Abstract
The ability of macrophages to control the growth of microorganisms is increased by macrophage activation. Previously, it was shown that epinephrine activated mouse macrophages to resist the growth of Mycobacterium avium via α2-adrenergic stimulation. In the present study, we show that the α2-adrenergic agonist (α2-agonist) clonidine induced resistance to M. avium growth in the RAW264.7 mouse macrophage cell line. The ability of catecholamines to induce resistance to mycobacteria was specific to α2-adrenergic stimulation, as α1-, β1-, and β2-agonists had no effect. Receptor signaling through Gi proteins was required. A G-protein antagonist specific for the α subunits of the Go/Gi family blocked the increased resistance induced by clonidine, while a Gs-protein antagonist was without effect. Both nitric oxide (NO) production and superoxide (O2−) production were required for the increased resistance to M. avium growth induced by clonidine. Although NO production was required, clonidine did not increase the level of NO in M. avium-infected cells. Since NO and O2− interact to produce peroxynitrite (ONOO−), we examined whether ONOO− mediates the increased resistance to M. avium induced by clonidine. 5,10,15,20-Tetrakis(4-sulfonatophenyl)prophyrinato iron (III) chloride (FeTPPS), a specific scavenger of ONOO−, inhibited the effect of clonidine on M. avium growth. Clonidine also increased the production of ONOO− in M. avium-infected RAW264.7 cells, as measured by the oxidation of 123-dihydrorhodamine and the production of nitrated tyrosine residues. We therefore conclude that α2-adrenergic stimulation activates macrophages to resist the growth of M. avium by enhancing the production of ONOO−.
Macrophages are among the first cells of the host to confront microbes and are important effector cells in innate resistance to intracellular microbial pathogens. The outcome of this initial encounter with an intracellular pathogen is that either the macrophage resists the growth of the microorganism or the microorganism adapts and replicates within the macrophage. The ability of the macrophage to resist the growth of the microorganism is dependent on the activation state of the macrophage. Cytokines, such as gamma interferon (IFN-γ), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha (TNF-α), activate macrophages to resist the growth of intracellular pathogens by enhancing the production of the major antimicrobial effector molecules, including reactive oxygen species and nitric oxide (38, 46, 53).
The sympathetic nervous system acts to maintain homeostasis during periods of stress by releasing norepinephrine at sympathetic nerve endings and epinephrine from the adrenal medulla (3, 20, 65). These catecholamine hormones modulate the activities of cells, including cells of the immune system (35). Macrophage function can be either activated (50, 59, 60) or suppressed (9, 31, 58, 63, 67) by catecholamines. A study by Boomershine et al. (9) showed that the addition of epinephrine to IFN-γ-activated mouse peritoneal macrophages inhibited the ability of the macrophages to resist the growth of Mycobacterium avium. This effect was shown to be mediated by the β2-adrenergic receptor and to be correlated with a decrease in nitric oxide production. In contrast, when resting peritoneal macrophages were treated with epinephrine, the inhibition of mycobacterial growth increased, suggesting that in this situation, the catecholamine activated the macrophages (50). The activation of the macrophages was mediated by α2-adrenergic stimulation. The α2-adrenergic agonist (α2-agonist) clonidine increased the ability of the macrophages to inhibit mycobacterial growth, and the effect of epinephrine was blocked by the α-adrenergic antagonist phentolamine.
In the present study, we examined the mechanism by which α2-adrenergic stimulation inhibited the growth of M. avium in the RAW 264.7 macrophage cell line. The effect of clonidine was shown to require both nitric oxide production and superoxide production and the formation of peroxynitrite. A specific peroxynitrite scavenger blocked the ability of macrophages stimulated with clonidine to inhibit mycobacterial growth.
MATERIALS AND METHODS
Reagents.
Iscove's modified Dulbecco's medium (IMDM) was obtained from Life Technologies (Gaithersburg, Md.). Fetal bovine serum was purchased from Harlan Bioproducts for Science (Indianapolis, Ind.). Clonidine (α2-agonist), methoxamine (α1-agonist), dobutamine (β1-agonist), salbutamol (β2-agonist), aminoguanidine, NG-monomethyl-l-arginine (l-NMMA), diphenyleneiodonium chloride (DPI), mannitol, biotinylated goat anti-mouse immunoglobulin G, and ExtrAvidin-alkaline phosphate conjugate were obtained from Sigma (St. Louis, Mo.). NF007, NF023, NF449, MN(III) tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), apocynin, and 5,10,15,20-tetrakis(4-sulfonatophenyl)prophyrinato iron (III) chloride (FeTPPS) were obtained from Calbiochem (La Jolla, Calif.). 123-Dihydrorhodamine (123-DHR) was obtained from Molecular Probes (Eugene, Oreg.). Antinitrotyrosine monoclonal antibody was obtained from Upstate Biotechnology (Lake Placid, N.Y.). p-Nitrophenyl phosphate was obtained from Zymed (South San Francisco, Calif.). 3H-uracil (40 to 60 Ci/mmol) and 32P-dCTP (3,000 Ci/mmol) were obtained from Amersham (Piscatawy, N.J.). The cDNA probe for inducible nitric oxide synthase (iNOS) was produced by reverse transcription-PCR with primers obtained from Clontech (Palo Alto, Calif.). The glyceraldehyde-3-phosphate dehydrogenase (G3PDH) cDNA probe was isolated from a murine macrophage cDNA library with a G3PDH-specific oligonucleotide.
Bacteria.
M. avium (ATCC 35712) was passaged through mice and grown in Middlebrook 7H9 medium (Difco, Detroit, Mich.) with oleic acid-albumin-dextrose-catalase enrichment (Difco) at 37°C in 5% CO2. Bacteria were stored in 1-ml aliquots at −70°C until used. The number of bacteria was confirmed by plate counting on 7H11 agar plates supplemented with oleic acid-albumin-dextrose-catalase.
Cell culture.
The macrophage cell line RAW264.7 was cultured in IMDM supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (complete IMDM) at 37°C under 5% CO2 humidified air.
Antimycobacterial activity.
The ability of the macrophages to inhibit the growth of M. avium was determined as previously described (20, 52). RAW264.7 macrophages in complete IMDM were plated in 96-well plates at 105 cells/well. After 24 h, the medium was replaced with IMDM without serum or antibiotics (incomplete IMDM). The macrophages were first treated with clonidine and other catecholamines and inhibitors and then immediately infected with M. avium at a 10:1 bacterium/macrophage ratio. On the next day, macrophage monolayers were washed vigorously to remove unphagocytized bacteria and incubated with fresh medium without serum or antibiotics. Clonidine was also added back to agonist-treated cultures. After 5 days of culturing, the macrophages were lysed and the bacteria were radiolabeled by the addition of lysis buffer, which contained incomplete IMDM and 5 μCi of 3H-uracil/ml diluted 1:1 with 1.2% saponin in 7H9 medium. Bacteria were incubated overnight and harvested onto glass fiber filter strips (Brandel, Gaithersburg, Md.) with a cell harvester. Radioactivity incorporated by the released bacteria was quantitated by liquid scintillation spectrometry. The inhibition of growth was calculated by dividing the radioactivity incorporated by bacteria obtained from cultures treated with clonidine and inhibitors by that incorporated by bacteria obtained from cultures not treated with clonidine and inhibitors and multiplying by 100.
Nitric oxide determination.
Nitric oxide production was determined by measurement of the accumulation of nitrite in culture supernatants with the Griess reagent (30).
RNA extraction and Northern blot hybridization.
Total macrophage RNA was isolated by the acid guanidinium thiocyanate-phenol-chloroform method of Chomczynski and Sacchi (13). RNA (15 μg) was separated in 1% formaldehyde-agarose gels and transferred to Hybond N+ membranes (Amersham). Northern blot hybridization was carried out as previously described (40). Gel-purifed insert cDNAs were radiolabeled with 32P-dCTP by the random primer method (High Prime; Roche, Indianapolis, Ind.). Blots that initially hybridized to the iNOS cDNA probe were stripped by boiling in 0.10% sodium dodecyl sulfate and rehybridized with a radiolabeled G3PDH cDNA probe.
Determination of peroxynitrite production.
RAW264.7 cells were plated at 3 × 106 cells/well in 24-well plates and cultured overnight at 37°C in complete IMDM without phenol red. On the following day, the medium was removed and replaced with incomplete IMDM. The cells were treated with 10−7 M clonidine and infected with M. avium at a 20:1 bacterium/macrophage ratio. 123-DHR was then added to the cultures at a concentration of 25 μM. The conversion of 123-DHR to 123-rhodamine was measured at 15, 30, 45, and 60 min by fluorimetric analysis at excitation and emission wavelengths of 485 and 530 nm, respectively, with a Cytofluor II multiwell fluorescence plate reader (PerSpective Biosystems). Fluorescence due to auto-oxidation of 123-DHR was subtracted by determining the fluorescence resulting from 125-DHR in medium without cells and subtracting this value from the measurements. The amount of 123-DHR converted to 123-rhodamine was determined from standard curves of 123-rhodamine.
Detection of nitrotyrosine.
The production of nitrated tyrosine residues was determined by a cellular enzyme-linked immunosorbent assay (ELISA). RAW264.7 cells were plated in 96-well plates at 200,000 cells/well and incubated overnight with M. avium and 10−7 M clonidine. The medium was removed, and the adherent macrophages were fixed with ethanol-acetic acid (95:5) for 1 min and washed with phosphate-buffered saline (PBS). The cells were incubated overnight with 2 μg of mouse antinitrotyrosine monoclonal antibody/well. The cells were washed with PBS, incubated with biotinylated goat anti-mouse immunoglobulin G for 30 min, and then incubated with ExtrAvidin-alkaline phosphatase conjugate for an additional 30 min. After the cells were washed in PBS, p-nitrophenyl phosphate substrate was added, and the reaction was read at 405 nm.
Statistical analysis.
Results were analyzed by a one-way analysis of variance (ANOVA) with SigmaSTAT (SPSS Science, Chicago, Ill.).
RESULTS
Clonidine increases resistance to mycobacterial growth.
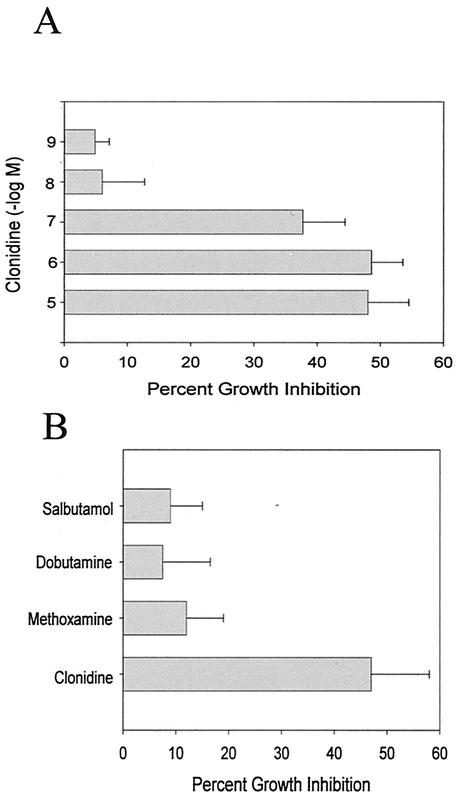
The results shown in Fig. 1A indicate that treatment of RAW264.7 macrophages with clonidine, an α2-agonist, inhibited the growth of M. avium in a dose-dependent manner. In this experiment, RAW264.7 cells were treated with clonidine and immediately infected with M. avium. After overnight incubation, the macrophage monolayers were washed to remove unphagocytized bacteria, and the medium was replaced with fresh medium containing clonidine. After 5 days of culturing, the cells were lysed and bacterial growth was determined by 3H-uracil incorporation. The data are expressed as the percent inhibition of mycobacterial growth by clonidine treatment. The effect of clonidine on mycobacterial growth in RAW264.7 macrophages was equivalent to that reported previously for resident peritoneal macrophages (50). The ability of catecholamines to increase resistance to mycobacterial growth is restricted to α2-adrenergic stimulation, as treatment with methoxamine (α1-agonist), dobutamine (β1-agonist), and salbutamol (β2-agonist) had no effect (Fig. 1B).
FIG. 1.
Effect of α2-adrenergic stimulation on the antimycobacterial activity of RAW264.7 cells. (A) RAW264.7 cells were treated with the α2-agonist clonidine and immediately infected with M. avium. After 5 days of culturing, cells were lysed and bacterial growth was assessed by overnight incubation with 3H-uracil. The data are expressed as the percent mycobacterial growth inhibition by clonidine, determined by dividing the radioactivity incorporated by bacteria in cultures treated with clonidine by the radioactivity incorporated by bacteria in cultures not treated with clonidine and multiplying by 100. The data represent the mean and standard error of four separate experiments. The effect of clonidine was significant, as determined by an ANOVA (P < 0.001). (B) The antimycobacterial activity of RAW264.7 macrophages was stimulated only by α2-agonist stimulation. RAW264.7 macrophages were treated as described above with 10−7 M salbutamol (β2-agonist), dobutamine (β1-agonist), methoxamine (α1-agonist), and clonidine (α2-agonist). The data represent the mean and standard error of three separate experiments. Only clonidine significantly increased antimycobacterial activity, as determined by an ANOVA (P < 0.001).
Increased resistance to mycobacterial growth induced by clonidine requires activation of Gi proteins.
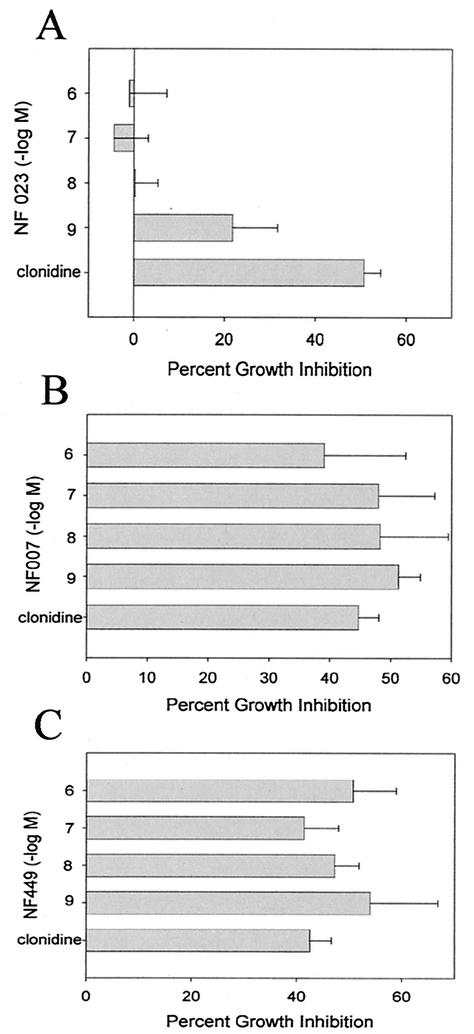
α2-Adrenergic receptors primarily couple to the Gi family of G proteins. To determine whether signaling through Gi proteins is required for the increased resistance to mycobacterial growth induced by clonidine, macrophages were treated with 10−7 M clonidine in the presence of G-protein antagonists and then infected with M. avium. After 5 days of culturing, the effect on mycobacterial growth was determined by 3H-uracil incorporation. As shown in Fig. 2, NF023, which is a selective G-protein antagonist specific for the α subunits of the Go/Gi family (25), blocked the increased resistance to mycobacterial growth of clonidine-stimulated macrophages, while NF007, an inactive form of NF023, and NF449, which is an antagonist specific for the α subunits of the Gs family (33), had no effect.
FIG. 2.
Effect of G-protein antagonists on the increased resistance to mycobacterial growth induced by clonidine. RAW264.7 cells were treated as described in the legend to Fig. 1 with 10−7 M clonidine in the presence of the G-protein antagonists NF023, which is specific for the α subunits of the Go/Gi family; NF007, which is an inactive form of NF023; and NF449, which is specific for the α subunits of the Gs family. The data represent the mean and standard error of five experiments. Only NF023 significantly inhibited the increase in antimycobacterial activity induced by clonidine, as determined by an ANOVA (P < 0.001).
Increased resistance to mycobacterial growth induced by α2-adrenergic stimulation requires both nitric oxide production and superoxide production.
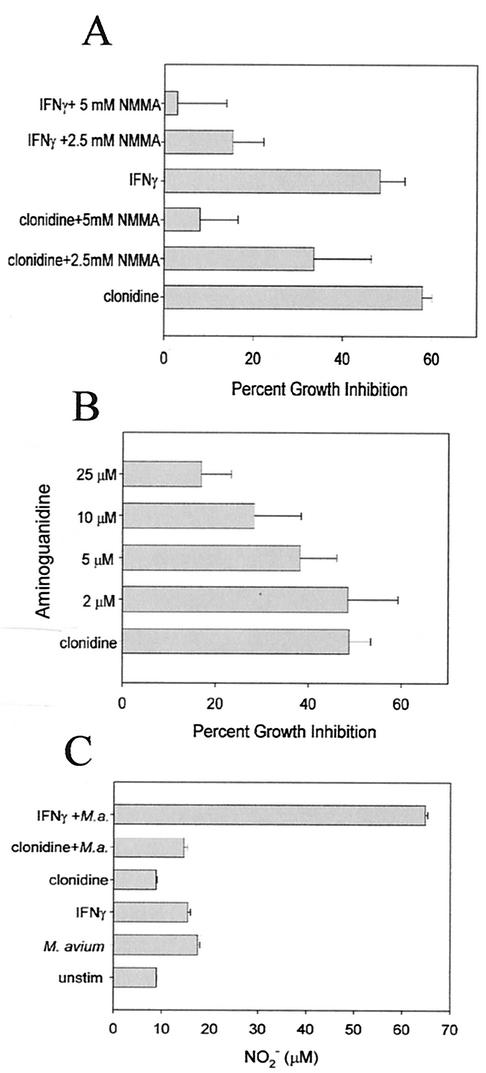
Nitric oxide has been shown to be a factor in the resistance of IFN-γ-activated macrophages to the growth of mycobacteria (8, 22, 56). To determine whether nitric oxide production is also a factor in the increased resistance induced by clonidine, we treated RAW264.7 macrophages with clonidine or IFN-γ in the presence of l-NMMA and aminoguanidine, competitive inhibitors of nitric oxide synthase. After infection with M. avium for 5 days, the effect on mycobacterial growth was assessed by 3H-uracil incorporation. Both l-NMMA (Fig. 3A) and aminoguanidine (Fig. 3B) inhibited the increased resistance to mycobacterial growth induced by clonidine and IFN-γ. Although this result suggests that nitric oxide production is required for the increased resistance to mycobacterial growth of clonidine-stimulated macrophages, clonidine alone did not induce the production of nitric oxide or increase the amount of nitric oxide produced in M. avium-infected macrophages, as determined by a nitric oxide assay of supernatants from 24-h cultures (Fig. 3C). Clonidine also did not increase the level of iNOS mRNA in M. avium-infected macrophages (data not shown).
FIG. 3.
Increased resistance to mycobacterial growth induced by clonidine requires nitric oxide production. (A) RAW264.7 cells were treated with IFN-γ (100 U/ml) or 10−7 M clonidine and the indicated concentrations of l-NMMA, and the effect on mycobacterial growth was determined. The data represent the mean and standard error of six separate experiments. l-NMMA significantly inhibited the increased antimycobacterial activity induced by clonidine and IFN-γ, as determined by an ANOVA (P < 0.001). (B) RAW264.7 cells were treated with 10−7 M clonidine and the indicated concentrations of aminoguanidine, and the effect on mycobacterial growth was determined. The data represent the mean and standard error of six separate experiments. Aminoguanidine significantly inhibited the increased antimycobacterial activity induced by clonidine, as determined by an ANOVA (P = 0.008). (C) NO production by RAW264.7 macrophages was stimulated with IFN-γ (100 U/ml) and 10−7 M clonidine, and cellswere infected with M. avium (M.a.) (bacterium/macrophage ratio, 10:1) for 24 h. Supernatants were collected and assayed for NO production by the Griess reaction. unstim, unstimulated. Results are representative of three independent experiments (mean and standard error). IFN-γ but not clonidine significantly increased NO production in M. avium-infected cells, as determined by an ANOVA (P < 0.0001).
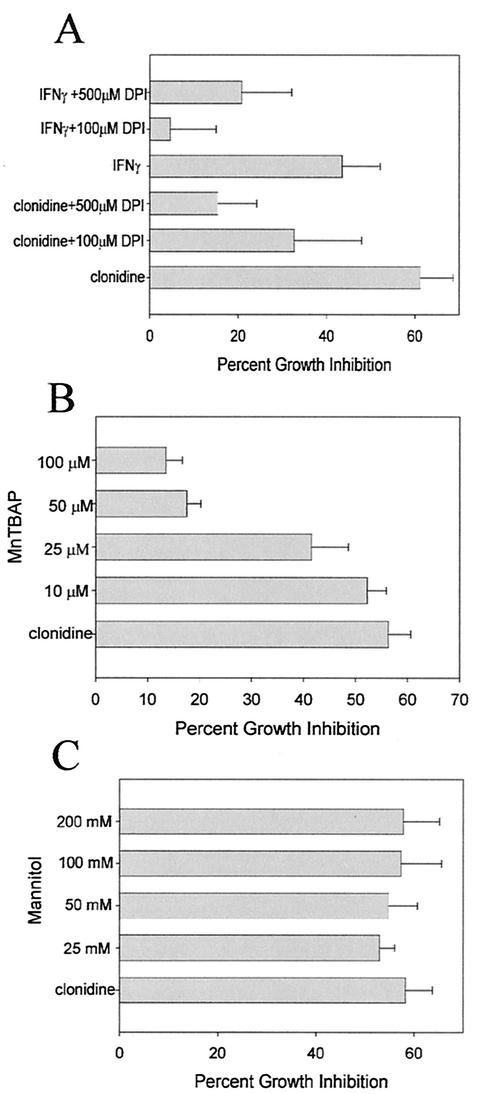
Macrophages also produce superoxide and other reactive oxygen intermediates by NADPH oxidase activation. To determine whether superoxide production is also a factor in the α2-agonist-induced increased resistance to mycobacterial growth, we treated macrophages with clonidine in the presence of DPI, an inhibitor of NADPH oxidase, and MnTBAP, a cell-permeating superoxide dismutase (SOD) mimetic agent which acts as a scavenger of superoxide (19). Macrophages were then infected with M. avium. After 5 days of culturing, the effect on mycobacterial growth was determined by 3H-uracil incorporation. Both DPI (Fig. 4A) and MnTBAP (Fig. 4B) inhibited the increased resistance to mycobacterial growth induced by clonidine, indicating that superoxide production is also required for the effect of clonidine. Another reactive oxygen species that has antimycobacterial activity is the hydroxyl radical, which is produced in phagosomes by the iron-catalyzed reaction of superoxide with H2O2. However, treatment with clonidine in the presence of mannitol, a scavenger of the hydroxyl radical, had no effect on the increased resistance to mycobacterial growth induced by clonidine (Fig. 4C).
FIG. 4.
Increased resistance to mycobacterial growth induced by clonidine requires superoxide but not hydroxyl radical production. (A) RAW264.7 cells were treated with IFN-γ (100 U/ml) or 10−7 M clonidine and the NADPH oxidase inhibitor DPI at 100 and 500 μM. The effect on mycobacterial growth was determined by 3H-uracil incorporation. The data represent the mean and standard error of six separate experiments. DPI significantly inhibited the increased antimycobacterial activity induced by clonidine, as determined by an ANOVA (P = 0.037), and IFN-γ, as determined by an ANOVA (P = 0.045). (B) RAW264.7 cells were treated with 10−7 M clonidine and the indicated concentrations of the superoxide scavenger MnTBAP. The effect on mycobacterial growth was determined by 3H-uracil incorporation. The data represent the mean and standard error of six separate experiments. MnTBAP significantly inhibited the increasedantimycobacterial activity induced by clonidine, as determined by an ANOVA (P < 0.001). (C) RAW264.7 macrophages were treated with 10−7 M clonidine and the indicated concentrations of mannitol, a hydroxyl radical scavenger, and the effect on mycobacterial growth was determined. The data represent the mean and standard error of five separate experiments.
A scavenger of peroxynitrite inhibits increased resistance to mycobacterial growth of clonidine-stimulated macrophages.
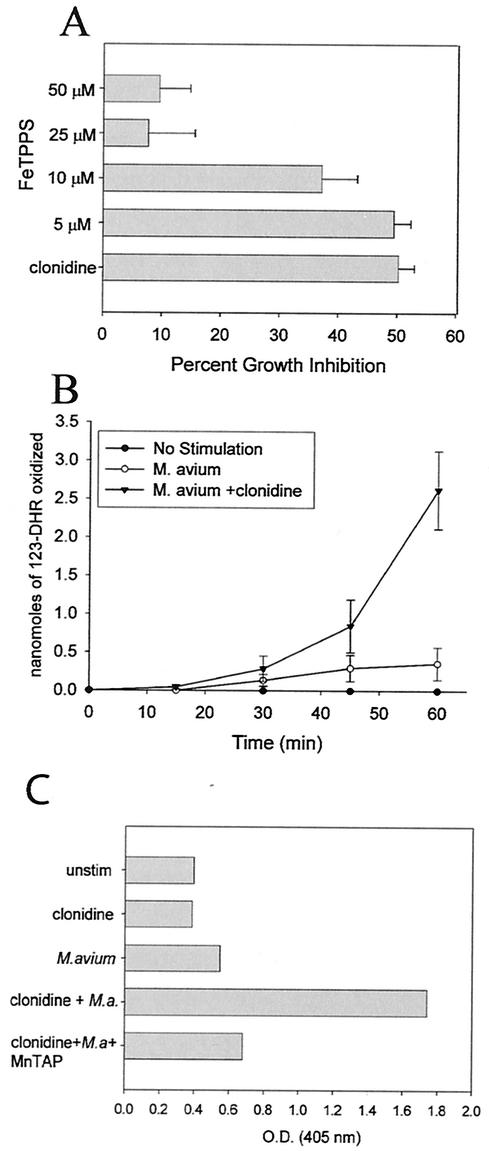
Superoxide is removed from cells by the enzyme SOD. However, nitric oxide competes with SOD for superoxide, forming peroxynitrite (ONOO−), which is a powerful oxidant. To determine whether peroxynitrite is involved in the resistance to mycobacteria induced by clonidine, RAW264.7 macrophages were treated with clonidine in the presence of FeTPPS, a specific scavenger of peroxynitrite with minimal SOD activity (51), and then infected with M. avium. The effect on mycobacterial growth was assessed after 5 days of culturing by 3H-uracil incorporation. As shown in Fig. 5A, FeTPPS inhibited the increased resistance to mycobacterial growth induced by both clonidine and IFN-γ.
FIG. 5.
Increased resistance to mycobacterial growth induced by clonidine requires peroxynitrite production. (A) RAW264.7 cells were treated with 10−7 M clonidine and the indicated concentrations of FeTPPS, a peroxynitrite scavenger, and the effect on mycobacterial growth was determined. The data represent the mean and standard error of five separate experiments. FeTPPS significantly inhibited the increased antimycobacterial activity induced by clonidine, as determined by an ANOVA (P < 0.001). (B) Clonidine increased the production of peroxynitrite. RAW264.7 cells were treated with 10−7 M clonidine and infected with M. avium. The production of peroxynitrite was determined by the oxidation of 123-DHR to 123-rhodamine. Thedata represent the mean and standard error of three separate experiments. Clonidine significantly increased the production of peroxynitrite, as determined by an ANOVA (P < 0.001). This production of peroxynitrite was completely inhibited by the inclusion of 300 μM apocynin or 1 mM aminoguanidine in the culture media (data not shown). (C) Clonidine increased the formation of nitrotyrosine. RAW264.7 cells were treated with 10−7 M clonidine and MnTBAP and infected with M. avium (M.a.). After overnight incubation, the nitration of tyrosines was determined by a cellular ELISA with a monoclonal antibody to nitrotyrosine. unstim, unstimulated; O.D., optical density. Results are representative of three experiments.
To confirm the role of peroxynitrite, we also measured the production of peroxynitrite by determining the oxidation of 123-DHR to 123-rhodamine by peroxynitrite (52). RAW264.7 cells were treated with clonidine and infected with M. avium. After the addition of 123-DHR, the production of 123-rhodamine was measured at various times over the next hour by fluorimetric analysis. As shown in Fig. 5B, M. avium infection of RAW264.7 macrophages induced the production of peroxynitrite. Infection with M. avium in the presence of 10−7 M clonidine substantially increased the production of peroxynitrite, while clonidine alone had no effect. To show that the oxidation of 123-DHR is specific for peroxynitrite, macrophages were stimulated with M. avium and clonidine in the presence of apocynin, an NADPH oxidase inhibitor (61), and aminoguanidine, a competitive inhibitor of nitric oxide. Both apocynin and aminoguanidine, when added separately, completely inhibited the oxidation of 123-DHR, confirming that the production of both nitric oxide and superoxide is required for the reaction (data not shown).
Since peroxynitrite reacts with proteins to form nitrated tyrosine residues (5), we also determined whether clonidine increased the production of nitrotryosine in M. avium-infected RAW264.7 cells. Infection with M. avium alone only marginally increased the production of nitrotyrosine, as detected by a cellular ELISA (Fig. 5C). The addition of clonidine resulted in a threefold increase in nitrotyrosine production. This increase in nitrotyrosine production was blocked by scavenging of superoxide with MnTBAP.
DISCUSSION
One of the factors controlling mycobacterial infections is the ability of macrophages to restrict the growth of intracellular bacteria. The ability of macrophages to restrict mycobacterial growth is greatly enhanced by IFN-γ produced by T cells and NK cells (8, 22, 56). Our investigations showed that catecholamines can also stimulate macrophage antimycobacterial activity. This stimulation was mediated via the α2-adrenergic receptor. Only the α2-agonist clonidine stimulated the antimycobacterial activity of RAW264.7 cells; α1-, β1-, and β2-agonists had no effect. These results confirm those of a previous study (50) in which it was shown that epinephrine stimulated the antimycobacterial activity of mouse peritoneal macrophages by binding to α2-adrenergic receptors.
The three main mechanisms by which activated macrophages restrict the growth of intracellular pathogens are the production of superoxide anions by NADPH oxidase, the production of NO by nitric oxide synthase, and the production of hydroxyl radicals via Fenton-Haber-Weiss reactions. We investigated which of these mechanisms was activated during clonidine stimulation of antimycobacterial activity. Our results showed that both superoxide production and NO production were required for the clonidine stimulation of antimycobacterial activity, while the production of hydroxyl radicals was not required. The requirement for superoxide anions was demonstrated by blocking increased antimycobacterial activity with the superoxide scavenger MnTBAP and inhibiting NAPDH oxidase activity with DPI. The requirement for NO production was demonstrated by inhibiting nitric oxide synthase with l-NMMA and aminoguanidine. Although NO production was required, clonidine did not increase NO levels above those found in infected cells alone. In contrast, IFN-γ dramatically increased the production of NO. NO and superoxide can react to form the potent oxidant peroxynitrite, which is highly reactive with biomolecules, resulting in oxidation and protein tyrosine nitration (5). We therefore examined whether peroxynitrite was produced following clonidine stimulation and was required for antimycobacterial activity. We found that the peroxynitrite scavenger FeTPPS inhibited the increased antimycobacterial activity induced by clonidine. Further, we showed that clonidine increased peroxynitrite production and protein tyrosine nitration. Thus, our results suggested that peroxynitrite production is responsible for the antimycobacterial activity of macrophages induced by α2-adrenergic stimulation.
This combination of NO- and superoxide anion-generating systems was previously shown to have a synergistic effect on the killing of microorganisms (18). Peroxynitrite has been shown to be better than NO at killing Escherichia coli (10), Salmonella enterica serovar Typhimurium (17), Mycoplasma pulmonis (32), Candida albicans (64), Plasmodium falciparum (27), and Rhodococcus equi (16). For other organisms, including Leishmania major (2), Giardia lamblia (21), and Cryptococcus neoformans (62), NO appears to be more toxic than peroxynitrite. Avirulent strains of M. bovis and M. smegmatis were found to be more susceptible than virulent strains of M. bovis and M. tuberculosis to peroxynitrite in an in vitro assay (66). Our in vivo studies with M. avium indicated that peroxynitrite does contribute to the resistance of macrophages to the growth of M. avium.
Through molecular cloning, three subtypes of α2-adrenergic receptors (α2A, α2B, and α2C) that differ in tissue expression have been described (39, 45, 54). Although the pattern of expression of these α2 subtypes in macrophages is not known, the presence of α2-adrenergic receptors on macrophages has been demonstrated by receptor binding of the α2-adrenergic antagonist yohimbine in a saturable and reversible manner (59). In most cells, α2-adrenergic receptors couple to Gi-protein α subunits. Our studies showed that increased resistance to mycobacterial growth requires signaling through Gi-protein α subunits, as a specific antagonist of the Go/Gi family blocked the increased resistance to mycobacterial growth induced by clonidine, while an antagonist of Gs-protein α subunits had no effect. α2-Adrenergic stimulation inhibits adenylyl cyclase (15, 44) and activates phospholipase C (15, 29), phospholipase D (PLD) (36, 47), and mitogen-activated protein (MAP) kinases (1, 23, 57). Which of these pathways is responsible for the increased production of peroxynitrite needs to be determined.
We also do not yet know the step in the synthesis of peroxynitrite affected by clonidine. However, our studies showed that it is not at the level of NO production. One possibility is that clonidine affects the production of superoxide anions. Superoxide is produced by NADPH oxidase via the reduction of molecular oxygen (4). The activation of NADPH oxidase by mycobacteria and the significance for controlling mycobacterial growth are controversial. Infection of mouse peritoneal macrophages with M. intracelulare was shown to result in the production of superoxide anions (28), but infection of differentiated U937 macrophages with M. kansasii did not trigger the production of superoxide anions (43). This difference was most likely due to differences in cell surface receptors that trigger superoxide production, such as Toll-like receptor 2, which is not expressed in human U937 cells but is expressed in mouse macrophages (11). Mycobacteria also have the means to evade the effects of reactive oxygen species (reviewed in reference 24). However, mice deficient in NADPH oxidase component p47phox have a reduced ability to control M. tuberculosis growth early in infection (14). Also, individuals with chronic granulomatous disease are more susceptible to tuberculosis (42). Thus, these studies support a role for NADPH oxidase in resistance to mycobacteria.
NADPH oxidase is a multiprotein complex consisting of membrane-bound cytochrome b558 and cytosolic p40phox, p47phox, p67phox, and Rac1 proteins. The activation of NADPH oxidase by stimuli results in the phosphorylation of p47phox and the migration of p40phox, p47phox, p67phox, and Rac1 proteins from the cytosol to the membrane, where they interact with cytochrome b558 to form an active enzymatic complex (34, 37). Thus, α2-adrenergic receptor signaling could increase the phosphorylation of p47phox and the migration of cytosolic proteins. This effect could occur through MAP kinase or PLD activation by the adrenergic receptor, as both MAP kinases and PLD have been shown to be involved in activating NADPH oxidase (41, 49). Although we did not measure superoxide production, we found that clonidine did not enhance the production of H2O2 by M. avium-infected mouse peritoneal macrophages (unpublished observations). H2O2 is formed by the dismutation of superoxide anions, and the level of H2O2 in macrophages is an indirect measure of superoxide production. This reaction is catalyzed by SOD, which exists in both cytoplasmic (Cu/Zn-SOD) and mitochondrial (Mn-SOD) forms (26). Since NO would be expected to compete with SOD for superoxide, clonidine could also favor the production of ONOO− by inhibiting SOD activity. Cu/Zn-SOD and Mn-SOD isolated in the presence of H2O2 have also been shown to break down NO into ONOO− (48). Clonidine could thus increase the ONOO− levels by increasing this enzymatic activity.
Studies have shown that catecholamines can either stimulate or inhibit macrophage function, depending on whether α2-adrenergic receptors or β2-adrenergic receptors are activated. A previous study (50) with peritoneal macrophages and this study with RAW264.7 cells found that catecholamines increase antimycobacterial activity via the stimulation of α2-adrenergic receptors. Spengler et al. (59, 60) also found, by using peritoneal macrophages, that the stimulation of α2-adrenergic receptors increases lipopolysaccharide stimulation of TNF-α production. In contrast, the stimulation of β2-adrenergic receptors inhibits macrophage function. Boomershine et al. found that catecholamine stimulation of IFN-γ-activated macrophages inhibits antimycobacterial activity and NO production (9). Spengler et al. (60) also reported that β2-adrenergic stimulation decreases lipopolysaccharide-induced TNF-α production. Similar effects of β2-adrenergic stimulation on the TNF-α production of human monocytes and the THP-1 monocytic cell line have been reported (58, 63). Others have reported that catecholamine stimulation of RAW264.7 macrophages through β2-adrenergic receptors inhibits the production of the chemokine macrophage inflammatory protein 1α (31) and increases macrophage arginase activity (7) and IL-10 production (12). These differences in responses to catecholamines may be due to the activation status of macrophages. For example, our study and the studies of Spengler et al. (59, 60) suggest that naive macrophages may be able to respond through both α2- and β2-adrenergic receptors, while in IFN-γ-activated macrophages, β2-adrenergic responses predominate. These findings could be due to changes in the expression of adrenergic receptors upon activation or through the induction of RGS (regulator of G-protein signaling) proteins. RGS proteins represent a large family of proteins that inhibit signaling by acting as GTPase-activating proteins for G-protein α subunits of the G0/Gi family and thus accelerating the rate of inactivation of GTP-bound G-protein α subunits (6, 55).
The results of this study add support to the view that catecholamines released as the sympathetic response to stress can act as macrophage activators for antimycobacterial activity. This activation of macrophages results from the α2-adrenergic modulation of the level of peroxynitrite produced by infected macrophages. The results also suggest that the sympathetic stress response can have a beneficial effect by enhancing the innate response to infection. However, once an acquired immune response is mounted and IFN-γ is produced, the sympathetic stress response acting through β2-adrenergic receptors inhibits the antimycobacterial activity of IFN-γ-activated macrophages, thus accounting for studies which show that stress limits the response to infection.
Acknowledgments
This work was supported by grants AI-42901, DK-57667, and HL-59795 from the National Institutes of Health to B.S.Z. and W.P.L.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alblas, J., E. J. van Corven, P. L. Hordijk, G. Milligan, and W. H. Moolenaar. 1993. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by α2-adrenergic receptors expressed in fibroblasts. J. Biol. Chem. 268:22235-22238. [PubMed] [Google Scholar]
- 2.Assreuy, J., F. Q. Cunha, M. Epperlein, A. Noronha-Dutra, C. A. O'Donnell, F. Y. Liew, and S. Moncada. 1994. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur. J. Immunol. 24:672-676. [DOI] [PubMed] [Google Scholar]
- 3.Autelitano, D. J., J. R. Lundblad, M. Blum, and J. L. Roberts. 1989. Hormonal regulation of POMC gene expression. Annu. Rev. Physiol. 51:715-726. [DOI] [PubMed] [Google Scholar]
- 4.Babior, B. M. 1995. The respiratory burst oxidase. Curr. Opin. Hematol. 2:55-60. [DOI] [PubMed] [Google Scholar]
- 5.Beckman, J. S., and W. H. Koppenol. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 40:C1424-C1437. [DOI] [PubMed] [Google Scholar]
- 6.Berman, D. M., and A. G. Gilman. 1998. Mammalian RGS proteins: barbarians at the gate. J. Biol. Chem. 273:1269-1272. [DOI] [PubMed] [Google Scholar]
- 7.Bernard, A. C., E. A. Fitzpatrick, M. E. Maley, G. L. Gellin, B. J. Tsuei, W. A. Arden, B. R. Boulanger, P. A. Kearney, and J. O. Ocha. 2000. Beta adrenoreceptor regulation of macrophage arginase activity. Surgery 127:412-418. [DOI] [PubMed] [Google Scholar]
- 8.Bonecini-Almeida, M. G., S. Chitale, I. Boutsikakis, J. Y. Geng, H. Doo, S. H. He, and J. L. Ho. 1998. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J. Immunol. 160:4490-4499. [PubMed] [Google Scholar]
- 9.Boomershine, C. S., W. P. Lafuse, and B. S. Zwilling. 1999. β-2-Adrenergic receptor stimulation inhibits nitric oxide generation by Mycobacterium avium infected macrophages. J. Neuroimmunol. 101:68-75. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli, L., J. P. Crow, and J. S. Beckman. 1995. The compartive toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch. Biochem. Biophys. 316:327-334. [DOI] [PubMed] [Google Scholar]
- 11.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H.-C. Reinecker, and D. K. Podolsky. 2000. Lipolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 12.Chen, L., C. Boomershine, T. Wang, W. P. Lafuse, and B. S. Zwilling. 1999. Synergistic interaction of catecholamine hormones and Mycobacterium avium results in the induction of interleukin-10 mRNA by murine peritoneal macrophages. J. Neuroimmunol. 93:149-155. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, A. M., B. H. Segal, A. A. Frank, S. M. Holland, and I. M. Orme. 2000. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect. Immun. 68:1231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotecchia, S., B. K. Kobilka, K. W. Daniel, R. D. Nolan, E. Y. Lapetina, M. G. Caron, R. J. Lefkowitz, and J. W. Regen. 1990. Multiple second messenger pathways of α-adrenergic receptor subtypes expressed in eukaryotic cells. J. Biol. Chem. 265:63-69. [PubMed] [Google Scholar]
- 16.Darrah, P. A., M. K. Hondalus, Q. Chen, H. Ischropoulos, and D. Mosser. 2000. Cooperation between reactive oxygen species and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect. Immun. 3587-3593. [DOI] [PMC free article] [PubMed]
- 17.DeGrote, M. A., D. Granger, Y. Xu, G. Cambell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang, F. C. 1997. Mechanisms of nitric oxide-related anti-mycobacterial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faulkner, K. M., S. I. Liochev, and I. Fridovich. 1994. Stable Mn (III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 269:23471-23746. [PubMed] [Google Scholar]
- 20.Felten, D. L., S. Y. Felten, D. L. Bellinger, S. L. Carlson, K. D. Ackerman, K. S. Madden, J. A. Olschowki, and S. Livant. 1987. Noradrenergic sympathetic neural interactions with the immune response: structure and function. Immunol. Rev. 100:225-260. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes, P. D., and J. Assreuy. 1997. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz. J. Med. Biol. Res. 30:93-99. [DOI] [PubMed] [Google Scholar]
- 22.Flesch, I. E. A., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flordellis, C. S., M. Berguerand, P. Gouache, Vér. Barbu, H. Gavras, D. E. Handy, G. Béréziat, and J. ël. Masliah. 1995. α2-Adrenergic receptor subtypes expressed in Chinese hamster ovary cells activate differentially mitogen-activated protein kinase by a p21ras independent pathway. J. Biol. Chem. 270:3491-3494. [DOI] [PubMed] [Google Scholar]
- 24.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 25.Freissmuth, M., S. Boehm, W. Beindl, P. Nickel, P. Ijzerman, M. Hohenegger, and C. Nanoff. 1996. Suramin analogues as subtype-selective G protein inhibitors. Mol. Pharmacol. 49:602-611. [PubMed] [Google Scholar]
- 26.Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64:97-112. [DOI] [PubMed] [Google Scholar]
- 27.Fritsche, G., C. Larcher, H. Schennach, H., and G. Wess. 2001. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J. Infect. Dis. 183:1388-1394. [DOI] [PubMed] [Google Scholar]
- 28.Gangadharam, P. R. J., and C. K. Edwards III. 1984. Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am. Rev. Respir. Dis. 130:834-838. [DOI] [PubMed] [Google Scholar]
- 29.Gesek, F. A. 1996. α2-Adrenergic receptors activate phospholipase C in renal epithelial cells. Mol. Pharmacol. 50:407-414. [PubMed] [Google Scholar]
- 30.Guevara, I., J. Iwanejko, A. Dembiñska-Kieæ, J. Pankiewicz, A. Wanat, A., P. Anna, I. Golabek, S. Bartuí, M. Malczewska-Malec, and A. Szczudlik. 1998. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 274:177-188. [DOI] [PubMed] [Google Scholar]
- 31.Hasko, G., T. P. Shanley, G. Egnaczyk, Z. H. Nemeth, A. L. Salzman, E. Sylvester, and C. Szabo. 1998. Exongenous and endogenous catecholamines inhibit the production of macrophage inflammatory protein (MIP) 1α via a β adrenoreceptor mediated mechanism. Br. J. Pharmacol. 125:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickman-Davis, J., J. Gibbs-Erwin, J. R. Lindsey, and S. Matalon. 1999. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl. Acad. Sci. USA 96:4953-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohenegger, M., M. Waldhoer, W. Beindl, B. Böing, A. Kreimeyer, P. Nickel, C. Nanoff, and M. Freissmuth. 1998. Gsα-selective G protein antagonists. Proc. Natl. Acad. Sci. USA 95:346-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inanami, O., J. L. Johnson, J. K. McAdara, J. El Bena, L. P. Faust, P. E. Newburger, and B. M. Babior. 1998. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 273:9539-9543. [DOI] [PubMed] [Google Scholar]
- 35.James, D. E., and F. P. Nijkamp. 2000. Neuroendocrine and immune interactions with airway macrophages. Inflammation Res. 49:254-265. [DOI] [PubMed] [Google Scholar]
- 36.Jinsi-Parimoo, A., and R. C. Deth. 2000. Protein kinase C-dependent coupling of α(2A/D)-adrenergic receptors to phospholipase D. Pharmacology 60:19-26. [DOI] [PubMed] [Google Scholar]
- 37.Johnson, J. L., J.-W. Park, J. El Benna, L. P. Faust, O. Inanami, and B. M. Babior. 1998. Activation of p47PHOX, a cytosolic subunit of the leukocyte NADPH oxidase. J. Biol. Chem. 273:35147-35152. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 39.Kobilka, B. K., H. Matsui, T. S. Kobilka, U. Francke, M. G. Caron, R. J. Lefkowitz, and J. W. Regan. 1987. Cloning, sequencing, and expression of the gene coding for the human platelet α2-adrenergic receptor. Science 238:650-656. [DOI] [PubMed] [Google Scholar]
- 40.Lafuse, W. P., D. Brown, L. Castle, and B. S. Zwilling. 1995. Cloning and characterization of a novel cDNA that is IFN-γ-induced in mouse peritoneal macrophages and encodes a putative GTP-binding protein. J. Leukoc. Biol. 57:477-482. [DOI] [PubMed] [Google Scholar]
- 41.Lal, A. S., A. D. Clifton, J. Rouse, A. W. Segal, and P. Cohen. 1999. Activation of the neutrophil NADPH oxidase is inhibited by SB 203580, a specific inhibitor of SAPK2/p38. Biochem. Biophys. Res. Commun. 259:465-470. [DOI] [PubMed] [Google Scholar]
- 42.Lau, Y. L., G. C. Chan, S. Y. Ha, Y. F. Hui, and K. Y. Yuen. 1998. The role of the phagocytic respiratory burst in host defense against Mycobacterium tuberculosis. Clin. Infect. Dis. 26:226-227. [DOI] [PubMed] [Google Scholar]
- 43.Le Cabec, V., C. Cols, and I. Mardionneau-Parini. 2000. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect. Immun. 68:4736-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limbird, L. E. 1988. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanism. FASEB J. 2:2686-2695. [DOI] [PubMed] [Google Scholar]
- 45.Lomasney, J. W., W. Lorenz, L. F. Allen, K. King, J. W. Regan, T. L. Yang-Feng, M. Brownstein, R. J. Lefkowitz, and M. G. Caron. 1990. Expansion of the α2-adrenergic receptor family: cloning and characterization of a human α2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc. Natl. Acad. Sci. USA 87:5094-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacMicking, J., Q.-W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 47.MacNulty, E. E., S. J. McClue, I. C. Carr, T. Jess, M. J. O. Wakelam, and G. Milligan. 1992. α2-C10 Adrenergic receptors expressed in rat 1 fibroblasts can regulate both adenylylcyclase and phosholipase D-mediated hydrolysis of phosphatidylcholine by interacting with pertussis toxin-sensitive guanine nucleotide-binding proteins. J. Biol. Chem. 267:2149-2156. [PubMed] [Google Scholar]
- 48.McBride, A. G., V. Borutaite, and G. C. Brown. 1999. Superoxide dismutase and hydrogen peroxide cause rapid nitric oxide breakdown, peroxynitrite production and subsequent cell death. Biochim. Biophys. Acta 1454:275-288. [DOI] [PubMed] [Google Scholar]
- 49.McPhail, L. C., K. A. Waite, D. S. Regier, J. B. Nixon, D. Qualliotine-Mann, W.-X. Zhang, R. Wallin, and S. Sergeant. 1999. A novel protein kinase target for the lipid second messenger phosphatidic acid. Biochim. Biophys. Acta 1439:277-290. [DOI] [PubMed] [Google Scholar]
- 50.Miles, B. A., W. P. Lafuse, and B. S. Zwilling. 1996. Binding of α-adrenergic receptors stimulates the anti-mycobacterial activity of murine peritoneal macrophages. J. Neuroimmunol. 71:19-24. [DOI] [PubMed] [Google Scholar]
- 51.Misko, T. P., M. K. Highkin, A. W. Veehuizen, P. T. Manning, M. K. Stern, M. G. Currie, and D. Salvemini. 1998. Characterization of the cyroprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 273:15646-15653. [DOI] [PubMed] [Google Scholar]
- 52.Muijsers, R. B. R., E. van den Worm, G. Folkerts, C. J. Beukelman, A. S. Koster, D. S. Postma, and F. P. Nijkamp. 2000. Apocynin inhibits peroxynitrite formation by murine macrophages. Br. J. Pharmacol. 130:932-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohmori, Y., and T. A. Hamilton. 1994. Regulation of macrophage gene expression by T cell derived lymphokines. Pharmacol. Ther. 63:235-264. [DOI] [PubMed] [Google Scholar]
- 54.Regan, J. W., T. S. Kobilka, T. L. Yang-Feng, M. G. Caron, R. J. Lefkowitz, and B. K. Kobilka. 1988. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc. Natl. Acad. Sci. USA 85:6301-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795-827. [DOI] [PubMed] [Google Scholar]
- 56.Sato, K., T. Akakai, T., and H. Tomioka. 1998. Differential potentiation of anti-mycobacterial activity and reactive nitrogen intermediate-producing ability of murine peritoneal macrophages activated by interferon-gamma and tumor necrosis factor-alpha. Clin. Exp. Immunol. 112:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schramm, N. L., and L. E. Limbird. 1999. Stimulation of mitogen-activated protein kinase by G protein-coupled α2-adrenergic receptors does not require agonist-elicited endocytosis. J. Biol. Chem. 274:24935-24940. [DOI] [PubMed] [Google Scholar]
- 58.Severn, A., N. T. Rapson, C. A. Hunter, and F. Y. Liew. 1992. Regulation of tumor ncerosis factor production by adrenaline and β-adrenergic agonists. J. Immunol. 148:3441-3445. [PubMed] [Google Scholar]
- 59.Spengler, R. N., R. M. Allen, D. G. Remick, R. M. Strieter, and S. L. Kunkel. 1990. Stimulation of α-adrenergic receptor augments the production of macrophage derived tumor necrosis factor. J. Immunol. 145:1430-1434. [PubMed] [Google Scholar]
- 60.Spengler, R. N., S. W. Chensue, D. A. Giacherio, N. Blenk, and S. L. Kunkel. 1994. Endogenous norepinephrine regulates tumor necrosis factor-α production from macrophages in vitro. J. Immunol. 152:3024-3031. [PubMed] [Google Scholar]
- 61.Stolk, J., T. J. Hiltermann, J. H. Dijkman, and A. J. Verhoeven. 1994. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 62.Tohyama, M., K. Kawakami, M. Futenma, and A. Saito. 1996. Enhancing effect of oxygen radical scavengers on murine macrophage anticryptococcal activity through the production of nitric oxide. Clin. Exp. Immunol. 103:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van der Poll, T., J. Jansen, E. Endert, H. P. Sauerwein, and S. J. Van Deventer. 1994. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 62:2046-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasquez-Torres, A. J., J. Jones-Carson, and E. Batlish. 1996. Peroxynitrite contributes to the candidacidal activity of nitric oxide-producing macrophages. Infect. Immun. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vizi, E. S., E. Orso, O. N. Osipenko, G. Hasko, and I. J. Eldenkov. 1995. Neurochemical electrophysical and immunocytochemical evaluation of the noradrenergic link between the sympathetic nervous system and thymocytes. Neuroscience 68:1263-1276. [DOI] [PubMed] [Google Scholar]
- 66.Yu, K., C. Mitchell, Y. Xing, R. S. Maglozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitric oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]
- 67.Zwilling, B. S., D. Brown, N. Feng, J. Sheridan, and D. Pearl. 1993. The effect of adrenalectomy on the restraint stressed induced suppression of MHC class II expression by murine peritoneal macrophages. Brain Behav. Immun. 7:29-35. [DOI] [PubMed] [Google Scholar]