Abstract
In chickens, colibacillosis is caused by avian pathogenic Escherichia coli (APEC) via respiratory tract infection. Many virulence factors, including type 1 (F1A) and P (F11) fimbriae, curli, aerobactin, K1 capsule, and temperature-sensitive hemagglutinin (Tsh) and plasmid DNA regions have been associated with APEC. A strong correlation between serum resistance and virulence has been demonstrated, but roles of virulence factors in serum resistance have not been well elucidated. By using mutants of APEC strains TK3, MT78, and χ7122, which belong to serogroups O1, O2, and O78, respectively, we investigated the role of virulence factors in resistance to serum and pathogenicity in chickens. Our results showed that serum resistance is one of the pathogenicity mechanisms of APEC strains. Virulence factors that increased bacterial resistance to serum and colonization of internal organs of infected chickens were O78 lipopolysaccharide of E. coli χ7122 and the K1 capsule of E. coli MT78. In contrast, curli, type 1, and P fimbriae did not appear to contribute to serum resistance. We also showed that the iss gene, which was previously demonstrated to increase resistance to serum in certain E. coli strains, is located on plasmid pAPEC-1 of E. coli χ7122 but does not play a major role in resistance to serum for strain χ7122.
Avian pathogenic Escherichia coli (APEC) belongs to the extraintestinal pathogenic group of E. coli. These bacteria cause airsacculitis, omphalitis, peritonitis, salpingitis, synovitis, and colisepticemia in poultry (17). APEC is also associated with cellulitis or necrotic dermatitis of the lower abdomen and thighs and with granuloma. APEC strains belong predominantly to three serogroups, O1, O2, and O78. Virulence factors associated with APEC strains include type 1 and P fimbriae, curli, aerobactin, K1 capsule, and temperature-sensitive hemagglutinin (Tsh) of the autotransporter family (9, 17). Serum resistance also appears to be an important virulence mechanism of APEC, and it may play a major role in the pathogenesis of avian colibacillosis. For instance, serum resistance has often been associated with isolates from septicemic turkeys and chickens (13, 33), and a correlation between serum resistance and virulence and lethality in isolates from septicemic chickens and turkeys has been observed (13, 15, 17).
At this time, it is not known if avian E. coli strains differ from mammalian isolates in their mechanisms of serum resistance and virulence. Studies carried out with mammalian E. coli showed that many virulence factors, such as capsules, lipopolysaccharide (LPS), and outer membrane proteins (OMPs), including OmpA and the ColV plasmid-encoded proteins TraT and Iss, are associated with complement resistance of E. coli (17). TraT is a surface exclusion protein encoded by conjugative plasmids (32), and Iss is a plasmid-encoded OMP homologous to the Bor protein of bacteriophage λ (32). In APEC, the role of different virulence factors in serum resistance has generally been speculative. Nolan et al. (22) produced an avirulent, complement-sensitive mutant from a virulent, complement-resistant APEC isolate. It differed from the wild-type strain in its OMP profile. Further characterization of this mutant suggested that the complement resistance of this isolate was due, at least in part, to its ability to restrict C3 deposition, but not to degrade C3, on the bacterial surface (19).
To clarify the role of different virulence factors in the pathogenicity of APEC, various mutants have been constructed (Table 1) and, in some cases, tested in infection studies with chickens. PA68 (fimH) (2) and DM34 (fim) (20) mutants of O2:K1 strain MT78 that no longer produce the type 1 fimbrial adhesin FimH or type 1 fimbriae, respectively, and strain χ7273 (tsh), an isogenic derivative of O78:K80 strain χ7122 that no longer produces the pAPEC-1 plasmid-encoded Tsh autotransporter (12), colonized internal organs of inoculated chickens to the same extent as their wild-type parent strains (2, 12, 20). By contrast, the pAPEC-1 cured derivative of strain χ7122, χ7274, persisted less than its wild-type parent in internal organs (12).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype and characteristicsa | Reference |
|---|---|---|
| χ7122 | APEC O78:K80:H9, gyrA Nalr | 7 |
| χ7273 | χ7122 tsh::tetAR(B), Nalr Tcr | 12 |
| χ7274 | χ7273 cured of virulence plasmid pAPEC-1, Nalr | 12 |
| χ7179 | O78-negative derivative of χ7122 hisG::Tn10 rfb | 7 |
| χ7186 | χ7122 curli-negative mutant; csgA::cat Nalr Cmr | 8 |
| χ6206 | Strain H30, O26:H11, SLT-1+ | 3 |
| χ7168 | χ7179 rfb+ (O26), prototroph by P1χ6206 lysate | This work |
| χ7112 | Strain A137, O1:K1:H7 ColV+ isolate from human septicemia | 1 |
| χ7193 | χ7179 rfb+ (O1), prototroph by P1χ7112 lysate | This work |
| χ289 | W1485 λ−glnV44 F− | 7 |
| χ7145 | χ7122 (χ289:hisG-zee), rfb deleted by replacement with E. coli K-12 region at 45 min | 7 |
| χ7145(pYA3255) | Cosmid pYA3255 complements the rfb mutation in χ7145 | 7 |
| MT78 | APEC O2:K1:H+, Nalr | 10 |
| PA68 | MT78 (fimH) | 2 |
| DM34 | MT78 (fim) | 20 |
| BEN2694 | MT78 spontaneous K1-negative mutant, Nalr | This work |
| BEN2700 | BEN2694 complemented with p2D12 (part of the kps cluster of MT78 cloned into pHC79), Nalr, Apr, Tcr | This work |
| TK3 | APEC O1:K1:H7 | 14 |
| TK37G | papG mutant of strain TK3 produced by allelic exchange using pKNG101 based suicide vector, Kmr | C. Martin, personal communication |
| 862 | Serum-sensitive strain (E. coli O115, K−, F165−) | 16 |
Ap, ampicillin; Cm, chloramphenicol; Km, kanamycin; Nal, nalidixic acid; Tc, tetracycline.
This report examines the specific contribution of P fimbriae, K1 capsule, and O78 LPS to colonization of internal organs of inoculated chickens by APEC strains and investigates the role of these and other virulence factors in serum resistance, by using mutants of three APEC strains belonging to the most predominant serogroups (O1, O2, and O78).
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. In order to obtain APEC strain χ7122 derivatives that express other O antigens instead of the native O78 antigen, strain χ7179, an O78− histidine-requiring (hisG::Tn10) auxotroph of strain χ7122 (Table 1), was used as the recipient for bacteriophage P1 clm clr100-mediated transduction. Since hisG is closely linked to the rfb O-antigen-encoding DNA region, some transduced derivatives would acquire both prototrophy and an O-antigen-encoding rfb gene cluster. Briefly, P1 phage lysates of strains χ6206 (O26) and χ7112 (O1) were used to transduce strain χ7179 as previously described (7). Transductants were selected for prototrophy by growth on minimal medium containing glucose, and loss of tetracycline resistance conferred by Tn10 was verified. O-antigen-positive clones were confirmed by slide agglutination with O-antigen-specific antisera using standard slide and tube agglutination techniques (24). The O1- and O26-expressing derivatives of strain χ7179 were named χ7193 and χ7168, respectively.
A cosmid library constructed in pHC79, representing the genome of MT78, was screened by PCR to identify clones carrying the kps region (17 kb). A positive cosmid (p2D12) was identified and was transferred by electroporation into BEN2694, generating strain BEN2700. The restoration of the K1 capsule in the complemented strain BEN2700 was demonstrated by the Wellcogen N. meningitidis B/E. coli K1 kit (Murex Biotech Limited) and lysis by K1-specific bacteriophage (data not shown).
Lennox broth and Lennox agar (21) were routinely used for growing E. coli and clones. For infection studies, strains were grown in brain heart infusion broth (Difco). Ampicillin (100 μg ml−1), kanamycin (25 μg ml−1), chloramphenicol (25 μg ml−1), nalidixic acid (12.5 μg ml−1), and tetracycline (10 μg ml−1) were used as required at the indicated concentrations unless stated otherwise. For the Nalr strains χ7122 and MT78, no significant difference in virulence was observed between the parent strains (Nals) and their respective Nalr derivatives in experimentally infected chickens (data not shown).
Bactericidal effect of serum.
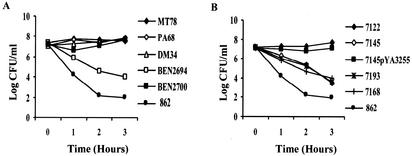
To identify virulence factors contributing to serum resistance, three APEC strains, their different mutants, and a control strain (862) (Table 1) were evaluated for their resistance to serum by examination of bacterial survival following a 3-h incubation in 90% serum from specific-pathogen-free chickens as previously described (13). The absence of specific antibodies for the APEC strains used in this study was confirmed by agglutination. A strain was considered serum resistant if the bacterial count increased or did not change during the 3-h incubation and serum sensitive if a decrease of more than 2 orders of magnitude in the bacterial count was observed. Results are presented as means of results of more than three experiments. Tukey's test was performed with commercially available software (SAS 8.1) (SAS Institute, Inc., Cary, N.C.), and post hoc 2-by-2 comparisons were done to assess differences between the strains. Our results confirmed that the three APEC strains MT78, χ7122, and TK3 were serum resistant (12, 29), whereas the nonpathogenic control strain 862 was serum sensitive (Fig. 1) (data for TK3 strain not shown). Significant differences in serum resistance between all APEC strains and the nonpathogenic control strain were observed from 1 h after inoculation (P < 0.0001). Sensitivity of the K1 mutant BEN2694 and resistance of its complemented strain BEN2700 to serum (Fig. 1A) demonstrated the role of K1 capsule in resistance of strain MT78 to serum. Furthermore, the growth of the K1 mutant in complement-inactivated serum showed more definitively that the K1 capsule interacted with complement (data not shown). Polysaccharidic capsules of E. coli may interact with C3b activators in the classical and alternative complement pathways, resulting in resistance of the bacteria to the bactericidal effects of complement. For instance, serum resistance of avian isolates has often been associated with the presence of K1 capsule. In fact, Pourbakhsh et al. (29) demonstrated a correlation between the presence of K1 capsule and bactericidal effects of serum in APEC strains. Our results confirm the role of K1 capsule in serum resistance.
FIG. 1.
Effect of 90% normal chicken serum on survival of strain MT78 and its mutants PA68 (fimH), DM34 (fim), and BEN2694 (K1−) and complemented strain BEN2700 (K1+) (A) and of strain χ7122 and its derivatives χ7145 (O78−), χ7193 (O1), and χ7168 (O26) and complemented strain χ7145 (pYA3255) (O78+) (B). Each point represents the mean for four to six experiments.
The absence of O78 antigen in χ7145 rendered this strain serum sensitive, and transformation of this strain with pYA3255, which encodes O78 LPS, rendered it as resistant to serum as wild-type strain χ7122 (Fig. 1B). On the other hand, the derivatives χ7193 (O1) and χ7168 (O26) were serum sensitive (Fig. 1B). Serum resistance can be mediated by particular O-antigen polysaccharide side chains (31). Resistance to the action of the membrane attack complex is often observed when bacteria change from a rough to a smooth phenotype. LPS of gram-negative bacteria are closely associated with the major OMPs (porins) (25, 30). It is probable that the length of the LPS chains determines the accessibility of complement to bacterial components such as porins and that effectiveness of complement fixation is also related to the nature of the LPS sugars. Nevertheless, the presence of long-chain smooth LPS is not sufficient for serum resistance in all O serotypes. In the present study, substitution of O78 with O1 or O26 antigen rendered the strain serum sensitive. Similarly, by testing different O serotypes, Pluschke et al. (28) and Pluschke and Achtman (27) showed that O1 polysaccharide did not contribute to serum resistance of an E. coli K1-positive strain. In fact, it fixed complement more efficiently than did isolated O7 or O18 LPS. By extrapolation, we can suppose that the O1 LPS alone does not protect the APEC strain TK3 (O1:K1) against the bactericidal effect of serum. Our results demonstrate that K1 capsule is not always needed for serum resistance, since strain χ7122 expressing O78 LPS was serum resistant even though it does not express K1 capsule. Also, as observed for strain χ7193, contrary to the case with O78 LPS, O1 LPS did not protect against the bactericidal effect of serum. Thus, the K1 capsule is probably required to prevent complement-mediated lysis of strains carrying certain O LPS antigens, such as O1 and O2, but is not needed to protect strains producing O LPS, such as O78, which would provide a protective role similar to that of K1 capsule.
Serum resistance of APEC strains has also been associated with the presence of type 1 fimbriae (13, 33). In contrast, our results showed that in the absence of FimH or type 1 fimbriae, the two mutant strains PA68 and DM34 remained serum resistant. Also, in spite of the presence of FimH and type 1 fimbriae, the mutant BEN2694 (K−) was serum sensitive (Fig. 1A). Hence, type 1 fimbriae do not appear to be important in serum resistance, at least for strain MT78. Similarly, it appears that the PapG adhesin of P fimbriae is not involved in serum resistance, since mutant TK37G remained resistant to the bactericidal effect of serum (data not shown).
Although curli bind to serum proteins (23), our results show that they do not contribute significantly to serum resistance, since inactivation of csgA, which encodes the curli subunit, did not affect resistance of strain χ7122 to serum (data not shown). Also, we have confirmed that tsh and plasmid pAPEC-1 do not contribute significantly to complement resistance of χ7122 (12).
Detection of iss and traT by PCR.
The iss and traT genes were amplified by PCR as previously described (18; H. Dezfullian, personal communication). Briefly, the DNA crude extracts prepared by a rapid boiling method were tested in a 25-μl PCR mixture containing 12.5 pmol (or 6.25 pmol each) of the forward and reverse primers, 5 nmol of each deoxynucleoside triphosphate, and 1 U of Taq DNA polymerase in 1× buffer. The PCR conditions were as follows: after initial incubation at 95°C for 5 min, temperature cycling was initiated with each cycle as follows: 94°C for 46 s, 63°C (traT) or 50°C (iss) for 46 s, and 72°C for 46 s for 24 cycles in a PTC-200 Peletier thermal cycler (MJ Research, Inc.). Samples were separated in 2% agarose gels and revealed with ethidium bromide. The sizes of the amplicons were determined by comparing them with a 100-bp DNA Ladder (Gibco). The three APEC wild-type strains, χ7122, MT78, and TK3, and all mutant strains used in this study (Table 1), with the exception of the pAPEC-1 plasmid-cured derivative χ7274, were iss and traT positive as demonstrated by PCR. Strain χ7274 was iss negative but traT positive. The nonpathogenic control strain 862 was iss and traT negative. Many OMPs (TraT, OmpA, and Iss) have been associated with complement resistance of APEC isolates (26). In our study, the presence of iss and traT genes in strains which had lost K1 or O serotype did not protect these bacteria against the bactericidal effect of serum. However, these strains were less sensitive than the control strain 862, which is iss and traT negative. Hence, Iss and TraT may play a limited role in the defense against serum killing, since it was suggested that the iss and traT products block the functioning of the terminal attack complex rather than its formation (5). The finding that iss was absent but traT was still present in the pAPEC-1 mutant χ7274 supported the hypothesis that the iss gene is located on the plasmid pAPEC-1, as has been already described for plasmid ColV,I-K94 (4, 5). However, in contrast to the finding of these authors, we found that the absence of iss in an APEC strain did not affect its resistance to complement. These results underline the multifactorial nature of bacterial resistance to the bactericidal effects of serum.
Experimental infection of chickens via the air sacs.
To study the involvement of the various virulence factors in pathogenicity, dissemination abilities of three APEC strains and their corresponding mutant strains were compared in the respiratory tract and internal organs of chickens. Briefly, groups of 8 or 10 3.5-week-old White Leghorn specific-pathogen-free chickens from the Institut National de la Recherche Agronomique experimental farm were inoculated with the appropriate strain into the right thoracic air sac as described previously (12). All birds were euthanatized at 48 h postinfection by injection of Nesdonal (Rhône-Mérieux, Lyon, France) and then necropsied. Organs were aseptically removed, and macroscopic fibrinous lesions were scored. The left lung, liver, and spleen were weighed, suspended in phosphate-buffered saline, and homogenized with an Ultra-Turrax apparatus as previously described (11). Dilutions of homogenates were plated onto Drigalski agar with appropriate antibiotics for bacterial quantification, and 1 ml was incubated in brain heart infusion for qualitative detection of E. coli. The presence of E. coli was also determined in air sacs, pericardial fluid, and blood. Lesion scores and bacterial counts in organs were compared between groups of chickens inoculated with the parent strain or its derivative(s) by analysis of variance. The exact Pearson's chi-square test was used to compare the number of contaminated chickens in each group in the case of qualitative detection of E. coli. In our previous studies, mutant strains DM34 (fim), PA68 (fimH), and χ7273 (tsh) persisted in internal organs as well as their corresponding wild-type strains (2, 12, 20), whereas χ7274 (pAPEC-1) persisted to a lesser extent than its wild-type parent strain (12). In this study, clinical signs of colibacillosis were reproduced with APEC strains χ7122, MT78, and TK3. Qualitative and quantitative results showed that all internal organs and fluids were colonized following inoculation of chickens with each of the three wild-type strains (Tables 2 and 3). On the other hand, mutants χ7145 (O78−) and BEN2694 (K1−) persisted less well or did not multiply in body fluids (pericardial fluid and blood) and colonized less well or did not colonize the internal organs (lungs, air sacs, spleen, and liver) in comparison to the corresponding parent strains. In contrast, no difference was noted in the ability of papG mutant TK37G to colonize internal organs and body fluids, compared with its corresponding parent strain. However, significant differences were apparent in lesion scores since the papG mutant induced less-severe lesions in the air sacs and liver (Table 3) than the wild-type strain TK3. Complemented strain χ7145 (pYA3255) (O78+) colonized internal organs and body fluids to the same extent as the wild-type strain, whereas the complemented BEN2700 (K1+) behaved in a manner similar to that of the corresponding mutant strain (Tables 2 and 3), in contrast to its behavior in the serum resistance experiments (Fig. 1A). This is possibly due to cosmid instability in vivo in the absence of antibiotic selection, as suggested by Brown and Curtiss (7). Also, we have observed that successive broth cultures of E. coli BEN2700 (K1+), in the absence of ampicillin selection, for longer periods than in the serum resistance experiments in which strain BEN2700 was complemented for, resulted in the loss of cosmid p2D12. The proportion of ampicillin-resistant clones decreased from 24 h (Δlog = −1.31) to day 4 (Δlog = −4.9) (data not shown).
TABLE 2.
Abilities of APEC strains and various mutants to colonize respiratory organs, invade internal organs, and disseminate in body fluids
| Strain | Inoculum (log CFU) | Air sacs, presence of E. colia | Pericardial fluid, presence of E. coli | Lung
|
Liver
|
Spleen
|
Blood
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Presence of E. coli | Mean no. of bacteriab | Presence of E. coli | Mean no. of bacteria | Presence of E. coli | Mean no. of bacteria | Presence of E. coli | Mean no. of bacteria | ||||
| χ7122 | 7.1 | 9/10 | 10/10 | 10/10 | 3.9 ± 1.5 | 10/10 | 2.4 ± 1.2 | 10/10 | 2.9 ± 0.8 | 10/10 | 3.1 ± 0.4 |
| χ7145 | 7.2 | 0/10c | 0/10c,d | 2/10c | 0.4 ± 0.8c | 0/10c | 0 ± 0c | 2/10c | 0.4 ± 0.8c | 0/10c | 0 ± 0c,d |
| χ7145(pYA3255) | 7.1 | 9/10c | 5/10c | 10/10 | 2.6 ± 1.1 | 9/10 | 1.8 ± 0.9 | 10/10 | 2.1 ± 0.4 | 10/10 | 1.9 ± 0.7c |
| MT78 | 7.1 | 9/10 | 10/10 | 10/10 | 3.3 ± 0.9 | 10/10 | 2.8 ± 1.0 | 10/10 | 3.2 ± 0.7 | 10/10 | 3.2 ± 0.5 |
| BEN2694 | 7.3 | 0/10c | 0/10c | 4/10c | 0.5 ± 0.7c | 0/10c | 0 ± 0c | 0/10c | 0 ± 0c | 0/10c | 0 ± 0c |
| BEN2700 | 7.3 | 1/10c | 1/10c | 0/10c | 0 ± 0c | 1/10c | 0.1 ± 0.5c | 2/10c | 0.2 ± 0.4c | 2/10c | 0.3 ± 0.7c |
| TK3 | 7.5 | 8/8 | NDe | 8/8 | 6.7 ± 0.9 | 8/8 | 4.1 ± 0.9 | 8/10 | 4.6 ± 0.6 | 4/4 | 3.6 ± 0.6 |
| TK37G | 7.3 | 8/8 | ND | 8/8 | 6.0 ± 1.6 | 7/8 | 4.1 ± 0.9 | 8/10 | 4.6 ± 0.9 | 4/4 | 3.5 ± 0.1 |
Number of chickens demonstrating bacterial colonization/total number of chickens.
Bacterial counts are presented as the mean log10 CFU per gram (organs) or milliliter (body fluids) ± standard deviation for 8 or 10 birds from each group. Counts were carried out at 48 h postinoculation with organs and pericardial fluid and at 6 h postinoculation with blood.
Significant difference (P < 0.01) versus the wild-type strain.
Significant difference (P < 0.01) versus the complemented strain.
ND, nondetermined.
TABLE 3.
Production of inflammatory lesions in air sacs and extrarespiratory organs of chickens inoculated with APEC strains and various mutants
| Strain | Mean lesional scores in air sacsa | Mean lesional scores in heart and liverb |
|---|---|---|
| χ7122 | 2.7 ± 1.3 | 2.8 ± 0.4 |
| χ7145 | 0.3 ± 0.5c | 0.2 ± 0.4c |
| χ7145(pYA3255) | 1.7 ± 0.8 | 2.5 ± 1.0 |
| MT78 | 2.7 ± 0.9 | 2.2 ± 0.6 |
| BEN2694 | 0.2 ± 0.6c | 0 ± 0c |
| BEN2700 | 0.2 ± 0.6c | 0 ± 0c |
| TK3 | 3.5 ± 0.5 | 4.0 ± 0 |
| TK37G | 0.8 ± 0.8c | 1.5 ± 1.6c |
Lesion scoring values for severity of aerosacculitis ± standard deviation: 0, normal; 1, slight edema; 2, mild diffuse thickening and neovascularization of air sacs with mild fibrinous exudate; 3, moderate fibrinous exudate; 4, severe extensive fibrinous exudate.
Combined lesion scoring values for severity of pericarditis and perihepatitis ± standard deviation. Heart and pericardium: 0, normal; 1, vascularization, opacity, cloudy fluid in the pericardial cavity; 2, acute pericarditis. Liver: 0, normal; 1, slight amounts of fibrinous exudate; 2, marked perihepatitis.
Significant difference (P < 0.01) versus the wild-type strain.
By using an intratracheal infection model in axenic chickens (6), we found that inactivation of csgA, which encodes the curli subunit, did not affect the ability of strain χ7186 to induce lesions and colonize internal organs as compared to wild-type strain χ7122 tested in the same conditions (data not shown).
Conclusion.
Taken together, our results show a correlation between serum resistance and the ability of bacteria to persist in body fluids and internal organs, since all bacteria when rendered serum sensitive were unable to infect internal organs. The use of mutant strains allowed us to demonstrate that the O78 LPS of strain χ7122, and the K1 capsule of strain MT78, are involved in the pathogenic process of these bacteria and that serum resistance is at least one mechanism used by these bacteria to reach internal organs of inoculated chickens. In contrast, the finding that the papG mutant strain TK37G induced less-severe lesions of airsacculitis and perihepatitis than the wild-type parent strain TK3 in infected chickens but nevertheless remained resistant to the bactericidal effect of serum suggests that the PapG adhesin is involved in another mechanism of the pathogenic process of this strain. The involvement of these virulence factors in other pathogenic steps, such as resistance to phagocytosis, could be examined to further elucidate the pathogenic process and lead to better control measures for colibacillosis.
Acknowledgments
We thank Guy Beauchamp for assistance in statistical analyses and Hojabr Dezfullian for providing Iss primers.
This work was supported by Formation des Chercheurs et à l'Aide de la Recherche du Québec grant 0214 and Natural Sciences and Engineering Research Council of Canada grant 2294 to J.M.F. and by U.S. Department of Agriculture National Research Initiative Competitive Grant Program grants 94-37204-1091, 97-35204-4512, and 00-35204-9224 to R.C., C.M.D., and P.B.
Editor: V. J. DiRita
REFERENCES
- 1.Achtman, M., A. Mercer, B. Kusecek, A. Pohl, M. Heuzenroeder, W. Aaronson, A. Sutton, and R. P. Silver. 1983. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect. Immun. 39:315-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arné, P., D. Marc, A. Brée, C. Schouler, and M. Dho-Moulin. 2000. Increased tracheal colonization in chickens without impairing pathogenic properties of avian pathogenic Escherichia coli MT78 with a fimH deletion. Avian Dis. 44:343-355. [PubMed] [Google Scholar]
- 3.Ashkenazi, S., and T. G. Cleary. 1989. Rapid method to detect shiga toxin and shiga-like toxin I based on binding to globotriosyl ceramide (Gb3), their natural receptor. J. Clin. Microbiol. 27:1145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binns, M. M., D. L. Davies, and K. G. Hardy. 1979. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature 279:778-781. [DOI] [PubMed] [Google Scholar]
- 5.Binns, M. M., J. Mayden, and R. P. Levine. 1982. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect. Immun. 35:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brée, A., M. Dho, and J. P. Lafont. 1989. Comparative infectivity for axenic and specific-pathogen-free chickens of O2 Escherichia coli strains with or without virulence factors. Avian Dis. 33:134-139. [PubMed] [Google Scholar]
- 7.Brown, P. K., and R. Curtiss III. 1996. Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 93:11149-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, P. K., C. M. Dozois, C. A. Nickerson, A. Zuppardo, J. Terlonge, and R. Curtiss III. 2001. MlrA, a novel regulator of curli (AgF) and extracellular matrix synthesis by Escherichia coli and Salmonella enterica serovar Typhimurium. Mol. Microbiol. 41:349-363. [DOI] [PubMed] [Google Scholar]
- 9.Dho-Moulin, M., and J. M. Fairbrother. 1999. Avian pathogenic Escherichia coli (APEC). Vet. Res. 30:299-316. [PubMed] [Google Scholar]
- 10.Dho-Moulin, M., J. F. Van den Bosch, J. P. Girardeau, A. Brée, T. Barat, and J. P. Lafont. 1990. Surface antigens from Escherichia coli O2 and O78 strains of avian origin. Infect. Immun. 58:740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dozois, C. M., N. Chanteloup, M. Dho-Moulin, A. Brée, C. Desautels, and J. M. Fairbrother. 1994. Bacterial colonization and in vivo expression of F1 (type 1) fimbrial antigens in chickens experimentally infected with pathogenic Escherichia coli. Avian Dis. 38:231-239. [PubMed] [Google Scholar]
- 12.Dozois, C. M., M. Dho-Moulin, A. Brée, J. M. Fairbrother, C. Desautels, and R. Curtiss III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect. Immun. 68:4145-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dozois, C. M., J. M. Fairbrother, J. Harel, and M. Bosse. 1992. pap-and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys. Infect. Immun. 60:2648-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dozois, C. M., S. A. Pourbakhsh, and J. M. Fairbrother. 1995. Expression of P and type 1 (F1) fimbriae in pathogenic Escherichia coli from poultry. Vet. Microbiol. 45:297-309. [DOI] [PubMed] [Google Scholar]
- 15.Ellis, M. G., L. H. Arp, and S. J. Lamont. 1988. Serum resistance and virulence of Escherichia coli isolated from turkeys. Am. J. Vet. Res. 49:2034-2037. [PubMed] [Google Scholar]
- 16.Fairbrother, J. M., A. Broes, M. Jacques, and S. Lariviere. 1989. Pathogenicity of Escherichia coli O115: K“V165” strains isolated from pigs with diarrhea. Am. J. Vet. Res. 50:1029-1036. [PubMed] [Google Scholar]
- 17.Gross, W. B. 1994. Diseases due to Escherichia coli in poultry, p. 237-259. In C. L. Gyles (ed.), Escherichia coli in domestic animals and man. CAB International, Wallingford, United Kingdom.
- 18.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 19.Kottom, T. J., L. K. Nolan, M. Robinson, J. Brown, T. Gustad, S. M. Horne, and C. W. Giddings. 1997. Further characterization of a complement-sensitive mutant of a virulent avian Escherichia coli isolate. Avian Dis. 41:817-823. [PubMed] [Google Scholar]
- 20.Marc, D., P. Arné, A. Brée, and M. Dho-Moulin. 1998. Colonization ability and pathogenic properties of a fim− mutant of an avian strain of Escherichia coli. Res. Microbiol. 149:473-485. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Nolan, L. K., R. E. Wooley, C. W. Giddings, and J. Brown. 1994. Characterization of an avirulent mutant of a virulent avian Escherichia coli isolate. Avian Dis. 38:146-150. [PubMed] [Google Scholar]
- 23.Olsèn, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338:652-655. [DOI] [PubMed] [Google Scholar]
- 24.Ørskov, F., and I. Ørskov. 1984. Serotyping of E. coli. Methods Microbiol. 14:43-112.
- 25.Pages, J. M., J. M. Bolla, A. Bernadac, and D. Fourel. 1990. Immunological approach of assembly and topology of OmpF, an outer membrane protein of Escherichia coli. Biochimie 72:169-176. [DOI] [PubMed] [Google Scholar]
- 26.Pfaff-McDonough, S. J., S. M. Horne, C. W. Giddings, J. O. Ebert, C. Doetkott, M. H. Smith, and L. K. Nolan. 2000. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 44:23-33. [PubMed] [Google Scholar]
- 27.Pluschke, G., and M. Achtman. 1984. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infect. Immun. 43:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pluschke, G., J. Mayden, M. Achtman, and R. P. Levine. 1983. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect. Immun. 42:907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pourbakhsh, S. A., M. Boulianne, B. Martineau-Doize, and J. M. Fairbrother. 1997. Virulence mechanisms of avian fimbriated Escherichia coli in experimentally inoculated chickens. Vet. Microbiol. 58:195-213. [DOI] [PubMed] [Google Scholar]
- 30.Sen, K., and H. Nikaido. 1991. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J. Bacteriol. 173:926-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stawski, G., L. Nielsen, F. Ørskov, and I. Ørskov. 1990. Serum sensitivity of a diversity of Escherichia coli antigenic reference strains. Correlation with an LPS variation phenomenon. APMIS 98:828-838. [PubMed] [Google Scholar]
- 32.Waters, V. L., and J. H. Crosa. 1991. Colicin V virulence plasmids. Microbiol Rev. 55:437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wooley, R. E., K. R. Spears, J. Brown, L. K. Nolan, and O. J. Fletcher. 1992. Relationship of complement resistance and selected virulence factors in pathogenic avian Escherichia coli. Avian Dis. 36:679-684. [PubMed] [Google Scholar]