Abstract
Reactive oxygen and nitrogen intermediates have critical, partially overlapping roles in host defense against a variety of pathogens. Using mice deficient in generating phagocyte superoxide (p47phox−/−) and mice deficient in generating inducible nitric oxide synthase (iNOS−/−), we examined the roles of these reactive species in host defense against Burkholderia cepacia and Chromobacterium violaceum, organisms known to have unusual virulence in chronic granulomatous disease. Intraperitoneal B. cepacia challenge (4.0 × 103 to 4.0 × 105 organisms/mouse) resulted in mortality in all p47phox−/− mice, with the survival interval being inversely proportionate to the amount of inoculum. Pretreatment with gamma interferon did not affect survival. C. violaceum was strikingly virulent in p47phox−/− mice (the 50% lethal dose [LD50] was <13 organisms). iNOS−/− and wild-type mice were resistant to B. cepacia challenges of at least 106 organisms per mouse, and the LD50 of C. violaceum was between 106 and 107 organisms per mouse. Consistent with the survival data, numbers of organisms in cultures of B. cepacia from multiple sites were higher for p47phox−/− mice than for iNOS−/− and wild-type mice at day 4 after challenge, but numbers of organisms for different B. cepacia strains varied. The recovery of C. violaceum was strikingly greater at 18 h after challenge for p47phox−/− mice than for iNOS−/− and wild-type mice, in which the organism burdens were virtually nil. In vitro, both B. cepacia and C. violaceum were sensitive to H2O2 and to reactive nitrogen intermediates but the sensitivities of different strains varied significantly. Host defense against B. cepacia and C. violaceum is critically dependent in vivo on reactive oxygen intermediates, and these species are model organisms to further dissect host and pathogen interactions related to the generation and scavenging of microbicidal reactive intermediates.
Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase in which phagocytes are defective in generating superoxide anion and downstream microbicidal metabolites, including hydrogen peroxide, hydroxyl anion, and hypohalous acid. As a result of the defect in this key antimicrobial pathway, patients with CGD suffer from recurrent life-threatening infections by catalase-producing bacteria and fungi (4, 28). Burkholderia (Pseudomonas) cepacia (23, 32) and Chromobacterium violaceum (4, 31; A. M. Macher, T. B. Casale, J. I. Gallin, H. Boltansky, and A. S. Fauci, Letter, Ann. Intern. Med. 98:259, 1983) are highly virulent pathogens in patients with CGD.
B. cepacia and other Burkholderia species typically cause pneumonia and, less commonly, sepsis in patients with CGD (3, 23, 25, 32). C. violaceum, a rare pathogen typically encountered in brackish waters, occurs with unusually high frequency in CGD patients from the southern United States (e.g., Florida and Louisiana), although C. violaceum infection in the northern United States has been reported (4, 31; Macher et al., letter). Clinical manifestations include soft tissue infection, pneumonia, and sepsis.
In the present study, we compared the levels of virulence of B. cepacia and C. violaceum in p47phox−/− mice and in mice with a targeted disruption of the inducible nitric oxide synthase (iNOS) gene to assess the relative importance of the reactive oxygen and reactive nitrogen intermediate pathways in host defense against these pathogens.
MATERIALS AND METHODS
Mice.
Mice with a targeted disruption of the p47phox gene have a defective NADPH oxidase, rendering phagocytes incapable of generating measurable amounts of superoxide (10). These mice are susceptible to a spectrum of pathogens similar to that of CGD patients (1, 10, 16). iNOS-deficient mice were generated as previously described (15). Both knockout strains were derived from C57BL/6 and 129 intercrosses. Wild-type strains were C57BL/6 × 129. Mice were maintained at the National Institute of Allergy and Infectious Diseases animal facility (Bethesda, Md.) under specific-pathogen-free conditions. All experiments were approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee. Mice were matched by age (2 to 6 months) and sex for each set of experiments.
Organisms.
B. cepacia strains (BS, 4A, and 8D) and C. violaceum strain BS were clinical isolates from CGD patients treated at the National Institutes of Health. C. violaceum 6357 was purchased from the American Type Culture Collection (Manassas, Va.). Bacteria were stored in Trypticase soy broth at −80°C until use. Frozen aliquots were inoculated into Trypticase soy broth and grown overnight with shaking at 37°C. Bacteria were washed twice in Hanks balanced salt solution (HBSS; Biofluids, Inc., Rockville, Md.) and diluted to an absorbance between 0.4 and 0.6 at 650 nm (corresponding to the linear portion of the absorbance-bacterial density curve). Bacterial concentration was calculated, and appropriate dilutions were made for each set of experiments. The bacterial concentration was confirmed by direct plating in duplicate and by counting the bacteria.
Culture medium and reagents.
Trypticase soy broth was purchased from Becton Dickinson (Sparks, Md.). Trypticase soy agar plates were purchased from Remel, Inc. (Lenexa, Kans.). B. cepacia selective agar plates were from the National Institutes of Health medium unit (Bethesda, Md.). Sodium nitrite (NaNO2) was purchased from Sigma (St. Louis, Mo.).
Mouse infection and sample collection.
Aliquots of frozen bacteria (stored at −80°C) were thawed, inoculated into Trypticase soy broth, and grown with shaking at 37°C overnight. The bacteria from overnight cultures were subcultured to log phase and then harvested by centrifugation. The bacteria were washed twice in HBSS, and bacterial density was determined by absorption at 650 nm (corresponding to the linear portion of the absorbance-bacterial density curve). Tenfold dilutions were prepared in HBSS and used to infect the mice. The dilutions were also plated on Trypticase soy agar plates to confirm the quantification.
Mice were injected intraperitoneally (i.p.) with 0.1 ml of diluted bacteria. Eighteen hours after challenge with C. violaceum and 4 days after challenge with B. cepacia, blood was collected retro-orbitally or from the tails of the mice into sterile heparinized tubes, mice were sacrificed by cervical dislocation, and peritoneal lavage was performed with 10 ml of phosphate-buffered saline. Spleens were homogenized in 2 ml of phosphate-buffered saline. Samples were diluted in serial 10-fold dilutions. Fifty microliters of peritoneal lavage and spleen homogenate and 20 μl of neat whole blood were inoculated in duplicate onto Trypticase soy agar plates. In experiments involving B. cepacia, B. cepacia selective agar plates were used to avoid contaminant bacteria (7). Since C. violaceum colonies are readily distinguished by their purple pigment, standard nonselective plates were used in experiments involving C. violaceum. After inoculation, plates were incubated for 24 to 48 h at 37°C and colonies were enumerated.
Antimicrobial activity of H2O2 and reactive nitrogen intermediates.
The antimicrobial activities of hydrogen peroxide (H2O2) and reactive nitrogen intermediates were measured as described elsewhere (30, 33), with some modifications. The antimicrobial activity of H2O2 was assayed by incubating 106 bacteria in a reaction mixture (1.0 ml) containing 0, 0.05, 0.1, 0.5, 1.0, 2.0, or 5.0 mM H2O2 in 100 mM sodium acetate buffer (pH 5.5) with continuous shaking at 37°C for 30 min. To evaluate the antimicrobial activity of reactive nitrogen intermediates, 106 bacteria were incubated in a reaction mixture (1.0 ml) consisting of 2 mg of NaNO2/ml in 100 mM sodium acetate buffer (pH 5.5) at 37°C for 30 min. Aliquots were serially diluted by 10-fold and plated in duplicate on Trypticase soy agar plates.
Statistical analysis.
Data were expressed as means ± standard deviations. The unpaired Student's t test was used to compare differences between two groups, and the differences were regarded as significant for P values of <0.05. The log rank (Mantel-Cox) test was applied to Kaplan-Meier survival data to evaluate differences between groups.
RESULTS AND DISCUSSION
B. cepacia challenge in vivo.
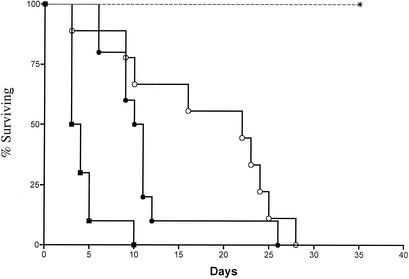
As shown in Fig. 1, the time to mortality following i.p. B. cepacia (strain BS) challenge in p47phox−/− mice was inversely related to the amount of inoculum (by the log rank test, P < 0.0001). Mortality occurred within 3 to 5 days following challenge with 4 × 105 organisms, whereas the mean intervals to mortality were 10 and 19 days following challenge with 4 × 104 and 4 × 103 organisms, respectively. B. cepacia pneumonia was present in all mice that were necropsied. In contrast to p47phox−/− mice, iNOS−/− mice (n = 4) challenged i.p. with 1.2 × 106 organisms showed no overt disease (Fig. 1). In a previous study, wild-type (C57BL/6 × 129) mice were resistant to challenge with 107 B. cepacia organisms (16).
FIG. 1.
Kaplan-Meier survival curves of p47phox−/− and iNOS−/− mice after i.p. challenge with B. cepacia. Open circles, p47phox−/− mice (n = 6) administered 4 × 103 CFU; closed circles, p47phox−/− mice (n = 6) administered 4 × 104 CFU; squares, p47phox−/− mice (n = 5) administered 4 × 105 CFU; *, iNOS−/− mice (n = 4) administered 1.2 × 106 CFU. By the log rank test, P was <0.0001. These data are representative of two independent experiments. Wild-type controls were previously shown to be resistant to i.p. challenge of 107 CFU/mouse (data not shown).
We next evaluated whether pretreatment with gamma interferon would improve survival, presumably through augmentation of nonoxidative pathways in p47phox−/− mice challenged with B. cepacia. In the present study, the subcutaneous administration of 20,000 U of mouse recombinant gamma interferon (Genentech, Inc., San Francisco, Calif.) three times a week beginning 6 weeks prior to and continuing after B. cepacia challenge (4.0 × 103, 4.0 × 104, or 4.0 × 105 organisms i.p.) did not affect the survival of p47phox−/− mice (data not shown).
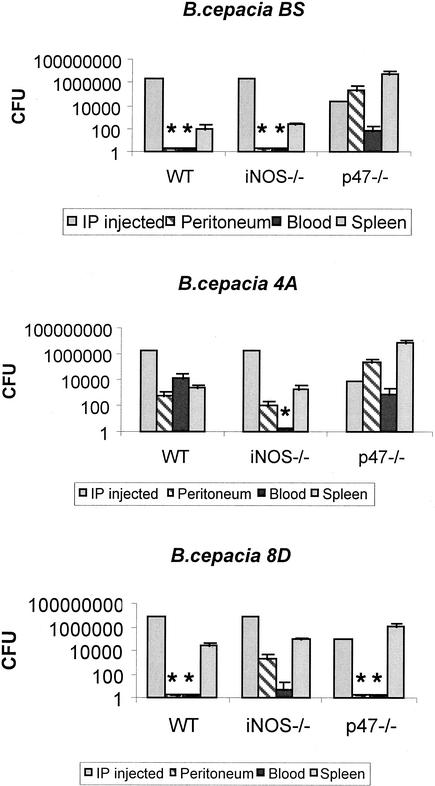
p47phox−/− mice were challenged i.p. with 104 CFU of B. cepacia, whereas wild-type and iNOS−/− mice were administered 106 CFU, and bacterial burdens in their peritoneums, blood, and spleens were determined on day 4 (Fig. 2). Despite the lower inoculum, organism recoveries of B. cepacia strain BS were dramatically higher from p47phox−/− mice than from iNOS−/− and wild-type mice. For B. cepacia strains 4A and 8D, the bacterial burdens in the spleen were consistently greater in p47phox−/− mice than in iNOS−/− and wild-type mice, whereas organism recoveries from the peritoneum and blood varied among the different genotypes. The organism recoveries from the peritoneum were strikingly different for different strains of B. cepacia in p47phox−/− mice. For B. cepacia strains BS and 4A, the mean number of peritoneal CFU was >105/mouse, whereas peritoneal cultures were sterile after B. cepacia strain 8D challenge.
FIG. 2.
Quantitative cultures of B. cepacia strains recovered from the peritoneums, blood, and spleens of p47phox−/−, iNOS−/−, and wild-type (WT) mice 4 days after i.p. challenge. p47phox−/− mice were administered 104 CFU/mouse, and iNOS−/− and wild-type mice were administered 106 CFU/mouse. Two to five mice per genotype per bacterial strain were used, and data shown are representative of two experiments. *, no organisms recovered.
C. violaceum challenge in vivo.
C. violaceum showed extraordinary virulence in p47phox−/− mice (Table 1). Administration of a mean of only 13 organisms to p47phox−/− mice resulted in ∼90% lethality, and all higher inocula killed 100% of p47phox−/− mice within 2 days (Table 1). Data in Table 1 are from one of two experiments, which showed virtually identical results. At necropsy, no obvious foci of infection were apparent, suggesting that the mice died of bacteremia. In contrast, the 50% lethal dose of C. violaceum was between 106 and 107 CFU for iNOS−/− mice and wild-type mice (Table 1). There may be a small survival advantage in wild-type versus iNOS−/− mice that could be confirmed with a larger number of mice.
TABLE 1.
Survival following i.p. inoculation of C. violaceuma
| Genotype | CFU administered per mouse | % Mortality (no. dead/total) | No. of days to deathb |
|---|---|---|---|
| Wild-type | 108 | 100 (8/8) | 1 |
| 107 | 71 (5/7) | 3, 3, 3, 7, 7 | |
| 106 | 0 (0/7) | NA | |
| 105 | 0 (0/4) | NA | |
| 104 | 0 (0/3) | NA | |
| 103 | 0 (0/3) | NA | |
| p47phax−/− | 1.5 × 103 | 100 (8/8) | ≤2 |
| 1.5 × 102 | 100 (8/8) | ≤2 | |
| 0.7 × 102 | 100 (8/8) | ≤2 | |
| 0.13 × 102 | 88 (7/8) | ≤2 | |
| iNOS−/− | 2.7 × 106 | 50 (2/4) | 16, 19 |
| 2 × 105 | 12.5 (1/8) | 26 | |
| 1.7 × 103 | 0 (0/4) | NA | |
| 1.7 × 102 | 0 (0/7) | NA |
Surviving mice were observed for at least 6 weeks following i.p. challenge. These data are pooled from two separate experiments which yielded similar results.
NA, not applicable.
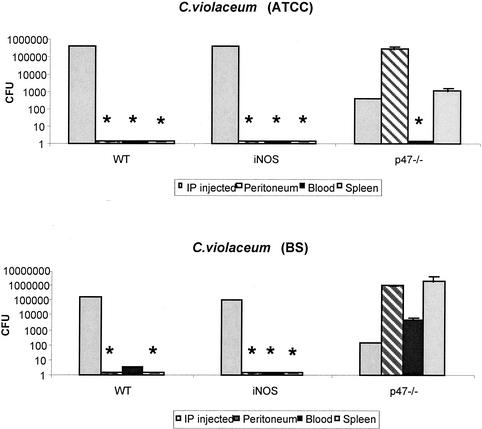
The recovery of C. violaceum was also determined. p47phox−/− mice were challenged i.p. with 102 organisms, and iNOS−/− and wild-type mice were challenged with 105 organisms. Eighteen hours after challenge, p47phox−/− mice appeared ill (hunched posture, reduced response to handling) whereas iNOS−/− and wild-type mice appeared well. Despite the lower inoculum in p47 phox−/− mice, the organism burden was dramatically higher than those in iNOS−/− and wild-type mice, from whom cultures were usually sterile (Fig. 3). In p47phox−/− mice, the organism burdens in the blood and spleens were significantly greater following challenge with C. violaceum strain BS than those resulting from challenge with C. violaceum strain ATCC 6357.
FIG. 3.
Quantitative cultures of C. violaceum strains recovered from the peritoneums, blood, and spleens of p47phox−/−, iNOS−/−, and wild-type (WT) mice 18 h after i.p. challenge with C. violaceum. p47phox−/− mice were administered 102 CFU/mouse, and iNOS−/− and wild-type mice were administered 105 CFU/mouse. Two to 10 mice per genotype per bacterial strain were used. Data shown are representative of three experiments. *, no organism recovered.
In vitro susceptibilities of B. cepacia and C. violaceum to reactive oxygen and nitrogen intermediates.
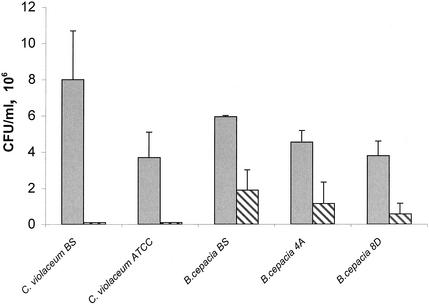
Taken together, the in vivo data show that host defense against experimental infection with B. cepacia and C. violaceum is critically dependent on the NADPH oxidase. Based on survival data (Table 1), there may exist a more subtle role for iNOS in host defense against C. violaceum. We next attempted to correlate these in vivo findings with in vitro susceptibility to H2O2 and nitric oxide (NO). As shown in Table 2, C. violaceum strain BS was significantly more sensitive to H2O2 than C. violaceum strain ATCC 6357. At 0.05 mM H2O2, a significant decrease in C. violaceum strain BS cells occurred, and at 0.5 mM H2O2, the organism recovery was about 1% of the recovery from the control suspension not exposed to H2O2. Among B. cepacia strains, strain BS was the least susceptible to exogenous H2O2 (Table 2). All C. violaceum and B. cepacia strains were sensitive to exogenous reactive nitrogen intermediates generated by NaNO2. C. violaceum strains were sterilized after a 30-min incubation with NaNO2, and the level of recovery of B. cepacia strains was less than 1% of that from untreated control suspensions (Fig. 4).
TABLE 2.
Bactericidal activity of H2O2
| Organism | CFU/ml (106) ± SDa in suspensions treated with:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 mMH2O2 | 0.05 mM H2O2 | 0.1 mM H2O2 | 0.5 mM H2O2 | 1mM H2O2 | 2mM H2O2 | 5 mM H2O2 | |
| C. violaceum BS | 6.6 ± 2.7 | 1.7 ± 0.4* | 0.85 ± 0.6* | 0.05** | 0.002** | ND | 0** |
| C. violaceum ATCC 6357 | 1.4 ± 0.5 | 1.67 ± 0.98 | 1.6 ± 0.17 | 1.1 ± 0.4 | 1.37 ± 0.13 | ND | 0** |
| B. cepacia BS | 7.4 ± 0.7 | 5.6 | 4.9 ± 0.14 | 5.2 ± 1 | 3.0** | 0** | 0** |
| B. cepacia 4A | 2.4 ± 0.4 | ND | ND | ND | 0.01** | 0** | ND |
| B. cepacia 8D | 7.65 ± 0.35 | ND | ND | ND | 0.15** | 0** | ND |
The results shown are the means ± standard deviations from three independent experiments, each performed in duplicate. ND, not done. Significant differences between results with 0 mM H2O2 and various concentrations of H2O2 are indicated by the following symbols: *P < 0.05, and **P < 0.01.
FIG. 4.
Activity of NaNO2-derived reactive nitrogen intermediates against C. violaceum and B. cepacia. Bacteria were treated with sodium acetate buffer alone (shaded bars) or with 2 mg of NaNO2 (hatched bars) at 37°C for 30 min. The results are expressed as the means ± standard deviations of three independent experiments, each performed in duplicate. A P of <0.05 was significant.
Previous studies have shown that in vitro, neutrophils from CGD patients were defective in killing B. cepacia and that killing by normal neutrophils was disabled by the addition of the reactive oxidant scavengers superoxide dismutase and catalase (32). In p47phox−/− mice (16) and mice with X-linked CGD (2), stem cell-directed gene therapy that restored NADPH oxidase function to a small proportion of myeloid cells conferred protection against similar B. cepacia challenges. Smith et al. (30) showed that NO potentiated the killing of B. cepacia by H2O2 in vitro and suggested that the lack of NO in the lungs of cystic fibrosis patients may lead to persistent infection with this pathogen. In our study, iNOS−/− mice did not show an increased sensitivity to B. cepacia compared with that of wild-type mice and, at most, may have had a small increase in susceptibility to challenge with C. violaceum. However, NO is produced from sources other than iNOS in vivo, and our data do not negate the potential importance of NO in host defense against these pathogens.
As shown in Table 2, individual strains of B. cepacia and C. violaceum vary considerably in their sensitivities to H2O2. Among B. cepacia strains, a significant reduction in viable organisms occurred at 1 mM H2O2, a concentration that far exceeds the level of reactive oxidants produced in vesicular fractions of neutrophils (35). In vitro, both B. cepacia and C. violaceum were sensitive to exogenous reactive nitrogen intermediates (Fig. 4). The major limitation of these in vitro studies relates to the difficulty in correlating the exogenously administered reactive oxidant and nitrogen species with those levels that are likely to be encountered in nature within the intracellular milieu, such as in phagolysosomes. In addition, the intact phagocyte generates multiple microbicidal downstream reactive metabolites, such as hydroxyl anion, hypohalous acid, and peroxynitrite anion, which are not encountered when a single reactive intermediate is added in vitro to bacteria.
B. cepacia primes the NADPH oxidase in phagocytes, probably through endotoxin (8, 26). In vitro resistance of B. cepacia strains to exogenous reactive oxygen species correlated with catalase and superoxide dismutase production (14). In addition, a melanin pigment from an epidemic strain of B. cepacia effectively scavenged superoxide anion produced by the NADPH oxidase (37). These antioxidant pathways likely facilitate intracellular survival of B. cepacia species within phagocytes (26). In vitro studies employing macrophage cell lines have shown that Burkholderia pseudomallei is susceptible to reactive oxidant and nitrogen intermediates following priming with gamma interferon (18) and that this pathogen may evade intracellular killing by interfering with iNOS production (34). In a comparative study of a clinical strain and an environmental strain of C. violaceum, the clinical strain produced higher levels of superoxide dismutase and catalase and was more resistant to intracellular killing by neutrophils, suggesting that virulence may be associated with the strain's ability to scavenge reactive oxidants (17).
In vivo, our study showed that host defense against B. cepacia and C. violaceum relies on NADPH oxidase function, suggesting that this pathway is essential in the defense against these pathogens. The NADPH oxidase likely functions in concert with other antimicrobial pathways to control infection in vivo (Table 2). However, pretreatment with gamma interferon provided no benefit for survival in p47phox−/− mice after B. cepacia challenge. Gamma interferon has been shown to reduce the rate of infections in CGD patients (9); it augments oxidative function in normal monocytes (21, 22) and enhances various oxidant-independent antimicrobial pathways, such as tumor necrosis factor alpha production, tryptophan metabolism, granule protein synthesis, major histocompatibility complex II expression, Fc gamma receptor I expression, and Fc gamma receptor-mediated phagocytosis (5, 27). Gamma interferon has not been shown to improve NADPH oxidase function or increase levels of its constituent proteins in patients with CGD (9, 19, 36). In p47phox−/− mice, subcutaneous recombinant gamma interferon did not lead to any detectable oxidant activity in thioglycolate-extracted peritoneal macrophages but did reduce the frequency of spontaneous infections, presumably through non-oxidation-dependent mechanisms (11).
The levels of reactive oxidant production in p47phox−/− mice and of reactive nitrogen intermediate production in iNOS−/− mice are not nil. Xanthine oxidase generates superoxide anion, and constitutively expressed NOS isoforms exist (reviewed in reference 20). Hepatic clearance of intravenous B. cepacia was reduced in p47phox−/− mice compared to that in wild-type mice and was further inhibited in p47phox−/− mice by pretreatment with the specific xanthine oxidase inhibitor allopurinol (29). Clearance and killing of intravenous Escherichia coli was intact in p47phox−/− mice and was unaffected by pretreatment with allopurinol (29). These data suggest that in patients with CGD, xanthine oxidase might contribute to host defense against a subset of reactive oxidant-sensitive pathogens.
NADPH oxidase and iNOS likely function to rapidly augment reactive oxidant and nitrogen intermediates, respectively, during states of emergency (infection). Reactive oxygen and nitrogen intermediates have synergistic bactericidal activities in a variety of in vitro systems (12, 13, 24). Superoxide anion, a relatively weak microbicidal agent, readily reacts with nitric oxide to form peroxynitrite anion, a highly cytotoxic molecule. Possible molecular targets of these species include genomic DNA, electron transport mechanisms, and sulfhydryl groups (24). All aerobic bacteria have evolved mechanisms to scavenge cytotoxic reactive oxidants and to protect against oxidant-targeted damage (6). Within the milieu of a phagocytic cell from a patient with CGD, it is possible that B. cepacia and C. violaceum can effectively scavenge the low level of ambient oxidants but that normal phagocytes rapidly generate high levels of NADPH oxidase-derived reactive oxidants which are fatal to the engulfed pathogens. B. cepacia and C. violaceum are likely to be excellent pathogen model systems to evaluate molecular targets of host-derived reactive oxidant species and strategies evolved by the pathogen to evade oxidant stress.
Editor: B. B. Finlay
REFERENCES
- 1.Chang, Y. C., B. H. Segal, S. M. Holland, G. F. Miller, and K. J. Kwon-Chung. 1998. Virulence of catalase-deficient aspergillus nidulans in p47(phox)−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J. Clin. Investig. 101:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinauer, M. C., M. A. Gifford, N. Pech, L. L. Li, and P. Emshwiller. 2001. Variable correction of host defense following gene transfer and bone marrow transplantation in murine X-linked chronic granulomatous disease. Blood 97:3738-3745. [DOI] [PubMed] [Google Scholar]
- 3.Dorman, S. E., V. J. Gill, J. I. Gallin, and S. M. Holland. 1998. Burkholderia pseudomallei infection in a Puerto Rican patient with chronic granulomatous disease: case report and review of occurrences in the Americas. Clin. Infect. Dis. 26:889-894. [DOI] [PubMed] [Google Scholar]
- 4.Gallin, J. I., E. S. Buescher, B. E. Seligmann, J. Nath, T. Gaither, and P. Katz. 1983. NIH conference. Recent advances in chronic granulomatous disease. Ann. Intern. Med. 99:657-674. [DOI] [PubMed] [Google Scholar]
- 5.Gallin, J. I., J. M. Farber, S. M. Holland, and T. B. Nutman. 1995. Interferon-gamma in the management of infectious diseases. Ann. Intern. Med. 123:216-224. [DOI] [PubMed] [Google Scholar]
- 6.Hassett, D. J., and M. S. Cohen. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 3:2574-2582. [DOI] [PubMed] [Google Scholar]
- 7.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35:614-619. (Erratum, 37:1237, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes, J. E., J. Stewart, G. R. Barclay, and J. R. W. Govan. 1997. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect. Immun. 65:4281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Chronic Granulomatous Disease Cooperative Study Group. 1991. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N. Engl. J. Med. 324:509-516. [DOI] [PubMed] [Google Scholar]
- 10.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, S. H., G. F. Miller, B. H. Segal, M. Mardiney III, J. B. Domachowske, J. I. Gallin, and S. M. Holland. 2001. IFN-gamma is effective in reducing infections in the mouse model of chronic granulomatous disease (CGD). J. Interferon Cytokine Res. 21:567-573. [DOI] [PubMed] [Google Scholar]
- 12.Klebanoff, S. J. 1993. Reactive nitrogen intermediates and antimicrobial activity: role of nitrite. Free Radic. Biol. Med. 14:351-360. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff, S. J., and C. F. Nathan. 1993. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem. Biophys. Res. Commun. 197:192-196. [DOI] [PubMed] [Google Scholar]
- 14.Lefebre, M., and M. Valvano. 2001. In vitro resistance of Burkholderia cepacia complex isolates to reactive oxygen species in relation to catalase and superoxide dismutase production. Microbiology 147:97-109. [DOI] [PubMed] [Google Scholar]
- 15.MacMicking, J. D., C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q. W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641-650. [DOI] [PubMed] [Google Scholar]
- 16.Mardiney, M., III, S. H. Jackson, S. K. Spratt, F. Li, S. M. Holland, and H. L. Malech. 1997. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood 89:2268-2275. [PubMed] [Google Scholar]
- 17.Miller, D. P., W. T. Blevins, D. B. Steele, and M. D. Stowers. 1988. A comparative study of virulent and avirulent strains of Chromobacterium violaceum. Can. J. Microbiol. 34:249-255. [DOI] [PubMed] [Google Scholar]
- 18.Miyagi, K., K. Kawakami, and A. Saito. 1997. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect. Immun. 65:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhlebach, T. J., H. J. Feickert, K. Welte, and R. A. Seger. 1991. Granulocyte-macrophage colony stimulating factor does not improve neutrophil oxidative metabolism in a patient with variant X-linked chronic granulomatous disease. Eur. J. Pediatr. 150:575-578. [DOI] [PubMed] [Google Scholar]
- 20.Nathan, C., and Q. W. Xie. 1994. Nitric oxide synthases: roles, tolls, and controls. Cell 78:915-918. [DOI] [PubMed] [Google Scholar]
- 21.Nathan, C. F., C. R. Horowitz, J. de la Harpe, S. Vadhan-Raj, S. A. Sherwin, H. F. Oettgen, and S. E. Krown. 1985. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc. Natl. Acad. Sci. USA 82:8686-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan, C. F., G. Kaplan, W. R. Levis, A. Nusrat, M. D. Witmer, S. A. Sherwin, C. K. Job, C. R. Horowitz, R. M. Steinman, and Z. A. Cohn. 1986. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N. Engl. J. Med. 315:6-15. [DOI] [PubMed] [Google Scholar]
- 23.O'Neil, K. M., J. H. Herman, J. F. Modlin, E. R. Moxon, and J. A. Winkelstein. 1986. Pseudomonas cepacia: an emerging pathogen in chronic granulomatous disease. J. Pediatr. 108:940-942. [DOI] [PubMed] [Google Scholar]
- 24.Pacelli, R., D. A. Wink, J. A. Cook, M. C. Krishna, W. DeGraff, N. Friedman, M. Tsokos, A. Samuni, and J. B. Mitchell. 1995. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 182:1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross, J. P., S. M. Holland, V. J. Gill, E. S. DeCarlo, and J. I. Gallin. 1995. Severe Burkholderia (Pseudomonas) gladioli infection in chronic granulomatous disease: report of two successfully treated cases. Clin. Infect. Dis. 21:1291-1293. [DOI] [PubMed] [Google Scholar]
- 26.Saini, L. S., S. B. Galsworthy, M. A. John, and M. A. Valvano. 1999. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology 145:3465-3475. [DOI] [PubMed] [Google Scholar]
- 27.Schiff, D. E., J. Rae, T. R. Martin, B. H. Davis, and J. T. Curnutte. 1997. Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood 90:3187-3194. [PubMed] [Google Scholar]
- 28.Segal, B. H., T. L. Leto, J. I. Gallin, H. L. Malech, and S. M. Holland. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79:170-200. [DOI] [PubMed] [Google Scholar]
- 29.Segal, B. H., N. Sakamoto, M. Patel, K. Maemura, A. S. Klein, S. M. Holland, and G. B. Bulkley. 2000. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47phox−/− mouse model of chronic granulomatous disease. Infect. Immun. 68:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, A. W., J. Green, C. E. Eden, and M. L. Watson. 1999. Nitric oxide-induced potentiation of the killing of Burkholderia cepacia by reactive oxygen species: implications for cystic fibrosis. J. Med. Microbiol. 48:419-423. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen, R. U., M. R. Jacobs, and S. B. Shurin. 1985. Chromobacterium violaceum adenitis acquired in the northern United States as a complication of chronic granulomatous disease. Pediatr. Infect. Dis. J. 4:701-702. [DOI] [PubMed] [Google Scholar]
- 32.Speert, D. P., M. Bond, R. C. Woodman, and J. T. Curnutte. 1994. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170:1524-1531. [DOI] [PubMed] [Google Scholar]
- 33.Tomioka, H., K. Sato, C. Sano, T. Akaki, T. Shimizu, H. Kajitani, and H. Saito. 1997. Effector molecules of the host defence mechanism against Mycobacterium avium complex: the evidence showing that reactive oxygen intermediates, reactive nitrogen intermediates, and free fatty acids each alone are not decisive in expression of macrophage antimicrobial activity against the parasites. Clin. Exp. Immunol. 109:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utaisincharoen, P., N. Tangthawornchiakul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophages. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 35.Wakeyama, H., K. Takeshige, R. Takayanagi, and S. Minakami. 1982. Superoxide-forming NADPH oxidase preparation of pig polymorphonuclear leucocyte. Biochem. J. 205:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodman, R. C., R. W. Erickson, J. Rae, H. S. Jaffe, and J. T. Curnutte. 1992. Prolonged recombinant interferon-gamma therapy in chronic granulomatous disease: evidence against enhanced neutrophil oxidase activity. Blood 79:1558-1562. [PubMed] [Google Scholar]
- 37.Zughaier, S. M., H. C. Ryley, and S. K. Jackson. 1999. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect. Immun. 67:908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]