Abstract
Group A streptococci (GAS) express a superantigen, SpeB, having cysteine protease activity. SpeB exhibits several properties that might contribute to virulence, the most recently discovered being the ability to cleave immunoglobulin G (IgG) in a manner similar to that of papain. In the present study, we confirmed this latter finding and found that the irreversible inhibition of SpeB protease activity completely abolishes IgG cleavage. SpeB cleavage of IgG was not species restricted since SpeB cleaved both human, rabbit, and mouse IgG. In order to investigate the nature of the SpeB cleavage of IgG, antibodies were immobilized prior to exposure to SpeB, either by unspecific binding of the Fc to GAS surface proteins or by antigen-specific binding. Analysis of the IgG molecules by SDS-PAGE showed that SpeB could cleave antigen-bound antibodies, while the IgG bound to IgG-binding proteins was protected from cleavage. In a phagocytosis assay using whole blood, the M49 GAS strain NZ131 showed a significantly higher survival than its isogenic speB mutant. Furthermore, the addition of extracellular supernatant derived from an overnight culture of native NZ131 increased the survival of its isogenic speB derivative. This indicates that SpeB's ability to cleave off the Fc part of antigen-bound IgG contributes to GAS escape from opsonophagocytosis while not interfering with the formation of a host-like coat by unspecific IgG binding.
The group A streptococcus (GAS) is one of the most common human pathogens to cause a broad spectrum of diseases, ranging from mild infections to severe invasive diseases like necrotizing fasciitis and streptococcal toxic shock syndrome (42). Despite the hostile environment of the blood, GAS has the ability not only to survive but to grow and multiply in nonopsonizing blood. The underlying mechanisms suggested are the ability to avoid recognition by the immune system and interference with complement activation. M proteins are one of the virulence factors thought to be involved, since mutants lacking these surface proteins have a decreased capacity to escape phagocytosis. However, the underlying mechanisms are not fully understood and M proteins are reported to exhibit several functions that might be of importance. M proteins bind C4BP and factor H, which inhibits activation of the complement system through the alternative pathway, while binding of the Fc part of immunoglobulin G (IgG) by M proteins inhibits activation through the classical pathway (6, 23, 25, 29). The M and M-like surface proteins bind several plasma proteins, including fibronectin, albumin, plasminogen, and the Fc part of IgG and IgA, thereby covering the bacteria with host proteins (1, 7, 18, 20, 38). Production of a hyaluronic capsule as a coat is another way by which GAS avoid the action of the immune system (3, 36, 41). Other proteins, like the streptococcal inhibitor of complement and the C5a peptidase present at the GAS surface, further interfere with the activation of the complement system and recruitment of immune cells (2, 13). Nevertheless, opsonizing antibodies specific for M and M-like proteins are believed to be protective against GAS infections and confer long-term protection (30).
During infection, GAS secretes a number of soluble proteins, including those highly potent immune modulators the streptococcal pyrogenic exotoxins (Spe's), which are also recognized as streptococcal superantigens (33). The streptococcal superantigen SpeB is a cysteine protease with a wide variety of functions (19, 27, 28, 34). SpeB is produced as an inactive 40-kDa proenzyme that undergoes autocatalytic cleavage to the active 28-kDa form. The crystal structure of the 40-kDa zymogen reveals that SpeB belongs to the papain family (26). The proteolytic activity can be irreversibly inhibited by the addition of a tripeptide that covalently binds the active site, but inhibition of SpeB's proteolytic activity has no effect on its T-cell mitogenicity (8, 17).
Secretion of SpeB induces a number of events, creating an altered bacterial surface. It has been shown that inactivation of speB affects the expression of the hyaluronic capsule (4, 41). SpeB releases surface-associated C5a peptidase, which blocks leukocyte migration towards the site of infection induced by the chemotactic peptide C5a and degrades both the streptococcal inhibitor of complement and serum opacity factor (5, 21, 35). Furthermore, SpeB cleaves protein H and parts of M proteins from the surface of GAS, thus inhibiting the binding of fibronectin and altering the IgG-binding specificity (6, 10, 37). IgG bound to membrane-associated protein H does not activate the complement system, while soluble IgG-protein H complexes do, suggesting a mechanism to avoid activation of the complement system at the surface of the bacteria (6). In addition, SpeB was recently shown to cleave the heavy chain of human IgG (14).
Phagocytosis can be triggered through two different pathways, either via complement receptor 3 (CR3), also referred to as CD11b/CD18, or via the Fcγ receptor (FcγR) (9). Activation of phagocytosis via CR3 is nonspecific and a very important first line of defense, while activation via FcγR requires humoral immunity (9, 15). CR3 also participates in IgG-mediated phagocytosis since binding of the complement factor C1q to the Fc region of IgG catalyzes the assembly of the C3 convertase C4b2a. Those GAS antiphagocytic mechanisms that have been described predominantly involve complement activation. Recently, a new streptococcal protease, IdeS, was described, which, as with SpeB, cleaves IgG in the hinge region and contributes to GAS escape from phagocytosis (40). In this study, we show that SpeB does not cleave the IgG associated with IgG-binding proteins while IgG bound by its antigen-binding site is cleaved. Furthermore, loss of SpeB activity significantly decreased GAS ability to survive in immune blood. This indicates that SpeB also contributes to escape from IgG-mediated phagocytosis.
MATERIALS AND METHODS
Bacterial strains and determination of phenotypic characteristics.
To study the SpeB cleavage of IgG bound to IgG-binding proteins on the GAS surface, the M1 streptococcal strain designated AP1 (40/58 strain; World Health Organization Streptococcal Reference Laboratory, Prague, Czech Republic) was used. In the phagocytosis assay, the M49 strain NZ131 and its speB isogenic mutant (kindly donated by J. J. Ferretti [11]) were used. Protease activity in ethanol-precipitated extracellular supernatant from overnight culture was determined in an azocasein assay as described previously (17). Expression of antiphagocytic protein M49 was determined in a semiquantitative immunoblot. Surface proteins from 100 ml of exponentially growing NZ131 and its speB-negative derivative (optical density at 520 nm [OD520] = 0.15) were released by the addition of HCl as previously described (31), and total protein concentration was measured at A280. Serial dilutions of surface proteins adjusted for A280 were blotted on nitrocellulose filters, and M49 proteins were detected with rabbit antisera towards a peptide covering the N-terminal amino acids 1 through 29 of the M49 protein, kindly provided by P. Cleary. The bound rabbit antisera were detected by AP-conjugated goat anti-rabbit IgG (Cappel, Stockholm, Sweden). Production of the hyaluronic capsule was detected with Stains All (Sigma-Aldrich, Stockholm, Sweden) as described elsewhere (39). Bacteria were grown to early exponential phase (OD520 = 0.15) in 100 ml of Todd-Hewitt broth and washed twice in water. Cells were resuspended in 1.5 ml of water and 1.5 ml of chloroform, mixed vigorously, and centrifuged. The hyaluronic acid content of 1 ml of aqueous phase was measured at A660 after the addition of 0.4 mg of Stains All in 2 ml of 50% formamide-1.2 μl of glacial acetic acid.
Purification of SpeB and proteolytic bacterial supernatant.
SpeB in the supernatant from an overnight culture of the streptococcal strain T1BRB was purified as described previously (17). The purity of the protein was >99% as determined by silver staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Protease activity was determined by degradation of AZO-casein, while T-cell mitogenicity was determined using a lymphocyte proliferation assay as previously described (17). Bacterial culture supernatants were recovered from overnight cultures of NZ131 and of its speB-deficient derivative by the precipitation of cell-free supernatant with EtOH (75%). Protein precipitates were kept at −20°C for 24 h, diluted in distilled water to 1/10 of their initial volume, sterile filtered, and stored at −20°C. Proteolytic activities in supernatants were determined in AZO-casein assays as previously described except that 40 mM l-Cys was used as the reducing factor instead of dithiothreitol (DTT) (17).
Cleavage of IgG in solution.
Purified SpeB (10 μg/ml) was activated in 0.1 M NaAc buffer (pH 5.5)-1 mM DTT at 37°C for 30 min. A portion of the activated protease was inactivated by the addition of 30 μg of tripeptide Z-LVG-CHN2/ml (Bachem AG, Bubendorf, Switzerland) as described elsewhere (17). Activated SpeB or inactivated SpeB (5 μg) was added to 100 μg of human polyclonal IgG (KABI-Pharmacia, Stockholm, Sweden) or 100 μg of mouse monoclonal IgG1, kindly provided by T. Stigbrandt, in 200 μl of 0.1 M NaAc buffer (pH 5.5)-1 mM DTT. Reaction mixtures were incubated at 37°C for 48 h to accomplish complete cleavage, dialyzed against phosphate-buffered saline (PBS) overnight, and separated on SDS-PAGE gels under reducing conditions. Peptide fragments were visualized with Coomassie blue staining and blotted on polyvinyldiene fluoride filters. The 32-kDa fragment of Fc chain was applied to N-terminal sequencing at the Department of Biochemistry, Umeå University, Umeå, Sweden. The cleavage products from mouse monoclonal IgG1 were separated on SDS-PAGE gels under nonreducing conditions. Peptides were detected in a Western blot by using horseradish peroxidase-conjugated anti-mouse Fab (Sigma-Aldrich).
IgG binding.
Binding of unspecific IgG to the bacteria was determined by the absorption of protein A-purified human IgG (KABI-Pharmacia) from solution. Heat-killed AP1 (5 × 108 CFU) or NZ131 (3 × 109 CFU) bacteria were incubated with known amounts of IgG in 200 μl of PBS for 1 h. Bacteria were pelleted, and unbound IgGs in the supernatant were titrated in 96-well enzyme-linked immunosorbent assay plates (Nunc, Roskilde, Denmark). IgG that remained in the supernatant was detected with secondary AP-conjugated rabbit anti-human γ-chain-specific antibodies (DAKO, Glostrup, Denmark) and measured at A405. Samples with known amounts of IgG were titrated as described above and used as standard. AP1 absorbed approximately 70 μg, while NZ131 absorbed less than 5 μg of human IgG. Keyhole limpet hemagglutinin (KLH; Scandinavian Peptide Synthesis, Köping, Sweden) gel was prepared by coupling KLH to Affi-10 Gel (N-hydroxysuccinimide-activated cross-linked agarose gel beads; Bio-Rad, Stockholm, Sweden). The ability of 100 μl of KLH gel to absorb antigen-specific IgG was determined by incubation with 100 μg of polyclonal rabbit IgG specific for KLH (16) in 200 μl of PBS at room temperature. IgG, which remained in supernatant from KLH gel, was measured at A280. KLH gel absorbed approximately 38 μg of rabbit anti-KLH antibodies as determined by the A280 in supernatants.
Cleavage of bound IgG.
Protein A-purified human IgG (100 μg) (KABI-Pharmacia) was absorbed to 5 × 108 CFU of heat-killed AP1 bacteria in 200 μl of PBS for 1 h at room temperature. Similarly, 100 μg of polyclonal rabbit IgG specific for KLH (16) was absorbed to 100 μl of KLH gel. Bacteria and gels were washed twice with 500 μl of PBS and resuspended in 200 μl of 0.1 M NaAc (pH 5.5) before the addition of 4.5 μg of SpeB, which had been preactivated in 0.1 M NaAc (pH 5.5)-2 mM DTT at 37°C for 1 h (final concentration of DTT was 0.1 mM). The reaction mixtures were incubated for 36 h at 37°C. As a positive control, 100 μg of polyclonal rabbit anti-KLH antibodies, as well as 100 μg of human IgG, was incubated with 4.5 μg of preactivated SpeB under the same conditions. The bacteria and gels were pelleted, the supernatants were collected, and the pellets were washed twice in 500 μl of PBS. IgG still bound to KLH gel was eluted by the addition of 50 μl of 0.2 M glycine-HCl (pH 2.5), and supernatant was neutralized with 1.5 M Tris-HCl (pH 8.8). All fractions including the bacterial pellet were boiled for 10 min in sample buffer containing SDS and β-mercaptoethanol before application on reducing SDS-PAGE gels and applied to a Western blot. Heavy chains of IgG were visualized by the addition of AP-conjugated rabbit anti-human γ-chain-specific antibodies (DAKO) or AP-conjugated goat anti-rabbit IgG antibodies (Cappel).
Selection of donor blood.
Five different donors were tested for opsonizing capacity against strain NZ131. Briefly, exponentially growing bacteria (OD520 = 0.15) were collected, diluted 1/10,000 in NaCl (0.9%), and inoculated as a 100-μl bacterial suspension in 1 ml of whole blood. Mixtures were rotated end over end at 37°C for 3 h before plating on blood agar plates. The multiplication factors were calculated as the mean CFU after 3 h divided by the mean CFU at start. All dilutions were plated in triplicate. The plasma from one donor, in whose blood the GAS strain NZ131 did not multiply, was further tested for opsonizing capacity and strain-specific antibodies. Presence of strain-specific antibodies in donor plasma was determined in an immunoblot of HCl-protein extracts as described above. To measure the opsonizing potential in donor plasma, 100 μl of plasma was added to the blood from one donor with a high multiplication factor in a phagocytosis assay.
Phagocytosis assay.
GAS M49 strain NZ131 and its isogenic speB mutant derivative were used in a phagocytosis assay. Overnight cultures of GAS strains were diluted to an OD520 of 0.06 in Todd-Hewitt broth and incubated at 37°C. A bacterial suspension at an OD520 of 0.15 was diluted to 1/100 and 1/1,000 in NaCl (0.9%). Whole blood from donor B, selected as having a high opsonizing capacity against NZ131, was collected in heparinized Vacutainer tubes, and 1 ml of it was immediately added to 100 μl of the bacterial suspensions. Prior to inoculation, 100 μl of preactivated ethanol precipitate of the proteolytic bacterial supernatant was added to test tubes containing SpeB-deficient-strain suspensions. To avoid discrepancies due to the action of l-Cys in the proteolytic supernatant, 100 μl of buffer was added to the other test tubes. The reaction mixtures were rotated end over end at 37°C and diluted in NaCl (0.9%) before plating out on blood agar plates. All dilutions were plated in triplicate; plates containing 50 to 200 CFU were counted. Percent survival was calculated as the mean CFU after 60 min divided by the mean CFU at start × 100. A shorter incubation time (60 min) was used in these experiments to avoid IgG-independent killing.
RESULTS
IgG is a substrate for SpeB cysteine protease activity.
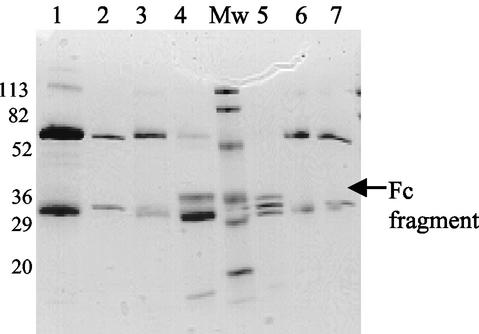
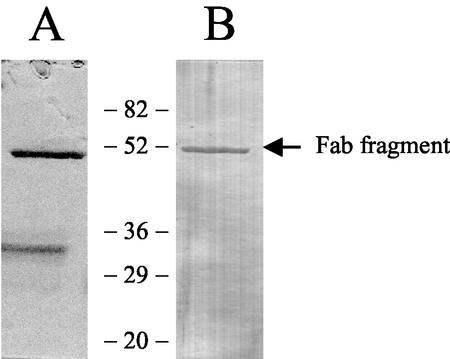
SpeB was able to cleave the heavy chain of both human polyclonal IgG and mouse monoclonal IgG1 under reducing conditions, since the 55-kDa heavy-chain fragments vanished when mixtures were analyzed on reducing-SDS-PAGE gels (Fig. 1, lanes 4 and 5). Furthermore, addition of the cysteine protease inhibitor Z-LVG-CHN2 totally inhibited cleavage (Fig. 1, lane 3). The experimental conditions used partially reduced IgG during the incubation time, as detected in the buffer control on nonreducing-SDS-PAGE gels (data not shown). However, two distinct fragments of approximately 32 and 50 kDa could be visualized by the Coomassie blue staining of a nonreducing-SDS-PAGE gel (Fig. 2). Western blot analysis showed that the goat anti-mouse Fab secondary antibody recognized the 50-kDa fragment, indicating that Fab fragments were produced (Fig. 2). SpeB cleaved the human, mouse, and rabbit IgG equally well, which indicated that the cleavage was not species restricted. The N-terminal sequence of the remaining 32-kDa Fc part of mouse IgG1 was TVPEVS, revealing that SpeB cleaves mouse IgG1 in the lower hinge region between C109 and T110 of the Fc part. This renders a Fab fragment, with the heavy chain 1 amino acid shorter than that after cleavage with papain (43).
FIG. 1.
Cleavage of human and mouse IgG by SpeB. One hundred micrograms of human polyclonal IgG or mouse monoclonal IgG1 was incubated with preactivated SpeB. As a control, human polyclonal IgG was incubated with protease-inactivated SpeB. The incubation mixtures were separated on SDS-12% PAGE gels under reducing conditions. The gel was stained with Coomassie brilliant blue to visualize the peptides. Lanes: 1, human polyclonal IgG; 2, human polyclonal IgG incubated in buffer alone; 3, human polyclonal IgG incubated with protease-inactivated SpeB; 4, human polyclonal IgG incubated with activated SpeB; 5, mouse monoclonal IgG1 with activated SpeB; 6, mouse monoclonal IgG1 in buffer alone; 7, mouse monoclonal IgG1. Molecular weight markers (Mw) are indicated.
FIG. 2.
Mouse Fab fragments produced by SpeB cleavage. (A) An IgG1 mouse monoclonal antibody was incubated with preactivated SpeB before separation on a nonreducing-SDS-PAGE gel. (B) The Fab fragment was visualized by the Western blot technique using horseradish peroxidase conjugate specific for mouse Fab. Molecular weights are indicated between the lanes.
SpeB cleaves antigen-bound IgG.
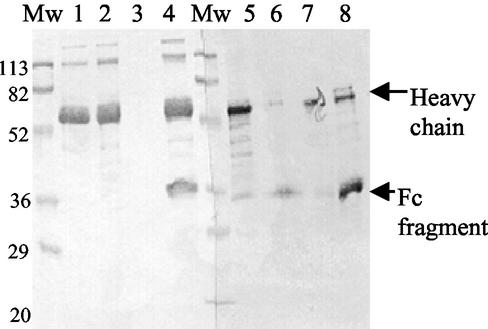
Binding of IgG to streptococcal IgG-binding proteins has been suggested to protect the bacteria from the deposition of complement at the bacterial surface by creating a host-like coat, while specific IgGs bound by the antigen-binding site are detrimental. In our laboratory, we observed that IgG bound to protein A-Sepharose was protected from cleavage; thus, we wanted to investigate further the ability of SpeB to cleave IgG that was bound in different ways, either via the Fc part to M and M-like proteins or via the antigen-binding site. KLH-specific polyclonal rabbit antibodies immobilized on KLH gel should adhere in an antigen-specific manner with the Fc part pointing outwards to the surrounding media. However, IgG absorbed by AP1 should predominantly be bound via interaction with Fc to protein H, which would result in exposure of the Fab parts. The heavy-chain fragment detected in the supernatant from rabbit polyclonal antibodies immobilized on KLH gel was approximately 35 kDa, compared to the intact heavy chain of 55 kDa detected in the untreated rabbit antibodies, and in the elute from KLH gel (Fig. 3, lanes 5, 6, and 7). This was comparable with the size of the heavy-chain fragment detected when the same rabbit polyclonal antibody was cleaved in solution (Fig. 3, lane 8). On the other hand, heavy chains of human IgG, in complex with the protein H present in supernatant from AP1 bacteria, were intact (Fig. 3, lanes 1 and 2). No IgG could be released from the AP1 bacterial pellet by boiling in sample buffer, indicating that SpeB had released the IgG-protein H complexes from the bacterial surface (Fig. 3, lane 3). To prevent the reduction of IgG into its heavy and light chains and protect other bacterial proteins and gel support the experimental conditions were modified and only 0.1 mM DTT was added to the reaction mixture. Under these conditions, SpeB cleavage of IgG in solution was not complete (Fig. 3, lanes 4 and 8).
FIG. 3.
SpeB cleavage of IgG in different complexes. The Western blot of a reducing-SDS-PAGE gel from antigen- or Fc-bound IgG exposed to SpeB is shown. Human polyclonal IgG was immobilized on GAS strain AP1, and rabbit polyclonal anti-KLH IgG was bound to KLH gel. Lanes: 1, human polyclonal IgG; 2, supernatant from SpeB-treated AP1-IgG; 3, AP1 pellet; 4, SpeB-treated human polyclonal IgG; 5, rabbit polyclonal anti-KLH IgG; 6, supernatant from SpeB-treated rabbit polyclonal anti-KLH IgG immobilized on KLH gel; 7, eluate from SpeB-treated rabbit polyclonal anti-KLH IgG immobilized on KLH gel; 8, SpeB-treated rabbit polyclonal anti-KLH IgG. Molecular weights (Mw) are indicated.
Contribution of SpeB to survival in blood.
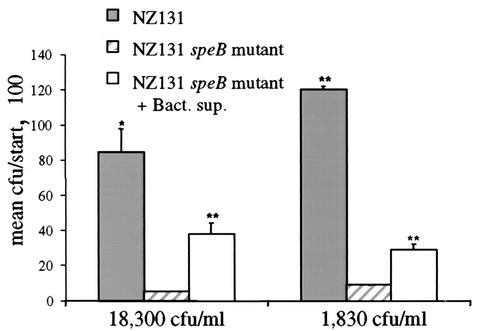
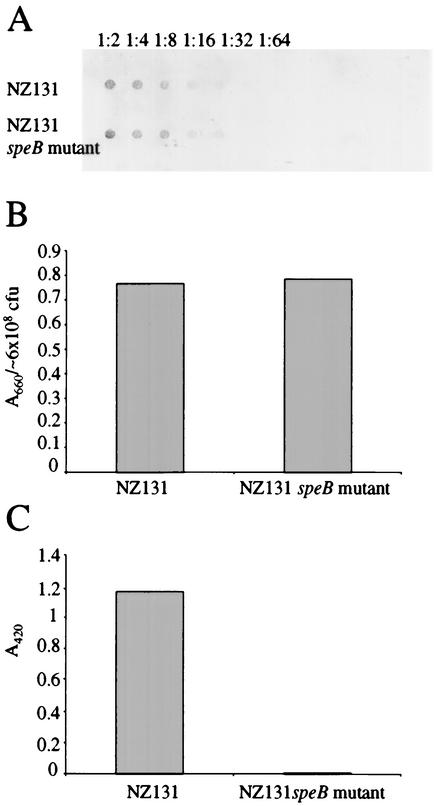
Blood from five donors was initially tested for opsonizing potential against GAS strain NZ131 (Table 1). One of the donors, donor B, in whose blood strain NZ131 persisted but did not multiply was selected for further opsonization experiments. Plasma from donor B was shown to contain strain-specific antibodies when applied to an immunoblot against whole-surface-protein-HCl extracts from NZ131 (data not shown). Furthermore, addition of plasma from donor B reduced the ability of strain NZ131 to multiply in the blood from donor A by 52% (Table 2). These results indicate that the inability of strain NZ131 to grow in the blood from donor B was due to the presence of strain-specific opsonizing antibodies. Thus, we used blood from donor B in the phagocytosis experiments. Figure 4 shows that strain NZ131 had a significantly higher survival at 60 min than its speB-deficient derivative: 85% compared to 5% when CFU at the start was 18,000 and 120% compared to 9% when 1,800 CFU was inoculated. Furthermore, the addition of preactivated-ethanol-precipitated proteolytic bacterial supernatant from NZ131 significantly increased escape from phagocytosis of the protease-deficient NZ131 derivative with a survival of 29 or 38%, respectively (Fig. 4). This was not due to the inability of the speB mutant to multiply in blood, since the mutant showed high multiplication in blood from a nonopsonizing donor (Table 2). The mutation did not interfere with other suggested antiphagocytic factors of GAS, since no significant difference in the expression of M49 protein or the hyaluronic capsule could be detected when the two strains were compared (Fig. 5A and B). Furthermore, we could confirm the findings of Chaussee et al. that the speB-deficient derivative of NZ131 lacks proteolytic activity in extracellular supernatant, which indicates that no other proteases are produced by the strain (Fig. 5C) (11). These results support the idea that SpeB's ability to cleave IgG contributes to the survival of NZ131 in opsonizing blood.
TABLE 1.
Multiplication factors of GAS strain NZ131 in the blood of various donors
| Donor | Multiplication factora |
|---|---|
| A | 91 |
| B | 0.8 |
| C | 0.04 |
| D | 0.7 |
| E | 0.2 |
| F | 1.5 |
| G | 2.7 |
Incubation time was 3 h.
TABLE 2.
Opsonic potential in donor plasma
| GAS strain | Multiplication factora |
|---|---|
| NZ131 | 91 |
| NZ131 + donor B plasma | 44 |
| NZ131 speB mutant | 258 |
Incubation time was 3 h.
FIG. 4.
SpeB contribution to the persistence of GAS in blood containing strain-specific antibodies. Exponentially growing NZ131 and its isogenic speB mutant were added to blood from donor B at the dilutions indicated, with or without addition of preactivated bacterial supernatant from NZ131. The data are shown, for a representative experiment, with percent survival calculated as mean CFU after 60 min divided by number of inoculums at start × 100. Significance was calculated by Student's paired t test. *, P < 0.05; **, P < 0.01.
FIG. 5.
Phenotypic characterization of the speB mutant. M49 protein and hyaluronic acid were extracted from exponentially growing bacteria, and extracellular proteolytic activity was measured in overnight cultures. (A) An immunoblot of serially diluted total-protein-HCl extracted from exponentially growing bacteria is shown. The M49 protein was detected by the addition of rabbit antisera against amino acids 1 through 29 of the M49 protein. Amount of protein was adjusted according to A280. The dilutions are indicated in the figure. (B) Hyaluronic acid was recovered from the aqueous phase of chloroform extracts of bacterial pellets and stained with Stains All. The data are presented as A660/(∼6 × 108 CFU). (C) The proteolytic activity in ethanol-precipitated overnight cultures was determined by AZO-casein cleavage and measured at A420.
DISCUSSION
The streptococcal superantigen SpeB is a cysteine protease and as such performs a number of proteolytic activities important for adherence, invasion, and spread of bacteria, including the ability to cleave IgG (5, 14, 27, 28, 32, 34). We confirm the findings that SpeB cleavage of IgG is performed at a specific site in the lower hinge region of the IgG heavy chain 1 amino acid downstream from the papain cleavage site. Analysis of the SpeB-treated mouse monoclonal IgG1 antibody on reducing SDS-PAGE gel resulted in three sharp bands, indicating that this was not due to unspecific degradation, even when the incubation was prolonged (Fig. 1). Furthermore, inhibition of the proteolytic activity by a tripeptide completely abolished IgG cleavage. SpeB revealed no species preferences since it cleaved human, rabbit, and mouse antibody equally well. In a Western blot analysis of a nonreducing SDS-PAGE gel, the mouse light chain was detected in the 50-kDa fragment, indicating that SpeB cleavage of IgG renders Fab fragments and not F(ab′)2 (Fig. 2).
In an effort to purify the Fab fragments created by the SpeB cleavage of IgG bound to protein A-Sepharose, we discovered that the IgG bound by its Fc part to protein A was not cleaved (data not shown). This was interesting since binding of IgG by M and M-like proteins is considered to be one mechanism by which GAS escape phagocytosis. It was shown previously that IgG bound to GAS surface protein H is not available for the activation of complement (6). However, the IgG-protein H complex released from the GAS surface activates complement, which might contribute to escape from phagocytosis by the consumption of complement far from the bacteria. SpeB degradation of IgG bound in this way would be a waste of energy, while SpeB cleavage of strain-specific IgG bound to GAS surface proteins by their antigen-binding site would be of great benefit to the bacteria. By immobilizing antibodies in different manners prior to exposure to SpeB, we show that only antibodies bound by their antigen recognition site are available for cleavage by SpeB; thus, SpeB cleavage of IgG does not interfere with other suggested antiphagocytic mechanisms (Fig. 3). Under conditions used in this experiment, the cleavage of IgG in solution was not complete (Fig. 3, lanes 4 and 8). This indicates that SpeB needs a reducing environment to be able to completely cleave IgG. However, at the site of deep microbial infections, sulfide levels of up to 1 mM have been detected, at which SpeB should be active (12, 22).
GAS are one of the most common human pathogens causing a wide variety of diseases. Large efforts have been made to determine the mechanisms by which this organism can survive in our blood. Many hypotheses have been suggested, predominantly involving the M proteins and the hyaluronic capsule, but the total picture has not yet been resolved. This study presents data indicating that SpeB, by its proteolytic activity, contributes to the enhanced escape from the specific immune response by the cleavage of strain-specific, antigen-bound IgG. Addition of a bacterial supernatant containing SpeB increased the survival of the speB mutant but not to the extent of that in the native strain (Fig. 4). A possible explanation for this might be that the concentration of SpeB was equal throughout the suspension when added in vitro while higher in the near proximity of the bacterial surface when produced in vivo.
The SpeB protein has been suggested to influence expression of the antiphagocytic hyaluronic capsule. However, in the literature, there are discrepancies concerning how SpeB affects hyaluronic acid production. Large capsular forms of an M64 strain were found to lack production of SpeB, and M3 speB mutant strains were reported to have reduced production of the hyaluronic capsule (4, 36, 41). Furthermore, the amount of capsule produced by the speB mutants of an M49 strain and an M3 strain was reported to be unaffected (41). The latter is in agreement with our findings that production of the hyaluronic capsule and colony morphology are unaffected by the inactivation of speB in NZ131 (Fig. 5A). Other proteins connected to the antiphagocytic properties expressed by GAS are the M and M-like proteins. Our results show no significant difference in the production of the M49 protein between NZ131 and its speB-deficient isogenic derivative, which indicates that lack of SpeB does not interfere with M49 protein expression (Fig. 5B).
M and M-like proteins, including the M49 protein, are also targets for SpeB proteolysis. Exposure to SpeB is thereby believed to alter the antigenic epitopes at the bacterial surface (10, 36). In the present study, we show that the SpeB-producing strain NZ131 was not able to multiply in opsonizing blood (Table 1). Furthermore, plasma from this donor reduced the multiplication rate of the bacteria in nonopsonizing blood by 52%, indicating the presence of opsonizing antibodies towards NZ131 in the plasma (Table 2). These results indicate that, in our study, the possible modification of the M49 protein by SpeB did not interfere with strain-specific recognition by the host immune system. M proteins differ in their ability to bind IgG, and the proteolytic actions of SpeB have been shown to alter the IgG-binding specificity of an M1 strain (37). However, NZ131 does not bind large amounts of IgG; thus, persistence of NZ131 in immune blood at levels higher than that of its speB-negative derivative is probably not due to the SpeB modification of the IgG-binding capacity of the M49 protein. M proteins also differ in their antiphagocytic capacity, and mutation of the mrp, emm, and enn genes in M49 strain CS101 only partially decreased GAS resistance to phagocytosis in an in vitro assay (24). However, the blood used in that study did not contain strain-specific antibodies, since the multiplication factor was high during the 3-h incubation. Thus, the effect of innate phagocytosis mediated by CR3 was studied. This is in agreement with our findings that the SpeB protein has no influence on bacterial growth in nonopsonizing blood (Table 2). However, when applied to blood with high opsonizing potential, the ability to produce SpeB was beneficial (Fig. 4).
The battle between the host immune defense and the GAS involves many participants, and loss of one agent might enhance the use of another. Many GAS determinants and mechanisms have been suggested to be important for the resistance to phagocytosis triggered by the CR3 receptor, which recognizes a variety of ligands (2, 6, 15, 25). In this study, we have shown that SpeB cleaves antigen-bound IgG and that a protease-deficient derivative of NZ131 has a significantly lower ability than the native strain to persist in immune blood. These results suggest a mechanism by which GAS can avoid FcγR-mediated phagocytosis, in a manner different from those previously described (3, 5). Another proteolytic streptococcal enzyme, IdeS, was recently shown to contribute to the antiphagocytic activity of GAS by a similar mechanism (40). However, the lack of proteolytic activity in the extracellular supernatant of the speB-negative derivative of NZ131 indicates that IdeS is not expressed by this strain. This antiphagocytic property of SpeB might be of importance, especially during the early stages of recurrent infections, when humoral immunity triggered during the primary infection operates.
Acknowledgments
This work was supported by the Swedish Research Council (grant 8675), the Magnus Bergwall Foundation, Insamlingsstiftelsen at Umeå University, and Västerbottens Läns Landsting.
We thank Carin Olofsson for technical support.
Editor: E. I. Tuomanen
REFERENCES
- 1.Akesson, P., J. Cooney, F. Kishimoto, and L. Bjorck. 1990. Protein H—a novel IgG binding bacterial protein. Mol. Immunol. 27:523-531. [DOI] [PubMed] [Google Scholar]
- 2.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh, C. D., T. J. Moser, M. H. Shearer, G. L. White, R. C. Kennedy, and M. R. Wessels. 2000. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cell Microbiol. 2:283-292. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh, C. D., and M. R. Wessels. 2001. Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group A streptococcal infection. Infect. Immun. 69:6683-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge, A., and L. Bjorck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. [DOI] [PubMed] [Google Scholar]
- 6.Berge, A., B. M. Kihlberg, A. G. Sjoholm, and L. Bjorck. 1997. Streptococcal protein H forms soluble complement-activating complexes with IgG, but inhibits complement activation by IgG-coated targets. J. Biol. Chem. 272:20774-20781. [DOI] [PubMed] [Google Scholar]
- 7.Berge, A., and U. Sjobring. 1993. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 268:25417-25424. [PubMed] [Google Scholar]
- 8.Bjorck, L., P. Akesson, M. Bohus, J. Trojnar, M. Abrahamson, I. Olafsson, and A. Grubb. 1989. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature 337:385-386. [DOI] [PubMed] [Google Scholar]
- 9.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 10.Chaussee, M. S., R. L. Cole, and J. P. M. van Putten. 2000. Streptococcal erythrogenic toxin B abrogates fibronectin-dependent internalization of Streptococcus pyogenes by cultured mammalian cells. Infect. Immun. 68:3226-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaussee, M. S., D. Gerlach, C.-E. Yu, and J. J. Ferretti. 1993. Inactivation of the streptococcal erythrogenic toxin B gene (speB) in Streptococcus pyogenes. Infect. Immun. 61:3719-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claesson, R., M. Granlund-Edstedt, S. Persson, and J. Carlsson. 1989. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect. Immun. 57:2776-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleary, P. P., U. Prahbu, J. B. Dale, D. E. Wexler, and J. Handley. 1992. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 60:5219-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collin, M., and A. Olsen. 2001. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69:7187-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson, A., S. E. Holm, and M. Norgren. 1998. Identification of domains involved in superantigenicity of streptococcal pyrogenic exotoxin F (SpeF). Microb. Pathog. 25:279-290. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson, A., and M. Norgren. 1999. The superantigenic activity of streptococcal pyrogenic exotoxin B is independent of the protease activity. FEMS Immunol. Med. Microbiol. 25:355-363. [DOI] [PubMed] [Google Scholar]
- 18.Frithz, E., L. O. Heden, and G. Lindahl. 1989. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol. Microbiol. 3:1111-1119. [DOI] [PubMed] [Google Scholar]
- 19.Hauser, A. R., and P. M. Schlievert. 1990. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J. Bacteriol. 172:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath, D. G., and P. P. Cleary. 1989. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc. Natl. Acad. Sci. USA 86:4741-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoe, N. P., P. Kordari, R. Cole, M. Liu, T. Palzkill, W. Huang, D. McLellan, G. J. Adams, M. Hu, J. Vuopio-Varkila, T. R. Cate, M. E. Pichichero, K. M. Edwards, J. Eskola, D. E. Low, and J. M. Musser. 2000. Human immune response to streptococcal inhibitor of complement, a serotype M1 group A Streptococcus extracellular protein involved in epidemics. J. Infect. Dis. 182:1425-1436. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz, A., and L. E. Folke. 1973. Hydrogen sulfide production in the periodontal environment. J. Periodontol. 44:390-395. [DOI] [PubMed] [Google Scholar]
- 23.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji, Y., N. Schnitzler, E. DeMaster, and P. Cleary. 1998. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect. Immun. 66:5399-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnsson, E., K. Berggard, H. Kotarsky, J. Hellwage, P. F. Zipfel, U. Sjobring, and G. Lindahl. 1998. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161:4894-4901. [PubMed] [Google Scholar]
- 26.Kagawa, T. F., J. C. Cooney, H. M. Baker, S. McSweeney, M. Liu, S. Gubba, J. M. Musser, and E. N. Baker. 2000. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. USA 97:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur, V., M. W. Majesky, L. L. Li, R. A. Black, and J. M. Musser. 1993. Cleavage of interleukin 1 beta (IL-1 beta) precursor to produce active IL-1 beta by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 90:7676-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur, V., S. Topouzis, M. W. Majesky, L. L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 29.Kihlberg, B. M., M. Collin, A. Olsen, and L. Bjorck. 1999. Protein H, an antiphagocytic surface protein in Streptococcus pyogenes. Infect. Immun. 67:1708-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancefield, R. C. 1962. Current knowledge of type-specific M-antigen of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 31.Lancefield, R. C. 1928. Demonstration of a type-specific substance in extracts of Streptococcus heamolyticus. J. Exp. Med. 47:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukomski, S., C. A. Montgomery, J. Rurangirwa, R. S. Geske, J. P. Barrish, G. J. Adams, and J. M. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:1066. [PubMed] [Google Scholar]
- 34.Matsuka, Y. V., S. Pillai, S. Gubba, J. M. Musser, and S. B. Olmsted. 1999. Fibrinogen cleavage by the Streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect. Immun. 67:4326-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinkney, M., V. Kapur, J. Smith, U. Weller, M. Palmer, M. Glanville, M. Messner, J. M. Musser, S. Bhakdi, and M. A. Kehoe. 1995. Different forms of streptolysin O produced by Streptococcus pyogenes and by Escherichia coli expressing recombinant toxin: cleavage by streptococcal cysteine protease. Infect. Immun. 63:2776-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raeder, R., E. Harokopakis, S. Hollingshead, and M. D. Boyle. 2000. Absence of SpeB production in virulent large capsular forms of group A streptococcal strain 64. Infect. Immun. 68:744-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raeder, R., M. Woischnik, A. Podbielski, and M. D. Boyle. 1998. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res. Microbiol. 149:539-548. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt, K. H., K. Mann, J. Cooney, and W. Kohler. 1993. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immunol. Med. Microbiol. 7:135-143. [DOI] [PubMed] [Google Scholar]
- 39.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Pawel-Rammingen, U., B. P. Johansson, and L. Bjorck. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21:1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woischnik, M., B. A. Buttaro, and A. Podbielski. 2000. Inactivation of the cysteine protease SpeB affects hyaluronic acid capsule expression in group A streptococci. Microb. Pathog. 28:221-226. [DOI] [PubMed] [Google Scholar]
- 42.The Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA 269:390-391. [PubMed] [Google Scholar]
- 43.Yamaguchi, Y., H. Kim, K. Kato, K. Masuda, I. Shimada, and Y. Arata. 1995. Proteolytic fragmentation with high specificity of mouse immunoglobulin G. Mapping of proteolytic cleavage sites in the hinge region. J. Immunol. Methods 181:259-267. [DOI] [PubMed] [Google Scholar]