Abstract
Staphylococcus aureus Cowan I and a clinically isolated coagulase-negative Staphylococcus strain, S. saprophyticus 10312, were found to have two fibronectin binding proteins, FnBPA and FnBPB. While both staphylococci bound to serum fibronectin to a similar extent, fibronectin binding significantly increased the phagocytic activity of macrophages against S. aureus (by ca. 150%) but not against S. saprophyticus. This enhancing effect of fibronectin was inhibited by an RGD sequence-containing peptide and also by anti-very late antigen 5 antibody. This suggests that the effect is mediated by very late antigen 5 expressed on macrophages. In macrophages ingesting fibronectin-bound Cowan I, α5 and β1 chains were associated with the cytoskeleton. Cytosolic signaling factors such as paxillin, c-Src, and c-Csk were also associated with the cytoskeleton. On the contrary, β3 integrin transiently disappeared from the cytoskeleton when macrophages ingested the fibronectin-treated S. aureus Cowan I. Furthermore, the Src kinase family tyrosine kinase Lyn dissociated from the cytoskeleton. These cellular components did not respond in a fibronectin-dependent manner when macrophages phagocytosed S. saprophyticus. This means that only fibronectin-treated S. aureus Cowan I induces the accumulation of very late antigen 5, which in turn induces the association of paxillin and tyrosine kinases. It is thought that the phagocytic activity of macrophages against fibronectin-treated S. aureus was increased by signaling via the activation of very late antigen 5.
Microbial pathogens use a number of mechanisms for interacting with their hosts. Adhesins, which are expressed on the surface of bacteria and bind to the surface of host cells, such as epithelial cells, endothelial cells, fibroblasts, and leukocytes, comprise a system that interacts with and colonizes on host tissues in order to invade cells in some cases. It has been demonstrated that bacterial adhesins recognizing integrins are categorized into three groups according their functions: (i) mimicry of a true ligand such as the RGD sequence in fibronectin (FN), (ii) recognition of an ancillary ligand of integrin such as gp63, and (iii) absorption of ligands consisting of extracellular matrix (ECM) (13). Staphylococcus aureus has a number of proteins that bind to extracellular matrix proteins, such as laminin, vitronectin, collagen, FN, elastin, and fibrinogen (9, 10, 19, 20, 25, 29, 32, 41). These receptors are thought to play a role in tropism, colonization of host tissues, invasion of host cells, and ingestion by host cells (31). FN-binding protein (FnBP) is a receptor of soluble and assembled FN that is expressed on staphylococci. There are two isoforms, FnBPA and FnBPB, which recognize the N-terminal sequence of FN at region D and also at region Du located in region C (15, 16, 38). Recently, it has been demonstrated that FnBPA has a third FN-binding site in region B (23). This activity is peculiar to FnBPA, because region B is not found in FnBPB.
A previous study showed that S. aureus Cowan I and two clinically isolated coagulase-negative staphylococci (CNS) expressing both FN-binding proteins, FnBPA and B, bound FN on their surfaces to similar extents. However, the number of bacteria ingested by macrophages increased only when the macrophages interacted with FN-bound S. aureus, whereas FN showed no effect on the ingestion of CNS (37). In the present study, the response of the adhesion architecture of macrophages after binding to FN-bound staphylococci was investigated.
MATERIALS AND METHODS
Bacteria.
S. aureus Cowan I or, in the case of immunoprecipitation, HLj, a protein A-deficient mutant strain of Cowan I (36), and a clinically isolated CNS strain, Staphylococcus saprophyticus 10312, were grown for 18 h in brain heart infusion at 37°C with shaking. After collection, the bacteria were washed three times with saline and suspended in PBS(+) (phosphate-buffered saline [PBS] containing 50 μM calcium chloride and 2 mM magnesium chloride) and protease inhibitors (1 mM benzamidine, 1 μg of pepstatin A/ml, 10 μg of aprotinin/ml, and 0.5 mM phenylmethylsulfonyl fluoride).
Macrophages.
Macrophages were obtained as previously described (37). In brief, 1 ml of 3% thioglycolate medium (Difco, Detroit, Mich.) was injected intraperitoneally into female ICR mice (5 weeks of age; purchased from Charles River Japan Inc.), and peritoneal exudate cells were collected on day 4 by flushing the cavity with 3 ml of ice-cold Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, N.Y.). The cells were washed twice, suspended in HEPES-buffered RPMI 1640 medium and plated onto plastic petri dishes (Nunc, Roskilde, Denmark). After 2 h of incubation at 37°C in a humidified atmosphere of 5% CO2 and 95% air, nonadherent cells were removed by rinsing. HEPES-buffered RPMI medium was then added to the cultures. The cell monolayers were found to contain >98% macrophages as determined from their morphology by use of a Giemsa stain or histochemical stain for nonspecific esterase.
Preparation of FN from fetal calf serum.
FN from fetal calf serum was prepared as previously described (37). Before the use of FN, gel-filtered fractions eluted by cellulofine GCL-2000-m (Seikagaku Co., Tokyo, Japan) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and fractions containing only dimers were selected, because spontaneous multimer formation sometimes occurred.
Quantification of ingested staphylococci in the presence of GRGDSP and GRADSP peptides and anti-VLA 5 antibody.
Staphylococci (1010 CFU) were suspended in 200-μg/ml FN dissolved in PBS(+) and were incubated for 1 h at 37°C. Bacteria were then washed three times with PBS(+). FN-treated bacteria were added to the macrophage cultures at a bacteria/macrophage ratio of 500 to 1. Before the addition of bacteria, a peptide with a GRGDSP or GRADSP sequence was added to the cultures at various concentrations. In another experiment, monoclonal anti-mouse very late antigen 5 (VLA-5) antibody (Chemicon International Inc., Temecula, Calif.) was added at various dilutions. After ingestion for 40 min, the cultures were washed with saline and treated with 20 μg of lysostaphin/ml for 30 min at 37°C to lyse bacteria outside of the macrophages. When bacteria were not completely lysed under this condition, as was especially the case with S. saprophyticus, the period of lysostaphin treatment was prolonged up to 45 min or 40 μg of lysostaphin/ml was used. The cultures were then washed, fixed with absolute methanol, and stained by Giemsa solution. Two hundred macrophages were selected randomly, and the number of ingested bacteria was counted under a light-field microscope (Nikon Optiphot-2). Statistical significance was evaluated by Student's t test.
Preparation of the cytoskeletal fraction from macrophages and Western blot analysis of associated proteins.
Macrophages which ingested the FN-treated or untreated bacteria were treated with 0.1% Triton X-100 solution containing 0.1 M sodium chloride, 1 mM EDTA, 10 mM piperazine-N-N′-bis(2-ethanesulfonic acid) (PIPES) (pH 7.0), 10 mM sodium orthovanadate, and protease inhibitors for 1 min at 37°C to solubilize the cytosolic fraction. The remaining Triton X-100-stable cytoskeletal fraction was dissolved in 0.1% SDS buffer with (for detection of β1 integrin, α5 integrin, paxillin, and tyrosine kinases) or without (for detection of β3 integrin) 0.1 M 2-mercaptoethanol, sonicated in ice-water, and boiled. These samples were separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore Co., Bedford, Mass.). In all of these experiments, proteins corresponding to 105 cells were loaded in each lane. The membranes were blocked and then treated with the first antibody followed by treatment with horseradish peroxidase-conjugated or alkaline phosphatase-conjugated secondary antibodies. Antibodies were purchased from the following companies: anti-β1 integrin, Chemicon International Inc.; anti-β3 integrin, Genex (Helsinki, Finland); anti-α5 integrin, anti-Src, anti-Csk, and anti-Lyn, Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.); and anti-phosphotyrosine (4G10), Upstate Biotechnology Inc. (Lake Placid, N.Y.). Quantification of signals was performed with a Densitograph AE-6920 M-FX (Atto Co., Tokyo, Japan). The intensity values of protein signals on a blot were analyzed by an image-analyzing system equipped with a Densitograph. This system calibrates the different background intensities among different blots.
Immunoprecipitation.
Proteins were immunoprecipitated from cell lysate supernatants by using goat anti-VLA-5 antibody. FN-treated or untreated HLj or FN-treated S. saprophyticus was added to the macrophage cultures as described above. After 2 or 20 min, cells were washed with ice-cold PBS and lysed with a solution containing 1% Nonidet P-40, 0.15 M sodium chloride, 1 mM EDTA, 2 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 10 mM sodium fluoride, and protease inhibitors. Cell lysates were rotated gently for 1 h at 4°C and centrifuged. Cleared supernatants were pretreated with goat IgG followed by protein G-agarose. Centrifuged supernatants were treated with anti-VLA-5 antibody followed by protein G-agarose. Precipitates were boiled in 0.1% SDS buffer, and the supernatants were subjected to SDS-PAGE. The separated proteins were blotted onto a membrane and then examined by Western blot analysis.
RESULTS
Effect of peptide containing the RGD sequence and anti-VLA-5 on phagocytosis of FN-treated staphylococci.
A previous study demonstrated that FN shows an opsonin-like effect on macrophage phagocytosis with S. aureus Cowan I but not with CNS, although FN can associate equally with these strains (37). It is thought that FN, which is bound on the surface of S. aureus, interacts with FN receptors expressed on macrophages. The principal FN receptors of macrophages are VLA-4 and VLA-5 (12). VLA-5 is known to associate with the RGD sequence in the FN molecule, whereas VLA-4 recognizes the EILDV sequence in the CS-1 region of FN (21, 46). αvβ3, which is known as a vitronectin receptor, also interacts with FN via binding to the RGD sequence (42). To determine the participation of the RGD sequence in the phagocytosis of FN-treated staphylococci, the effect of the RGD-containing peptide GRGDSP was examined, and the results are shown in Table 1. As described above, FN treatment increased the number of ingested Cowan I cells but did not affect the ingestion of S. saprophyticus. GRGDSP peptide suppressed the number of ingested FN-treated Cowan I cells in a peptide concentration-dependent manner. In contrast, this peptide had little effect on the ingestion of untreated Cowan I or that of S. saprophyticus regardless of FN treatment. In another experiment, an analogue peptide with a GRADSP sequence was investigated. This peptide had no effect on the phagocytosis of FN-treated Cowan I by macrophages, as the mean number of ingested bacteria per macrophage (± standard deviation) without the peptide was 78.4 ± 56.2 and that with 1 μg of GRADSP peptide/ml was 82.6 ± 45.0 (P = 0.57). These results indicate that the FN receptor, which recognizes the RGD sequence, is associated with the FN-dependent ingestion of S. aureus Cowan I. Thus, VLA-5 or αvβ3 may be the FN receptor mediating the FN-dependent ingestion. To determine which receptor was responsible for this function, the effect of anti-VLA-5 antibody was investigated (Table 1). Addition of anti-VLA-5 antibody suppressed the ingestion of FN-treated Cowan I, but not that of untreated Cowan I, in a dose-dependent manner. In the case of S. saprophyticus, this antibody did not affect ingestion irrespective of FN treatment. This suggests that VLA-5 on macrophages is responsible for the FN-activated ingestion of S. aureus Cowan I.
TABLE 1.
Effect of RGD peptide and anti-VLA-5 on the ingestion of FN-treated staphylococci
| Peptide or antibody | No. of ingested bacteria/macrophage without or with FN treatmenta
|
|||
|---|---|---|---|---|
|
S. aureus Cowan I
|
S. saprophyticus
|
|||
| − | + | − | + | |
| None | 33.1 ± 27.0b | 45.5 ± 40.5c | 16.3 ± 14.2d | 14.4 ± 11.2e |
| GRGDSP | ||||
| 0.05 μg/ml | 36.7 ± 32.3 | |||
| 0.1 μg/ml | 32.8 ± 28.7 | 31.2 ± 29.9 | 16.9 ± 17.4 | 13.3 ± 13.1 |
| Anti-VLA-5 | ||||
| 1:1,000 dilution | 41.2 ± 44.3 | |||
| 1:500 dilution | 34.3 ± 34.3 | |||
| 1:200 dilution | 34.1 ± 32.1 | 30.9 ± 34.2 | 15.4 ± 12.4 | 13.2 ± 13.0 |
Mean numbers of ingested bacteria per macrophage ± standard deviations are shown.
Compared with the value obtained with FN treatment, P was 0.030; compared with the value obtained with 0.1 μg of GRGDSP/ml, P was 0.810; and compared with the value obtained with anti-VLA-5 at a 1:200 dilution, P was 0.263.
Compared with the values obtained with 0.05 and 0.1 μg of GRGDSP/ml, P values were 0.095 and 0.005, respectively, and compared with the values obtained with anti-VLA-5 at dilutions of 1:1,000, 1:500, and 1:200, P values were 0.470, 0.035, and 0.007, respectively.
Compared with the value obtained with FN treatment, P was 0.305; compared with the value obtained with 0.1 μg of GRGDSP/ml, P was 0.750; and compared with the value obtained with anti-VLA-5 at a 1:200 dilution, P was 0.627.
Compared with the value obtained with 0.1 μg of GRGDSP/ml, P was 0.529, and compared with the value obtained with anti-VLA-5 at a 1:200 dilution, P was 0.627.
β1 integrin associates with the cytoskeleton upon ingestion of FN-treated Cowan I.
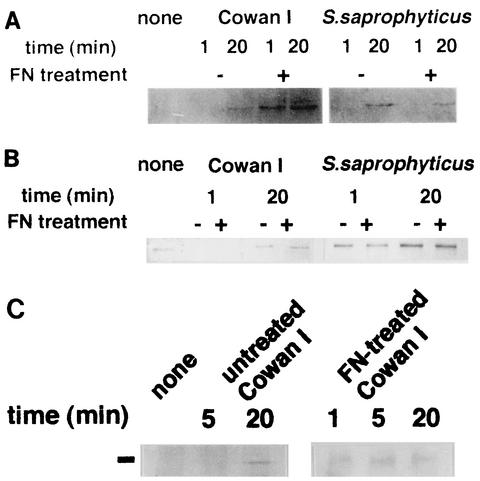
Since the interaction of integrin with its ligands induces its association with the cytoskeletal structure (47), integrins in the Triton-stable cytoskeletal fraction of macrophages were analyzed (Fig. 1). In macrophages ingesting FN-treated Cowan I, a β1 integrin signal was detected at 1 and 20 min after the addition of bacteria. With untreated Cowan I, however, the signal for this integrin was not observed at 1 min and was detected faintly at 20 min (about one-fifth of the strength of that detected with FN-treated Cowan I). In S. saprophyticus-ingesting macrophages, the signal of β1 integrin was not detected at 1 min but appeared at 20 min regardless of the FN treatment (Fig. 1A). Figure 1B shows the results of our analysis of β3 integrin. This integrin was already associated with the cytoskeleton in nonstimulated macrophages and disappeared from the cytoskeletal fraction at 1 min followed by reassociation at 20 min after Cowan I addition. This response was independent of FN treatment. In macrophages ingesting S. saprophyticus, this integrin did not respond at 1 min. At 20 min, the amount of associated integrin increased to about three times the level at 1 min. However, no difference was observed between macrophages that ingested untreated and FN-treated S. saprophyticus. Figure 1C shows the action of α5 integrin. The association of this integrin was not seen up to 5 min after the ingestion of untreated Cowan I, but a signal appeared at 20 min. FN-treated Cowan I, however, rapidly induced the association at 1 min after the start of ingestion (the level of association was five times greater than that of untreated bacteria). This seems to be in good agreement with the association of β1 integrin with the cytoskeleton. The results shown in Table 1 and Fig. 1 suggest that the integrin responsible for the enhancement of FN-treated Cowan I ingestion is VLA-5, although this integrin seems to function even when macrophages ingest untreated Cowan I or S. saprophyticus. The receptor consisting of β3 integrin does not function in the FN-dependent ingestion of Cowan I; nevertheless, this integrin also responds in an FN-independent manner.
FIG. 1.
Association of integrins with the macrophage cytoskeleton upon ingestion of staphylococci. The Triton-stable cytoskeletal fraction was prepared from macrophages which ingested FN-treated or untreated S. aureus Cowan I or S. saprophyticus 10312. The association of β1, β3, and α5 integrins with this fraction was analyzed by Western blot analysis. (A and B) Signals of β1 (A) and β3 (B) integrins in the cytoskeletal fraction of macrophages that ingested Cowan I or S. saprophyticus. (C) α5 integrin in the cytoskeleton of Cowan I-ingesting macrophages. The position of the α5 signal is shown by the black line at left. The lower-molecular-mass signals were nonspecific signals of the first antibody.
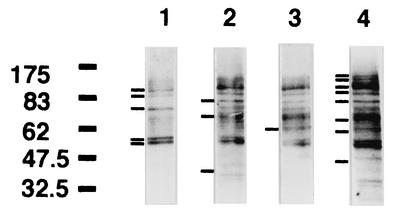
Some distinct proteins are tyrosine phosphorylated during ingestion of FN-treated S. aureus Cowan I.
Figure 2 shows the results of Western blot analysis, with anti-phosphotyrosine antibody, of the macrophage cytoskeletal fraction 20 min after the addition of FN-treated or untreated S. aureus Cowan I. In the nonstimulated state, some proteins, whose molecular masses were estimated to be 100, 90, 70, 58, and 57 kDa, were definitely tyrosine phosphorylated (Fig. 2, lane 1). Addition of FN to the culture also induced tyrosine phosphorylation of proteins of 83 and 40 kDa (lane 2). Signals at approximately 65 to 68 kDa were also observed (lane 2). Addition of untreated Cowan I induced tyrosine phosphorylation of the same proteins as those affected by FN addition, and a new phosphorylation signal at 60 kDa was seen (lane 3). FN-treated Cowan I enhanced the phosphorylation of the 58- and 57-kDa proteins, and the level of phosphorylation of the proteins whose molecular masses were estimated to be 100, 90, 83, 65 to 68, and 60 kDa was markedly increased. Furthermore, 130-, 120-, and 50-kDa proteins were also phosphorylated (lane 4). These proteins should be included in the β1 integrin-associated cytoskeletal apparatus.
FIG. 2.
At 20 min after the start of ingestion, tyrosine-phosphorylated proteins in the cytoskeletal fractions from macrophages that ingested FN-treated or untreated Cowan I were examined by Western blot analysis. Lane 1, no treatment; lane 2, 200 μg of FN/ml added to macrophage culture; lane 3, untreated Cowan I added to macrophage culture; lane 4, FN-treated Cowan I added to macrophage culture. Bars at the left side of each lane indicate the signals (with molecular masses in kilodaltons indicated) mentioned in the text.
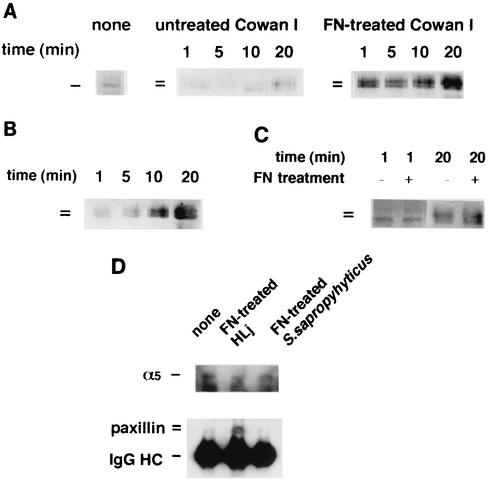
Paxillin associates with cytoskeleton and forms a complex containing VLA-5 upon ingestion of FN-treated Cowan I.
Paxillin, whose molecular mass is approximately 60 to 70 kDa, localized in focal adhesions. This is one of the docking proteins having multiple binding sites for FAK, Src, vinculin, etc. (44). Binding of this protein to these factors is regulated by tyrosine phosphorylation. We analyzed whether this protein associated with the cytoskeletal structure upon ingestion of bacteria, because proteins of approximately 65 to 68 kDa were markedly tyrosine phosphorylated in FN-treated Cowan I-ingesting macrophages, as shown in Fig. 2. Figure 3A show the results for Cowan I. In nonstimulated macrophages, only a 65-kDa signal was observed. Upon ingestion of Cowan I, the 65-kDa signal was observed mainly during the first 10 min. In this period, the signal intensity scarcely changed. A 68-kDa signal appeared 20 min after the start of ingestion. In macrophages that ingested FN-treated Cowan I, a 65-kDa signal remarkably increased at 1 min (to a level about 10 times greater than that in the nonstimulated state) and a 68-kDa signal also appeared. At 20 min, the 65-kDa signal increased to a level 15 to 20 times greater than that in the nonstimulated macrophages and the 68-kDa signal had almost the same intensity as the 65-kDa signal. Figure 3B shows the results of an analysis of paxillin in the cytoskeletal fraction when only FN was added to the culture. Lower- and higher-molecular-mass components gradually associated with the cytoskeletal fraction, although the signals were not very strong during the first 5 min. At 20 min, the 65-kDa component was detected at a level 10 times higher than during the first 5 min, and the 68-kDa component was detected at a similar level. The action of paxillin in S. saprophyticus-ingesting macrophages is shown in Fig. 3C. Neither component of paxillin responded at 1 min, irrespective of FN treatment, and the intensity of a 65-kDa signal remained unchanged during the first min. At 20 min, both the higher- and lower-molecular-mass components were detected but there was no marked difference between the results with FN-treated and untreated bacteria. These data indicate that FN-bound Cowan I induces notable association between the cytoskeleton and paxillin, especially the 68-kDa component. Furthermore, the response of paxillin is thought to be in good accordance with the behavior of β1 integrin.
FIG. 3.
Association of paxillin with the cytoskeleton and VLA-5 after ingestion of FN-treated staphylococci. (A to C) Paxillin in the cytoskeletal fractions from macrophages that ingested FN-treated or untreated Cowan I (A), that were treated with 200 μg of FN/ml (B), and that ingested FN-treated or untreated S. saprophyticus 10312 (C) were analyzed by Western blot analysis. (D) Immunoprecipitation of S. aureus- or S. saprophyticus-treated macrophage lysates by anti-VLA-5 antibody. S. aureus HLj with or without FN treatment or FN-treated S. saprophyticus was added to the macrophage culture for 2 min. Macrophage lysates were prepared and immunoprecipitated with anti-VLA-5 antibody as described in Materials and Methods. The immunoprecipitates were subjected to Western blot analysis with anti-paxillin antibody or anti-α5 antibody.
To determine whether VLA-5 formed the complex containing paxillin, immunoprecipitation, using anti-VLA-5 antibody, was performed with macrophages that ingested untreated HLj, a protein A-deficient mutant strain of Cowan I, FN-treated HLj, or FN-treated S. saprophyticus for 2 min (Fig. 3D). In each of the immunoprecipitates, similar amounts of α5 integrin were detected. However, the precipitate from macrophages ingesting FN-treated HLj contained more paxillin, including a higher-molecular-mass component, than did the other precipitates. This result indicates that VLA-5 quickly formed the complex containing paxillin, especially the 68-kDa isoform, when macrophages started to ingest FN-treated S. aureus.
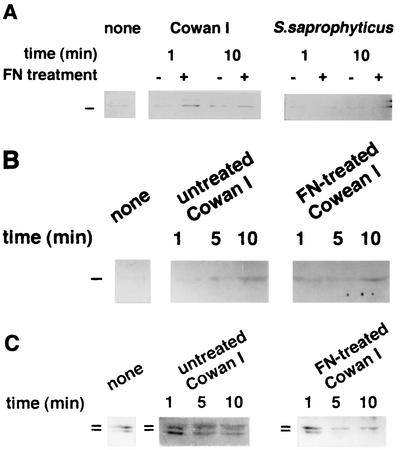
Analysis of the action of the tyrosine kinases Src, Lyn, and Csk in macrophages.
As shown in Fig. 2, some proteins whose molecular masses were approximately 60 kDa were tyrosine phosphorylated after exposure to FN-treated Cowan I. Since the molecular masses of many nonreceptor tyrosine kinases are within this range and some kinases are known to associate with the focal contact apparatus (48), we investigated the action of tyrosine kinases. Figure 4A shows the results of Western blot analysis of Src kinase associated with the cytoskeletal fraction. In nontreated macrophages, a small amount of kinase was present in this fraction. Ingestion of untreated Cowan I did not result in much increase in the amount of Src; however, in macrophages ingesting FN-treated Cowan I, this kinase increased to twice the level present in macrophages ingesting untreated Cowan I, even at 1 min after the start of ingestion. In the case of S. saprophyticus, FN treatment did not affect the association of this kinase with the cytoskeletal fraction. Another tyrosine kinase, Csk, which is known to regulate the activity of the Src kinase family (28), also started to associate with the cytoskeleton only in macrophages that ingested FN-treated Cowan I (with association beginning at 1 min and continuing until 10 min after ingestion), whereas untreated Cowan I did not induce a rapid association (Fig. 4B). These results suggest that Src and Csk respond in a manner similar to that of α5 and β1 integrins in macrophages ingesting FN-treated S. aureus. The association of Lyn with the cytoskeleton in Cowan I-ingesting macrophages is shown in Fig. 4C. This kinase belongs to the Src kinase family and is known to be highly expressed in hematopoietic cells (43). Two isoforms, of 56 and 53 kDa, are known to result from alternative splicing (49). In untreated macrophages, both isoforms were associated with the cytoskeletal fraction, but the 53-kDa isoform disappeared from the fraction at 5 min after the start of ingestion of FN-treated Cowan I (but not of untreated Cowan I). This result suggests that the 53-kDa Lyn also functions during the FN-mediated ingestion of Cowan I.
FIG. 4.
Association of Src (A), Csk (B), and Lyn (C) with the cytoskeleton in macrophages that ingested FN-treated or untreated Cowan I or S. saprophyticus as indicated.
DISCUSSION
The results of this study show that FN bound on the surface of S. aureus Cowan I has an opsonin-like effect on phagocytosis by macrophages via VLA-5, because the phagocytosis of FN-treated Cowan I was inhibited by an RGD sequence-containing peptide or anti-VLA-5 antibody and because both α5 and β1 chains were shown to be associated with the cytoskeletal structure immediately after the ingestion of FN-treated S. aureus Cowan I. Since α5 and β1 chains were also associated with the cytoskeleton after the ingestion of untreated S. aureus, VLA-5 seems to participate in this ingestion, although the association was very slow compared with that seen upon ingestion of FN-treated Cowan I. Furthermore, this integrin was implicated in the ingestion of S. saprophyticus because its association with the cytoskeleton was similar to that seen upon ingestion of untreated S. aureus. It has been demonstrated that some surface molecules, such as teichoic acid or lipoteichoic acid on staphylococci, seem to bind to integrin (7); hence, the response of α5 and β1 integrins observed upon the ingestion of untreated S. aureus or S. saprophyticus may have been caused by direct interaction between the integrin and surface molecules of these bacteria. The amount of β3 integrin in the cytoskeletal fraction did not increase after the addition of S. aureus; in contrast, this integrin transiently disappeared from the fraction upon the addition of bacteria in an FN-independent manner. This indicates that αvβ3 does not have an essential function in the FN-enhancing ingestion of S. aureus Cowan I. The contrasting behaviors of β1 and β3 integrins with regard to association with the cytoskeleton upon FN-treated S. aureus ingestion may reflect cross talk between β1 and β3 integrin functions, that is, one integrin influences the function of another integrin, as has been demonstrated to occur between α5β1 and αvβ3 (2, 39). In S. saprophyticus, the amount of β3 integrin increased regardless of FN treatment. It is possible that interaction between S. saprophyticus and macrophages is mediated by αvβ3 and that a surface molecule on the bacteria, not FN, is responsible for the interaction.
Previous reports have demonstrated that integrins which accumulate at the focal contact region in the adherent cell associate with FAK and tensin and that binding of the accumulated integrins with ECM induces the association of additional components constituting the adhesion apparatus, including talin and paxillin, which mediate the interaction of integrins with the cytoskeletal structure (47). These factors are tyrosine phosphorylated to associate with the apparatus and for regulation of their activity (48). The present study shows that FN-treated S. aureus Cowan I immediately induced a number of tyrosine-phosphorylated proteins in the cytoskeleton and the association of paxillin with the cytoskeleton, whereas untreated Cowan I and FN-treated and untreated S. saprophyticus did not bring about such intense paxillin-cytoskeleton interaction. Two paxillin isoforms with molecular masses of approximately 65 and 68 kDa were observed. Judging from their molecular masses and from the results of a previous report demonstrating that the γ isoform is absent from murine tissues (23), the higher- and lower-molecular-mass isoforms are thought to be α and β paxillin, respectively. It has been established that β isoform takes part in cell movement (24). In nonstimulated cells, only the lower-molecular-mass component was associated with the cytoskeleton. Only in the case of FN-treated Cowan I, but not FN alone or bacteria alone, was the quick and marked accumulation of both isoforms in the early period of ingestion observed. In this period, VLA-5 formed complexes containing both isoforms of paxillin, indicating that FN bound to the S. aureus surface enables VLA-5 to form an adherent apparatus, as described above.
In the present study, we also found rapid association of Src and Csk with the cytoskeleton and slow dissociation of 53-kDa Lyn from the cytoskeleton in macrophages that ingested FN-treated S. aureus Cowan I. This suggests that FN-enhanced ingestion of S. aureus was regulated by these tyrosine kinases. It is known that various molecules concerned with signal transduction accumulate in the integrin-mediated focal adhesion apparatus (11, 18, 30). In macrophages, it has been reported that adhesion to ECM via integrin causes activation of Src kinases (26). Binding of paxillin in the adhesion apparatus is regulated by the cytoplasmic tyrosine kinases c-Src and c-Csk (14, 35, 47, 48). Furthermore, it is reported that Lyn is tyrosine phosphorylated after adhesion of macrophages to FN via β1 integrin (26). The fact that FN-treated S. aureus but not FN-treated S. saprophyticus strongly induced the responses of these intracellular signaling factors and VLA-5 suggests that the structural basis of FN binding to S. aureus and that to S. saprophyticus are quite different and that FN bound on S. aureus possibly forms multimers, which induce the ligation of integrins and their interaction with the cytoskeleton and signaling factors as well as ECM, whereas FN on CNS does not form such structures. If this is the case, then it is thought that the “outside-in signal” via VLA-5 is transmitted into macrophages by FN bound on S. aureus so that phagocytic activity is enhanced (3). In this case, ingestion would be mediated by another phagocytic receptor, for example, Fc receptor or complement receptor. Another possibility is that VLA-5, like other phagocytic receptors, directly wraps up FN-bound S. aureus. A previous report demonstrated that α5 and β1 integrins are present in endocytic vesicles and are recycled by an intracellular trafficking system in polymorphonuclear neutrophils (33). Also, in our preliminary study, we observed a large amount of α5 integrin in detergent-permeabilized macrophages compared with that in untreated cells. This suggests that α5 integrins in macrophages are also recycled together with trafficking vesicles. In this case, FN-bound S. aureus would be incorporated into macrophages via recycled integrins. These possibilities are now being investigated.
It has been demonstrated that certain kinds of bacteria modulate host cell function. With enteropathogenic Escherichia coli, interaction of intimin with host epithelial cells triggers tyrosine phosphorylation of some signaling factors and actin assembly beneath the bacteria, followed by bacterial infection (17). With Bordetella pertussis, the RGD sequence in filamentous hemagglutinin interacts with integrin CR3 (αMβ2 and CD11b/CD18) to adhere to the host cells (34). Invasin, the outer membrane protein in Yersinia spp., interacts with VLA-5 to enter the host cells efficiently (8). By using Ipa proteins, Shigella flexneri invades cells via VLA-5 (45). These previous findings suggest that systems to modulate or utilize host cell function constitute a useful mechanism for survival in host cells. In addition to these intracellular pathogens, a variety of extracellular pathogens are known to enter host cells and survive within them. Streptococcus pyogenes is internalized into epithelial cells through interactions among FN, bacterial FN-binding proteins, and VLA-5 (4). This is thought to be one of the reasons for the frequent failure of β-lactam antibiotics to eradicate these organisms from infected patients. It has been reported that host cells such as fibroblasts or epithelial cells ingest S. aureus (5, 27). Some strains of S. aureus escape from endosomes and induce apoptosis of these cells (1, 27). Internalization of S. aureus within epithelial cells depends on the expression of FnBP and on tyrosine kinase activity in the host cells (5). This internalization is inhibited by the addition of anti-β1 integrin antibody (6). It has also been demonstrated that FnBP deletion mutants lose their invasiveness, that expression of FnBP confers invasiveness, that soluble FnBP blocks invasion, and that anti-α5 antibody blocks invasion into epithelial cells (40). It has been reported that FnBP (FnBPA in particular) mediates the invasion of S. aureus into endothelial cells via α5β1 (22). From these observations, it can be concluded that FnBP on S. aureus must play a major role in the interaction of bacteria with integrins on host cells through FN. Furthermore, it is probable that another factor related to FnBP or FN binding may be expressed on S. aureus, because different signals were observed between macrophages ingesting S. aureus and those ingesting CNS, although both staphylococci possess FnBPs and bind FN equally as previously described (37).
Acknowledgments
This work was supported by the Bio-Venture Research Fund Project Aid grant from the Ministry of Education, Science and Culture of Japan.
We thank Y. Yasui and T. Iwasaki for their excellent assistance in the preparation of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blystone, S. D., S. E. Slater, M. P. Williams, M. T. Crow, and E. J. Brown. 1999. A molecular mechanism of integrin crosstalk: αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J. Cell Biol. 145:889-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark, E. A., and J. S. Brugge. 1995. Integrins and signal transduction pathways: the road taken. Science 268:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Cue, D., S. O. Southern, P. J. Southern, J. Prabhakar, W. Lorelli, J. M. Smallheer, S. A. Mousa, and P. P. Cleary. 2000. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin α5β1-fibronectin-M1 protein complexes. Proc. Natl. Acad. Sci. USA 97:2858-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dziewanowska, K., A. R. Carson, J. M. Patti, C. A. Deobald, K. W. Bayles, and G. A. Bohach. 2000. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68:6321-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgavish, A., A. Pattanaik., K. Lloyd, and R. Reed. 1994. Integrin-mediated adhesive properties of uroepithelial cells are inhibited by treatment with bacterial toxins. Am. J. Physiol. 266:1552-1559. [DOI] [PubMed] [Google Scholar]
- 8.Fällman, M., C. Persson, and H. Wolf-Watz. 1997. Yersinia proteins that target host cell signaling pathways. J. Clin. Investig. 99:1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flock, J.-I., G. Fröman, K. Jönsson, B. Guss, C. Signäs, B. Nilsson, G. Raucci, M. Höök, T. Wadström, and M. Lindberg. 1987. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 11.Guinebault, C., B. Payrastre, C. Racaud-Sultan, H. Mazarguil, M. Breton, G. Mauco, M. Plantavid, and H. Chap. 1995. Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85α with actin filaments and focal adhesion kinase. J. Cell Biol. 129:831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemler, M. E. 1990. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 8:365-400. [DOI] [PubMed] [Google Scholar]
- 13.Hoepelman, A. I. M., and E. I. Tuomanen. 1992. Consequences of microbial attachment: directing host cell functions with adhesins. Infect. Immun. 60:1729-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamoto, A., and P. Soriano. 1993. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73:1117-1124. [DOI] [PubMed] [Google Scholar]
- 15.Joh, D., P. Speziale, S. Gurusiddappa, J. Manor, and M. Höök. 1998. Multiple specificities of the staphylococcal and streptococcal fibronectin-binding microbial surface components recognizing adhesive matrix molecules. Eur. J. Biochem. 258:897-905. [DOI] [PubMed] [Google Scholar]
- 16.Jönsson, K., C. Signäs, H.-P. Müller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 17.Kenny, B., and B. B. Finlay. 1997. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect. Immun. 65:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharbanda, S., A. Saleem, Z. Yuan, Y. Emoto, K. V. S. Prasad, and D. Kufe. 1995. Stimulation of human monocytes with macrophage colony-stimulating factor induces a Grb2-mediated association of the focal adhesion kinase pp125FAK and dynamin. Proc. Natl. Acad. Sci. USA 92:6132-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, O. D., F. Ascencio, L. Å. Fransson, and T. Wadström. 1992. Binding of heparan sulfate to Staphylococcus aureus. Infect. Immun. 60:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes, J. D., M. D. Reis., and R. R. Brentani. 1985. Presence of laminin receptors in Staphylococcus aureus. Science 229:275-277. [DOI] [PubMed] [Google Scholar]
- 21.Main, A. L., T. S. Harvey, M. Baron, J. Boyd, and I. D. Campbell. 1992. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell 71:671-678. [DOI] [PubMed] [Google Scholar]
- 22.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. J. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Höök, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3:839-851. [DOI] [PubMed] [Google Scholar]
- 23.Mazaki, Y., H. Uchida, O. Hino, S. Hashimoto, and H. Sabe. 1998. Paxillin isoforms in mouse. Lack of the γ isoform and developmentally specific β isoform expression. J. Biol. Chem. 273:22435-22441. [DOI] [PubMed] [Google Scholar]
- 24.Mazaki, Y., S. Hashimoto, and H. Sabe. 1997. Monocyte cells and cancer cells express novel paxillin isoforms with different binding properties to focal adhesion proteins. J. Biol. Chem. 272:7437-7444. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 26.Meng, F., and C. A. Lowell. 1998. A β1 integrin signaling pathway involving Src-family kinases, CbI and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17:4391-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murai, M., J. Sakurada, K. Seki, H. Shinji, Y. Hirota, and S. Masuda. 1999. Apoptosis observed in BALB/3T3 cells having ingested Staphylococcus aureus. Microbiol. Immunol. 43:653-661. [DOI] [PubMed] [Google Scholar]
- 28.Okada, M., and H. Nakagawa. 1989. A protein tyrosine kinase involved in regulation of pp60c-src function. J. Biol. Chem. 264:20886-20893. [PubMed] [Google Scholar]
- 29.Park, P. W., J. Rosenbloom, W. R. Abrams, J. Rosenbloom, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin binding protein (elbS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 30.Parsons, J. T. 1996. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr. Opin. Cell Biol. 8:146-152. [DOI] [PubMed] [Google Scholar]
- 31.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 32.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 33.Pierini, L. M., M. A. Lawson, R. J. Eddy, B. Hendy, and F. R. Maxfield. 2000. Oriented endocytic recycling of α5β1 in motile neutrophils. Blood 95:2471-2481. [PubMed] [Google Scholar]
- 34.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 35.Sabe, H., A. Hata, M. Okada, H. Nakagawa, and H. Hanafusa. 1994. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc. Natl. Acad. Sci. USA 91:3984-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki, K., S. Nishihara, H. Ikigai, and S. Masuda. 1988. Effect of intravenous administration of heat-killed bacterial cells on blood clearance and kidney lodgement property of Staphylococcus aureus organisms subsequently injected to mice. Jikeikai Med. J. 35:275-284. [Google Scholar]
- 37.Shinji, H., J. Sakurada, K. Seki, M. Murai, and S. Masuda. 1998. Different effects of fibronectin on the phagocytosis of Staphylococcus aureus and coagulase-negative staphylococci by murine peritoneal macrophages. Microbiol. Immunol. 42:851-861. [DOI] [PubMed] [Google Scholar]
- 38.Signäs, C., G. Raucci, K. Jönsson, P.-E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptide. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon, K. O., E. M. Nutt, D. G. Abraham, G. A. Rodan, and Le T. Duong. 1997. The αvβ3 integrin regulates α5β1-mediated cell migration toward fibronectin. J. Biol. Chem. 272:29380-29389. [DOI] [PubMed] [Google Scholar]
- 40.Sinha, B., P. P. Francois, O. Nüβe, M. Foti, O. M. Hartford, P. Vaodaux, T. J. Foster, D. P. Lew, M. Herrmann, and K.-H. Krauze. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-107. [DOI] [PubMed] [Google Scholar]
- 41.Smeltzer, M. S., A. F. Gillaspy, F. L. Pratt, Jr., M. D. Thames, and J. J. Iandolo. 1997. Prevalence and chromosomal map location of Staphylococcus aureus adhesin genes. Gene 196:249-259. [DOI] [PubMed] [Google Scholar]
- 42.Smith, J. W., and D. A. Cheresh. 1990. Integrin (αvβ3)-ligand interaction. J. Biol. Chem. 265:2168-2172. [PubMed] [Google Scholar]
- 43.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 44.Turner, C. E. 2000. Paxillin interactions. J. Cell Sci. 113:4139-4140. [DOI] [PubMed] [Google Scholar]
- 45.Watarai, M., S. Funato, and C. Sasakawa. 1996. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wayner, E. A., A. Garcia-Pardo, M. J. Humphries, J. A. McDonald, and W. G. Carter. 1989. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J. Cell Biol. 109:1321-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng, Z., J. A. Taylor, C. E. Turner, J. S. Brugge, and C. Seidel-Dugan. 1993. Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed Balb/c 3T3 cells. J. Biol. Chem. 268:14956-14963. [PubMed] [Google Scholar]
- 48.Yamada, K. M., and B. Geiger. 1997. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol. 9:76-85. [DOI] [PubMed] [Google Scholar]
- 49.Yi, T., J. B. Bolen, and J. N. Ihle. 1991. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol. Cell. Biol. 11:2391-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]