Abstract
Intestinal M cells deliver macromolecules, particles, and pathogens into the subepithelial dome (SED) region of Peyer's patch mucosa, an area rich in dendritic cells (DCs). We tested whether uptake of the mucosal adjuvant cholera toxin (CT) or live Salmonella bacteria can induce DC migration within Peyer's patches. Virus-sized, fluorescent polystyrene microparticles were efficiently transported by M cells and ingested by CD11c+, CD11b−, and CD8a− DCs in the SED region. DCs loaded with microparticles remained in the SED for up to 14 days. CT (but not the CT B subunit) and live attenuated Salmonella enterica serovar Typhimurium bacteria induced migration of the microparticle-loaded DCs from the SED region into underlying B-cell follicles and adjacent parafollicular T-cell zones. Our data provide the first demonstration that DCs move in response to enterotoxin adjuvants and live bacteria that enter the mucosa via M cells.
In the gastrointestinal tract, specialized M cells in the follicle-associated epithelium (FAE) deliver samples of foreign material to organized mucosal lymphoid tissues (15). This local sampling of antigens is believed to be critical to the induction of adaptive mucosal immunity (2), but little is known about the fates of antigens or pathogens transported by M cells. Phagocytic dendritic cells (DCs) are numerous in the subepithelial dome (SED) region underlying the FAE (13, 27). It is likely that most incoming antigens and pathogens are captured by these antigen-presenting cells. Indeed, live attenuated Salmonella enterica serovar Typhimurium (9) bacteria, Listeria monocytogenes bacteria, and microparticles (24) that entered Peyer's patches (PP) after oral administration were taken up by DCs in the SED region.
The paradigm of DC function is their acquisition of antigen in peripheral tissues such as skin as immature DCs and their subsequent maturation and migration via lymphatic or blood vessels to T-cell areas in secondary lymphoid organs, where they interact with naïve T lymphocytes (1). The behavior of DCs in PP may be unique, because the site of initial antigen entry and capture (the SED region) is in close proximity to organized T- and B-cell zones. It has been proposed that luminal antigens transported by M cells and endocytosed by immature DCs in the SED region would be ferried by DCs to the adjacent interfollicular T-cell zones where DC maturation and antigen presentation would occur (10, 13), but this has not been directly demonstrated. In skin, migration of DCs to draining lymph nodes is accelerated and immune responses are enhanced after topical application of cholera toxin (CT) (G. M. Glenn, M. Rao, G. R. Matyas, and C. R. Alving, Letter, Nature 391:851, 1998). CT is a powerful adjuvant when delivered mucosally (5), and CT in the intestinal lumen is rapidly transported by M cells into PP (15). Although CT is known to have multiple effects on PP cells, the possibility that CT or other adjuvants may induce migration of PP DCs has not been tested.
DCs are known to migrate constitutively from the intestinal mucosa and other peripheral tissues into lymphatic vessels and migration can be accelerated by systemic injection of lipopolysaccharide (LPS) (18, 19, 26), but the major source of the intestinal DCs recovered in lymph is likely to be the lamina propria. DC emigration specifically from the PP was detected only recently, by identifying cells in mesenteric lymph nodes (MLN) that had ingested bacteria along with fluorescent particles (24). Recently, a subpopulation of DCs were shown to migrate within the PP mucosa, moving from the SED region to the interfollicular T-cell zones upon systemic administration of Toxoplasma gondii soluble trophozoite antigen (10). Whether specific DC populations move within the PP after M-cell uptake of particles, live pathogens, or immunostimulants such as CT is not known, however.
To follow the movements of PP DCs after uptake of nonliving particles, we exploited the ability of M cells to efficiently transport hydrophobic polystyrene microparticles from the intestinal lumen into PP (23). Water and food were withheld from 8- to 10-week-old, specific pathogen-free BALB/cAnNCr mice (Charles River Laboratories, Wilmington, Mass.) for 3 to 4 h prior to oral administration of 1012 Fluoresbrite Yellow Green microspheres (diameter, 0.2 μm; Polysciences Inc., Warrington, Pa.). The microspheres and all other agents used in these studies were administered in a volume of 0.5 ml of saline solution by using a 1-ml syringe and a disposable 20-g, 1.5-in.-long blunt-ended feeding needle (Popper, New Hyde Park, N.Y.), and mouse chow was returned to the cages approximately 1 h after intragastric administration. At least three mice were included in each treatment group, and multiple sections from at least three distal PP from each mouse were documented. At intervals from 1 h to 14 days after the feeding of the particles, the mice were sacrificed and their PP were harvested, embedded in Tissue-Tek OCT embedding medium (Sakura Finetek, Torrance, Calif.), frozen in liquid-nitrogen-cooled isopentane, and stored at −20°C. Frozen sections (7 μm) were cut using a Leica Cryostat model CM3050 apparatus (Nussloch, Germany) and mounted on Fisher Superfrost microscope slides. To visualize the fluorescent microspheres, sections were temporarily mounted in saline, examined, and photographed. The coverslips were then rapidly removed, and the slides were air dried, fixed in acetone, and stained with biotinylated anti-CD3, B220, CD8α, CD11b, or CD11c (Pharmingen, San Diego, Calif.) for 45 min as described previously (10, 14). After three washes, the slides were incubated with streptavidin-conjugated horseradish peroxidase (Sigma, St. Louis, Mo.) for 30 min, washed, incubated with diaminobenzidine (Sigma) substrate solution, and mounted in Immumount (Dako, Carpenteria, Calif.). DCs were identified by anti-CD11c, since this marker specifically labels mouse PP DCs in both SED and interfollicular T-cell regions (10). Other candidate DC markers were not used, because all had drawbacks for the purposes of this study: for example, CCR6 is expressed by other cell types, DEC-205 is not expressed by DCs in the SED region of mice, and the ability of antibodies against human Langerin to label mouse PP DCs is not established. The results reported below are based on observations that were consistent for all three mice of a given treatment group.
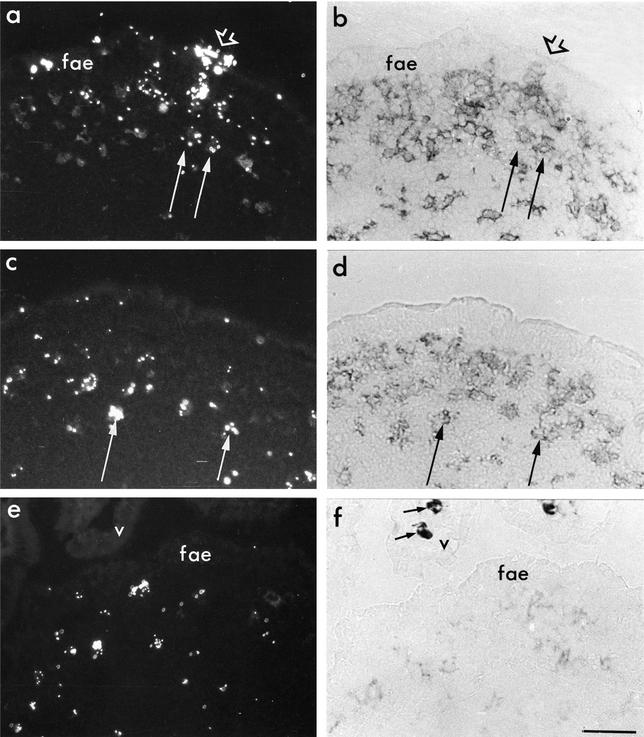
At early time points (1 to 2 h) after feeding, we observed microparticles within the FAE and in some subepithelial cells (Fig. 1a). At later time points (4 h onward), cells containing clusters of microparticles were present in the SED region (shown at 12 and 24 h in Fig. 1e and c, respectively) but not in deeper regions of PP. Through-focusing of sections at high power suggested that microparticle clusters were consistently intracellular (Fig. 2), an observation consistent with previous studies in which DCs were shown to efficiently phagocytose latex microparticles (16). Single microparticles were also present in the SED (Fig. 2), but whether they were intra- or extracellular could not be determined with certainty. No microparticles were observed in the epithelium or lamina propria of villi at any time point (Fig. 1e). All of the SED region cells containing microparticle clusters expressed CD11c (Fig. 1b and d) but little or no CD11b, indicating that they were not macrophages. Macrophages intensely stained with CD11b were present in the lamina propria of adjacent villi (Fig. 1f) and at the serosal margins of follicles and T-cell zones, but these cells contained no microparticles. It was of interest to determine the subtype of the particle-containing cells, because distinct subpopulations of DCs may determine the nature of immune responses in vivo (20, 25). At least three DC subpopulations in PP have previously been described (10, 13, 14) in addition to the follicular DCs (1). CD11c+/CD8α+ DCs are located in the interfollicular T-cell areas but not in the SED region (10). CD11c+/CD11bint DCs are present in the SED region, but the majority of CD11c+ DCs in the SED region of PP are double negative, expressing neither CD11b nor CD8α (10, 11). Intestinal mucosal DC subpopulations differ in their cytokine profiles: CD8α+ and CD11b−/CD8α− DCs produced much higher levels of interleukin-12 p70 in vitro, whereas CD11b+ DCs produced higher levels of interleukin-10 (11). In the mice examined here, the majority of microparticle-containing cells in the SED region were double-negative DCs but a few were CD11c+/CD11bint. Thus, we could not conclude that uptake of microparticles was limited to a specific DC subset in the SED region. The CD8α+ cells of the parafollicular T-cell areas contained no microparticles.
FIG. 1.
Polystyrene microparticles transported into the PP are ingested by DCs in the SED region. BALB/c mice were orally fed 1012 Fluoresbrite polystyrene microparticles and sacrificed at 1 h (a and b), 12 h (e and f), or 24 h (c and d). PP were harvested, cryosectioned, and temporarily mounted in saline to visualize fluorescent microparticles (a, c, and e). Coverslips were then removed and sections were dried, fixed in acetone, and immunostained with anti-CD11c (b and d) or CD11b (f) antibodies. (a and b) At 1 h, microparticles were present at sites in the epithelium that presumably corresponded to M cells and M-cell pockets (short arrows) and in subepithelial CD11c+ cells (long arrows). (c to e) At later times, cells in the subepithelial region that had ingested the fed microparticles were consistently CD11c+ (panel d, long arrow), did not contain high levels of CD11b (f), and were CD8α− (data not shown). Macrophages in neighboring villi, identified by intense CD11b staining (f), contained no microparticles (e). v, villus epithelium; fae, follicle-associated epithelium. Bar, 50 μm.
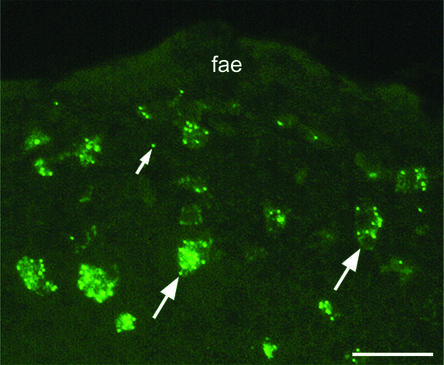
FIG. 2.
At 14 days after feeding, DCs in the SED region below the FAE contained multiple microparticles. Background fluorescence of the DC cytoplasm shows that microparticles lay within the limits of the cell (large arrows). In addition, a few single microparticles (small arrow) are visible in the tissue. Bar, 50 μm.
Unexpectedly, DCs containing microparticles remained in the SED region for up to 14 days after feeding and their numbers did not seem to diminish over time (Fig. 3a). Throughout this time course, all of the microparticle clusters were contained in CD11c+ DCs in the SED region and no microparticle-containing cells were seen in the interfollicular T-cell zones (Fig. 3b), in the B-cell follicles (data not shown), or in adjacent villi (Fig. 1f). In sections of MLN, microparticle-containing cells were rarely seen (data not shown), but we could not rule out the possibility that particle-loaded DCs did move to MLN but were not detected in sections due to their dispersion in the relatively large volume of MLN tissue.
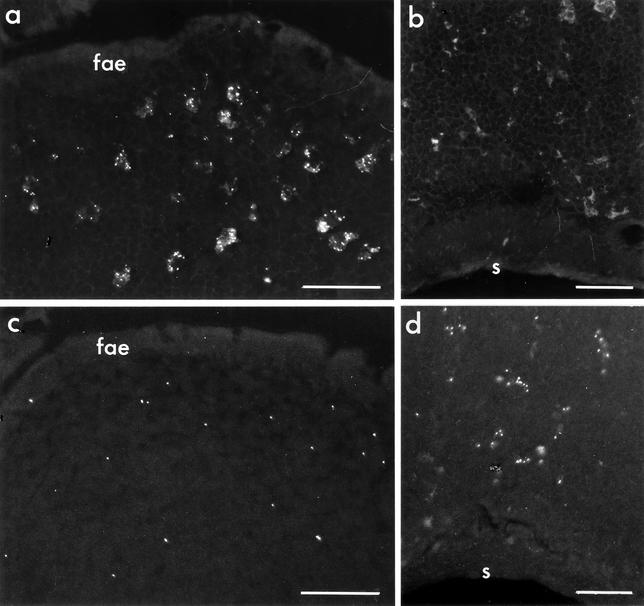
FIG. 3.
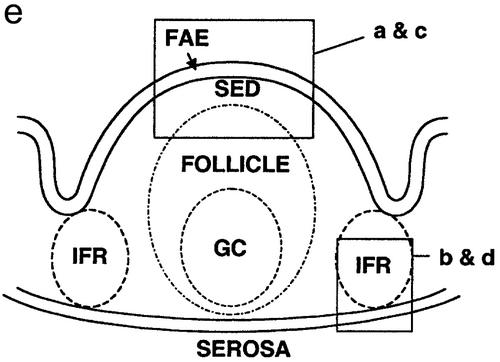
CT stimulates migration of microparticle-containing DCs from the SED to T-cell areas in the PP. BALB/c mice were orally fed 1012 Fluoresbrite polystyrene microparticles as described for Fig. 1. The areas shown in panels a to d are outlined in panel e. (a) The SED region of control mice fed saline contained numerous microparticle-loaded cells from 6 h to day 14. A PP harvested 14 days after feeding is shown here. (b to d) PP of mice fed microparticles on day 0 and then fed either 50 μg of CT or phosphate-buffered saline (PBS) on day 2 and sacrificed 24 h later. (b) In PP of a control mouse fed PBS, interfollicular T-cell regions had no detectable microparticle-containing cells. (c) The SED regions of mice fed CT were largely depleted of microparticle-containing cells. (d) The interfollicular T-cell regions of mice fed CT contained numerous microparticle-loaded cells. Small arrows indicate FAE. s, serosal surface of the intestine. Bars, 50 μm.
The fact that DCs with ingested microparticles remained in the SED region raised the possibility that these cells require an additional signal to initiate migration which the inert particles did not provide (8). We therefore tested the effect of the presence of CT, a strong antigen and powerful mucosal adjuvant (5) that has recently been shown to induce maturation of DCs and expression of migration-associated chemokines but without the inflammatory response evoked by other bacterial products such as LPS (7). The nontoxic B subunit of CT (CTB), which generally lacks adjuvant activity in the gastrointestinal tract, was tested in parallel. We also tested an attenuated strain of S. enterica serovar Typhimurium that is transported into PP (9) and might be expected to affect PP DCs. Certain viruses can also trigger DC maturation and/or migration (17), and DCs can carry viruses such as human immunodeficiency virus to lymph nodes (21). Thus, we expected that reovirus, a mouse pathogen that enters the mucosa exclusively via M cells (29) and induces a vigorous local mucosal immune response (28), might also trigger movement of PP DCs.
To determine whether CT, CTB, or live microorganisms can drive microparticle-loaded DCs from the SED region, mice were first fed microparticles to load and label the SED DCs. Two days later they were fed one of the following: 50 μg of CT holotoxin, 50 μg of CTB (both from Calbiochem, La Jolla, Calif.), live attenuated S. enterica serovar Typhimurium (1010 CFU of a PhoPc mutant [22] kindly provided by John J. Mekalanos, Harvard Medical School), 2 mg of S. enterica serovar Typhimurium LPS (Sigma), or 1011 PFU of reovirus (Type 1 Lang) in 0.5 ml of saline. Control mice received 0.5 ml of saline. Mice were sacrificed 24 h later, and microparticle-containing cells were visualized in frozen sections of PP. The results are summarized in Table 1. In mice fed CT holotoxin, most cells containing clusters of microparticles were no longer present in the SED region, although some single microparticles remained (Fig. 3c). Instead, microparticle-loaded cells were present in the parafollicular T-cell zones (Fig. 3d) and also in B-cell follicles. In contrast, no changes in the distribution of microparticle-containing cells were observed after feeding of a comparable dose of CTB. Mice fed live S. enterica serovar Typhimurium showed the same changes in microparticle-labeled DC distribution as did mice fed CT. Our observation that oral administration of live S. enterica serovar Typhimurium induced DC migration to parafollicular T-cell zones is consistent with previous findings that these bacteria enter PP via M cells (3, 12), are endocytosed by SED DCs (9), and evoke strong mucosal immune responses.
TABLE 1.
Relative frequencies of cells containing microparticle clusters in various regions of PP 24 h after peroral administration of test agents
| Treatment group | PP region
|
||
|---|---|---|---|
| SED | IFRa | FOLLb | |
| Control (PBS) | ++++ | − | +/− |
| CT | +/−c | +++ | ++ |
| CTB | ++++ | − | +/− |
| S. enterica serovar Typhimurium | +/− | +++ | ++ |
IFR, interfollicular T-cell region.
FOLL, B-cell follicle.
+/−, small number of microparticles in some, but not all, samples.
Neither S. enterica serovar Typhimurium LPS nor reovirus resulted in discernible DC movement, but it should be emphasized that a single dose was tested in these preliminary experiments and that uptake into PP may have been insufficient to produce an effect. Experiments with LPS were repeated using C57BL/6 mice that are more LPS sensitive than BALB/c mice, but again no effect was observed. The dose of reovirus administered was previously shown to be sufficient for entry and infection of PP cells (28). Whether reovirus replication in PP would eventually result in migration of DCs from the SED region is to be examined in future studies.
Migration of the microparticle-loaded cells into T-cell zones upon CT treatment has important implications for mucosal vaccine development. The strong adjuvant action of CT in the intestine has previously been shown to be due to multiple effects of the toxin on B cells, T cells, and epithelial cells (5). Recently, it was shown that CT also acts directly on DCs to induce expression of maturation-associated surface components such as HLA-DR and the costimulatory molecules B71 and B72 as well as expression of CCR7, a chemokine associated with DC migration and interaction with T cells in secondary lymphoid organs (7). In the PP, expression of CCR7 by DCs was associated with their movement into parafollicular T-cell areas (10). In future studies it will be important to determine the maturation state of the microparticle-loaded cells. Nevertheless, the observations reported here are consistent with the idea that the strong adjuvant effect of CT administered along with antigen is due in part to the toxin's ability to drive DCs to locations where interaction with naïve T cells might occur. The fact that the B subunit alone had no effect suggests that DC migration, like many other effects of CT, involves activation of adenylate cyclase.
Oral administration of CT also resulted in movement of some DCs from the SED region into mucosal B-cell follicles. DCs are known to directly stimulate the production of antibodies by B cells, and DCs can augment the proliferation of B cells that have been stimulated by CD40L on activated T cells (4). DCs also orchestrate immunoglobulin class switching: there is evidence that immunoglobulin A2, a vital component of mucosal immune responses, is dependent on the direct interaction between DCs and B cells (6, 30). Many vaccines consist of inactive pathogens, inert particles, and soluble antigens that would not be expected to trigger DC migration or maturation. Thus, our observations help to explain why CT is a highly effective mucosal adjuvant when coadministered with antigens and open the way for future studies testing the effects of other adjuvants such as mutated toxins on DC migration in the mucosa.
Acknowledgments
We gratefully acknowledge the technical assistance of Daniel Mielcarz.
This work was supported by NIH research grants HD17557, AI34757, and AI35365 and NIH center grant DK34854 to the Harvard Digestive Diseases Center.
Editor: J. D. Clements
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg, P., E. S. Baekkevold, I. N. Farstad, F. L. Jahnsen, F. E. Johansen, E. M. Nilsen, and T. Yamanaka. 1999. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol. Today 20:141-151. [DOI] [PubMed] [Google Scholar]
- 3.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145:543-552. [DOI] [PubMed] [Google Scholar]
- 4.Dubois, B., B. Vanbervliet, J. Fayette, C. Massacrier, C. Van Kooten, F. Briere, J. Banchereau, and C. Caux. 1997. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 185:941-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elson, C. O., and M. T. Dertzbaugh. 1999. Mucosal adjuvants. p. 817-838. In R. Ogra, J. Mestecky, J. McGhee, J. Bienenstock, M. Lamm, and W. Strober (ed.), Mucosal immunology. Academic Press, New York, N.Y.
- 6.Fayette, J., B. Dubois, S. Vandenabeele, J. M. Bridon, B. Vanbervliet, I. Durand, J. Banchereau, C. Caux, and F. Briere. 1997. Human dendritic cells skew isotype switching of CD40-activated naïve B cells towards IgA1 and IgA2. J. Exp. Med. 185:1909-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licenses them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 8.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249-1255. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins, S., F. Niedergang, I. E. Corthésy-Theulaz, and J.-P. Kraehenbuhl. 2000. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell. Microbiol. 2:56-68. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki, A., and B. L. Kelsall. 2001. Unique functions of CD11b+, CD8a+ and double-negative Peyer's patch dendritic cells. J. Immunol. 166:4884-4890. [DOI] [PubMed] [Google Scholar]
- 12.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelsall, B., and W. Strober. 1999. Gut-associated lymphoid tissue: antigen handling and T cell responses, p. 293-318. In R. Ogra, J. Mestecky, J. McGhee, J. Bienenstock, M. Lamm, and W. Strober (ed.), Mucosal immunology. Academic Press, New York, N.Y.
- 14.Kelsall, B. L., and W. Strober. 1996. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of murine Peyer's patches. J. Exp. Med. 183:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraehenbuhl, J.-P., and M. R. Neutra. 2000. Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16:301-332. [DOI] [PubMed] [Google Scholar]
- 16.Lennon-Duménil, A.-M., A. H. Bakker, R. Maehr, E. Fiebiger, H. S. Overkleeft, M. Rosemblatt, H. L. Ploegh, and C. Lagaudrière-Gesbert. 2002. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196:529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 18.MacPherson, G. G., and L. M. Liu. 1999. Dendritic cells and Langerhans cells in the uptake of mucosal antigens. Curr. Top. Microbiol. Immunol. 256:33-54. [DOI] [PubMed] [Google Scholar]
- 19.MacPherson, G. G., C. D. Jenkins, M. J. Stein, and C. Edwards. 1995. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J. Immunol. 154:1317-1322. [PubMed] [Google Scholar]
- 20.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Hierman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8 alpha+ and CD8 alpha-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masurier, C., B. Salomon, N. Guettari, N. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, S. I. 1991. PhoP/PhoQ: macrophage-specific modulator of Salmonella virulence? Mol. Microbiol. 5:2073-2078. [DOI] [PubMed] [Google Scholar]
- 23.Pappo, J., and T. H. Ermak. 1989. Uptake and translocation of fluorescent latex particles by rabbit Peyer's patch follicle epithelium: a quantitative model for M cell uptake. Clin. Exp. Immunol. 76:144-148. [PMC free article] [PubMed] [Google Scholar]
- 24.Pron, B., C. Boumalia, F. Jaubert, P. Berche, G. Milon, F. Geissman, and J. L. Gaillard. 2001. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell. Microbiol. 3:331-340. [DOI] [PubMed] [Google Scholar]
- 25.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, E. Pettit, E. Maraskovsky, and C. R. Malizcewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune responses in vivo. Proc. Natl. Acad. Sci. USA 96:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roake, J. A., A. S. Rao, P. J. Morris, C. P. Larsen, D. F. Hankins, and J. M. Austyn. 1995. Dendritic cell loss from non-lymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor and interleukin 1. J. Exp. Med. 181:2237-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruedl, C., C. Reiser, G. Bock, G. Wick, and G. Wolf. 1996. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer's patches. Eur. J. Immunol. 26:1801-1806. [DOI] [PubMed] [Google Scholar]
- 28.Silvey, K. J., A. B. Hutchings, M. Vajdy, M. M. Petzke, and M. R. Neutra. 2001. Role of IgA in protection against reovirus entry into murine Peyer's patches. J. Virol. 75:10870-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf, J. L., D. H. Rubin, R. Finberg, R. S. Kauffman, A. H. Sharpe, J. S. Trier, and B. N. Fields. 1981. Intestinal M cells: a pathway for entry of reovirus into the host. Science 212:471-472. [DOI] [PubMed] [Google Scholar]
- 30.Wykes, M., A. Pombo, C. Jenkins, and G. G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313-1319. [PubMed] [Google Scholar]