(Suppl. 2):S136-S141. [DOI] [PubMed]
- 49.Turbyfill, K. R., A. B. Hartman, and E. V. Oaks. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect. Immun. 68:6624-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van De Verg, L. L., C. P. Mallett, H. H. Collins, T. Larsen, C. Hammack, and T. L. Hale. 1995. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect. Immun. 63:1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaki, A. M., H. L. DuPont, M. A. El Alamy, R. R. Arafat, K. Amin, M. M. Awad, L. Bassiouni, I. Z. Imam, G. S. El Malih, A. El Marsafie, M. S. Mohieldin, T. Naguib, M. A. Rakha, M. Sidaros, N. Wasef, C. E. Wright, and R. G. Wyatt. 1986. The detection of enteropathogens in acute diarrhea in a family cohort population in rural Egypt. Am. J. Trop. Med. Hyg. 35:1013-1022. [DOI] [PubMed] [Google Scholar]