Abstract
Surface layer proteins (SLPs) are essential for induction of abortion by Campylobacter fetus subsp. fetus in experimentally challenged ewes. These proteins are encoded by multiple sap genes and vary in size and antigenicity. The role of SLP antigenic variation during experimental ovine infection was investigated. Following subcutaneous challenge, the SLPs were highly antigenic, and antibodies were detected in serum, milk, bile, and urine. Fecal anti-SLP antibodies were detected only in animals challenged orally. Ewes challenged with wild-type strain 23D with variable SLPs developed detectable circulating anti-SLP immunoglobulin G (IgG) antibodies by 2 weeks postchallenge. In contrast, ewes challenged with mutants of 23D that had fixed expression of a single SLP developed antibodies within 1 week postchallenge, suggesting that antigenic variation in SLPs may delay the host antibody response. Although not statistically significant, the data from challenge experiments in which vaccinated ewes were used suggested that SLP-expressing vaccines could protect animals from abortion and that this effect was independent of the SLP expressed, indicating involvement of conserved epitopes in the SLP. The conserved 184-amino-acid N-terminal region of the SLP, identified from previously published sequences, was epitope mapped with rabbit anti-SLP antisera by using overlapping synthetic 20-mer peptides. Two putative epitopes were identified at amino acids 81 to 110 and 141 to 160. Amino acids 81 to 100 also bound serum IgG antibodies from experimentally challenged sheep. Conserved antigenic regions of the SLP that induce protective immune responses may enable development of synthetic vaccine candidates for C. fetus subsp. fetus-associated ovine abortion.
Campylobacter fetus is a microaerophilic bacterium that is able to colonize a variety of mucosal sites (24). There are two subspecies, C. fetus subsp. fetus and C. fetus subsp. venerealis, which are distinguishable by a variety of techniques (27). In humans, C. fetus subsp. fetus infections can result in serious systemic disease and even death (2), especially in immunocompromised people. Both subspecies can cause substantial veterinary problems associated with ruminant infertility. In particular, C. fetus subsp. fetus is associated with sporadic cases of bovine abortion and outbreaks of ovine abortion (24).
Ovine abortion is a worldwide problem, particularly in those countries where lamb is the predominant meat food source or has economic significance (19). In the United Kingdom about 18% of ovine abortions diagnosed in 1999 were campylobacter related (1). Most of these infections were caused by C. fetus subsp. fetus (12).
Little is known about the host-pathogen interactions during C. fetus subsp. fetus infections. However, recently, an experimental model of ovine campylobacter-associated abortion has been developed and used to demonstrate the essential role of the S-layer proteins (SLPs) of this organism in the pathogenesis of this infection (13). The SLPs appear to protect the bacterium from phagocytosis and serum killing (5, 14, 29) and comprise a family of highly antigenic proteins with variable molecular masses (96 to 147 kDa) (7, 20, 28). Each SLP is encoded by one of nine homologous genes (sapA1 to sapA8) with a single promoter (6, 10). Rearrangements in the sapA locus, primarily (8) but not solely (22) mediated by RecA, enable variations in SLP expression to occur. This also allows variation in antigenicity (11). This antigenic diversity is believed to permit pathogen persistence in an immunologically hostile environment (4). However, it appears that the extent of the antigenic diversity is limited to the eight proteins expressed. It is difficult to envision how such limited diversity could allow persistence of infection.
In this study, we investigated the immunogenicity of the SLPs and the role of the antigenic diversity of these proteins in sheep challenged with C. fetus subsp. fetus 23D or mutants of this strain that expressed only one fixed SLP. The results indicated that SLP switching delayed the host antibody response. Nevertheless, previous studies (16) have indicated that antibody responses to the SLPs may provide some protection against abortion in previously exposed animals; our preliminary results support this possibility and suggest that this effect is independent of S-layer switching. Our results suggest that important conserved antigenic regions also are present in these proteins. The presence of these conserved epitopes in the SLPs was investigated by using sera from hyperimmunized rabbits and experimentally challenged sheep.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains and mutants used in this study have been described previously (13). C. fetus subsp. fetus 23D is a wild-type strain originally isolated from a bovine vagina. A series of sapA and recA deletion mutants of this wild type were used. 23D:600(2) and 23D:600(4) are both sapA+ recA mutants that express only 97- and 127-kDa SLPs, respectively. 23D:501 is a sapA recA+ mutant which initially expresses no SLP, but RecA function enables SLP switching to occur. 23D:502 has deletions in both sapA and recA and therefore is unable to express any SLP.
All strains were cultured on Columbia blood agar (Oxoid Ltd.) at 37°C for 48 h under microaerobic conditions. For the defined mutants, the culture medium contained kanamycin (40 μg/ml) or chloramphenical (50 μg/ml) or both.
Antisera and antibodies.
Two New Zealand White rabbits were immunized subcutaneously at four sites with 50 μg of purified SLP (see below) per ml; the SLP was emulsified in Freund's complete adjuvant. The immunization procedure was repeated twice at 4-week intervals by using antigen emulsified in Freund's incomplete adjuvant. Production and characterization of mouse monoclonal antibody 1D1 directed against SLPs and mouse monoclonal antibody CF15 directed against a genus-specific epitope of campylobacter flagellin have been described previously (17, 28).
Ovine abortion model.
Infection of pregnant female Welsh mountain sheep with C. fetus subsp. fetus to induce abortion has been described previously (13). Briefly, at day 126 of pregnancy, 108 CFU of C. fetus subsp. fetus 23D or a mutant was administered to each animal in groups of five or six ewes by the subcutaneous or oral route. Blood (for serum) and feces were collected twice weekly until a few weeks after lambing. Milk samples were collected at parturition or abortion (colostrum samples) and twice weekly for a total of 8 weeks. Bile and urine were collected postmortem. All animal experiments were performed under license under the Animals (Scientific Procedures) Act 1986 of the United Kingdom.
Ovine immune protection studies.
Six nonpregnant ewes were injected twice at times 6 weeks apart subcutaneously with C. fetus subsp. fetus 23D by using a dose of 108 CFU. Similarly, five ewes were injected with the SLP-negative mutant 23D:502, and another five ewes were injected with only FBP broth. The ewes then were mated and challenged with strain 23D as described above at 105 day of gestation. In another experiment, 12 ewes were injected as described above with mutant 23D:600(2) and subsequently challenged with 23D:600(4) (n = 6) or with 23D:600(2) (n = 6).
Purification of the 97-kDa SLP.
Water extraction of the 97-kDa SLP was performed by the method of Pei et al. (21). Briefly, the bacterial growth of strain 23D from two agar plates was suspended in 10 ml of distilled water and mixed gently for 10 min. The suspension was centrifuged at 4,000 × g for 5 min, and the supernatant was discarded. This process was repeated three times, and the supernatant was retained each time. The pooled supernatants were passed through a 0.2-μm-pore-size filter and concentrated (Centriprep; Amicon Ltd.) to a protein concentration of 500 μg/ml. The protein concentration was determined with a Bio-Rad protein estimation kit (Bio-Rad Ltd.). The total protein profile of the water extract was analyzed by sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and Western blotting as described previously (13) by using monoclonal antibodies 1D1 and CF15. Gel filtration high-performance liquid chromatography (HPLC) was performed by using a Sepharose 12 column (Pharmacia LKB Biotechnology Inc.) with 20 mM Tris buffer (pH 7.5) containing 50 mM sodium chloride at a flow rate of 0.15 ml/min. Subsequently, ion-exchange HPLC was performed by using a Mono-Q column (Pharmacia) with a flow rate of 0.5 ml/min in 20 mM Tris HCl (pH 7.5) buffer with a linear gradient to 350 mM sodium chloride over 40 min. The purified SLP was stored at −20°C and used for the immunological assays.
Extraction of secretory immunoglobulin A (IgA) from feces.
Feces (2 g) were mixed with 4 ml of phosphate-buffered saline containing 0.01% Thimersol (Sigma Ltd.). The mixture was incubated at 37°C for 30 min and centrifuged at 1,200 × g for 20 min, and the supernatant was collected. The procedure was repeated twice without further incubation. The pooled supernatants were centrifuged at 20,000 × g for 1 h at 40°C, and the resulting supernatant was stored at −20°C until it was used.
Preparation of milk whey.
Milk whey was prepared by rennet coagulation of milk (26), followed by centrifugation at 3,500 × g for 15 min at 4°C. The material was stored at −20°C until it was used.
ELISA.
Purified SLP (5 μg/ml) was coupled to micro enzyme-linked immunosorbent assay (ELISA) plates (100 μl/well) by using 0.1 M carbonate buffer (pH 9.6) overnight at 4°C. Unbound antigen was removed by washing the plates three times with 200 μl of ELISA wash (145 mM sodium chloride containing 0.05% Tween 20) per well. Sheep sera (final dilution, 1:50 in 0.05 M Tris-acetate buffer [pH 7.6] containing 1% bovine serum albumin, 145 mM sodium chloride, and 0.05% Tween 20 [Tris-BSA buffer]) were added to duplicate antigen-coated wells (100 μl/well) and incubated at 37°C for 2 h. The wells were washed again, and bound sheep IgG was detected by incubation with rabbit anti-sheep IgG coupled to peroxidase (Amersham Pharmacia Biotech, Little Chalfont, England) (diluted 1:1,000 in Tris-BSA buffer) for 30 min at 37°C. After washing, the remaining peroxidase conjugate was detected with the chromogenic substrate tetramethylbenzidine (Cambridge Veterinary Services, Cambridge, England) added to each well (100 μl/well). After 15 min the reaction was stopped by addition of 50 μl of 2 M sulfuric acid, and the optical density at 450 nm was determined with a micro ELISA reader.
This assay was modified to investigate the isotype specificity of serum antibody responses. Specific sheep IgG1, IgG2, IgM, and IgA were detected by using serum dilutions of 1:50, 1:40, 1:100, and 1:200, respectively, and anti-sheep IgG1 (1:200 dilution), anti-sheep IgG2 (1:200 dilution), anti-sheep IgM (1:200 dilution), and anti-sheep IgA (1:25 dilution) monoclonal antibodies (Serotec Ltd.), respectively. Specific IgA in bile, urine, and feces was detected by using sample dilutions of 1:200, 1:50, and 1:40, respectively, and anti-sheep IgA monoclonal antibody at dilutions of 1:25, 1:100, and 1:25, respectively. Specific IgG in milk was detected by using a sample diluted 1:10 and rabbit anti-sheep IgG antibody at a dilution of 1:100.
Epitope mapping.
The conserved regions of the N-terminal amino acid sequences of three SLPs (SapA, SapA1, and SapA2) (3, 8) were identified by using DNAStar software (DNAStar Inc.). Comparison of SapA2 with SapA or SapA1 revealed identical N-terminal 184-amino-acid sequences. From this sequence 17 20-mer peptides overlapping by 10 amino acids were synthesized by using Fmoc technology (432A peptide synthesizer; Applied Biosystems Plc.) according to the manufacturer's instructions. The identities and purities of the peptide products were confirmed by mass spectrometry (kindly performed by Jeff Keen, University of Leeds, Leeds, United Kingdom). For peptide mapping, the synthetic peptides were covalently bound to the wells of Covalink-NH plates as recommended by the manufacturer (Nunc Ltd.). Briefly, a peptide-n-hydroxysuccinimide stock solution was made by dissolving 50 mg of peptide and 184 μg of n-hydroxysuccinimide in 60% dimethyl sulfoxide. This solution was added (50 μl) to the Covalink plates to give a final concentration of 2.5 μg of peptide per well. The reaction was initiated by adding 50 μl of a 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (Sigma Ltd.) solution (1.23 mg/ml) to each well. The plates were incubated for 2 h at 22°C and then washed three times in Covabuffer (16.9 g of NaCl, 20.59 g of MgSO4 · 7H2O, and 0.5 ml of Tween 20 dissolved in 1 liter of phosphate-buffered saline). At the final washing stage the wells were incubated in the buffer for 15 min at 22°C. Rabbit anti-SLP antiserum (100 μl diluted 1:200) was added to each well and incubated for 1 h at 22°C. The wells were washed three times with Covabuffer; during the final wash the buffer remained on the wells for 15 min. Goat anti-rabbit IgG conjugated to peroxidase (100 μl diluted 1:10,000 in Covabuffer; Amersham Pharmacia Biotech) was added to each well for 30 min at 22°C, and then the wells were washed as described above. The bound peroxidase was detected as described above for the ELISA. Epitope mapping with the mouse monclonal antibodies and the sheep sera was performed by using the appropriate dilutions and peroxidase conjugates as described above for the ELISA.
RESULTS
Antigen preparation for serological testing.
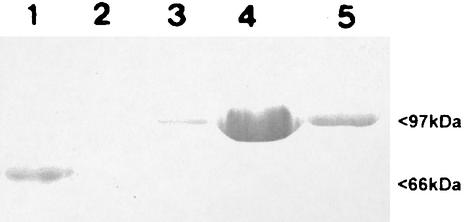
The SLP was initially recovered from the bacterial surface by water extraction. Upon gel filtration HPLC this material produced four peaks (data not shown). The fractions containing the 97-kDa SLP were pooled and subjected to ion-exchange HPLC, and two major peaks were obtained. SDS-PAGE and Western blotting demonstrated that the major peak was SLP and the minor was peak flagellin (Fig. 1). The fractions containing the SLP were pooled and used as the ELISA antigen.
FIG. 1.
Total protein profiles, as determined by SDS-PAGE, of gel filtration HPLC fractions of water-extracted SLPs. Fractions 3 to 5 were pooled to obtain the ELISA antigen.
Anti-S-layer IgG serum antibodies during experimental ovine infection.
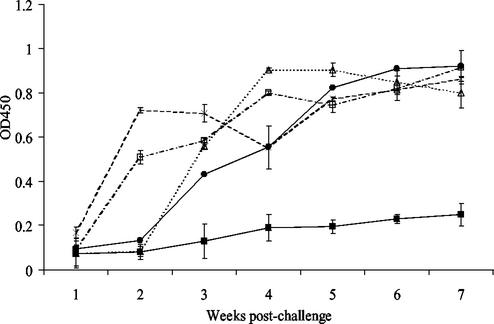
The outcomes of experimental challenges of pregnant ewes with 23D and its sapA recA deletion mutants have been described previously (13). When the ELISA technique described above was used, the serum IgG antibody levels in animals challenged with wild-type strain 23D (five of five had culture-positive placentas and one of five had abortions) increased sharply by 3 weeks postchallenge, reached a plateau at 4 weeks (Fig. 2), and then declined from 5 to 7 weeks. In contrast, the antibody levels in sera from all animals challenged with 23D:502 (sapA recA mutant) (none of five had culture-positive placentas and none of five had abortions) and from five of the six animals challenged with 23D:501 (sapA recA+ mutant) (one of five had culture-positive placentas and none of five had abortions) were identical, exhibiting only a small increase (P < 0.05) in the specific IgG levels (Fig. 2) over the 7 weeks of the experiment. However, in another animal challenged with 23D:501 there was an increase in the antibody levels, detectable by 3 weeks postchallenge, that was similar to the increase observed in the animals challenged with strain 23D. This animal aborted, and C. fetus cells expressing a 97-kDa SLP were recovered from the fetus and the placenta.
FIG. 2.
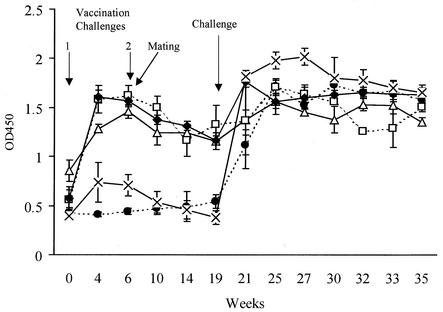
Serum IgG antibodies directed against SLPs following challenge of pregnant ewes with wild-type strain C. fetus subsp. fetus 23D (▵), with mutants 23D:501 and 23D:502 (▪), with mutant 23D:600(2) (×), and with mutant 23D:600(4) (□). Note that the antibody response of the one ewe that aborted following challenge with 23D:501 (•) is shown separately. The error bars indicate standard errors. OD450, optical density at 450 nm.
In ewes challenged with either 23D:600(2) (five of five had culture-positive placentas and two of five had abortions) or 23D:600(4) (five of five had culture-positive placentas and two of five had abortions) (sapA+ recA mutants) there was a more rapid increase in the antibody response, and both groups had significantly greater antibody levels (P < 0.001) that were detectable within 1 week after challenge (Fig. 2). This response then was maintained or slightly increased over the subsequent 6 weeks. These results indicate that infection with organisms in which recA was intact and which were therefore able to vary their SLP was associated with a short delay in host immune responses directed against the SLP.
Isotypes of the serum antibody response.
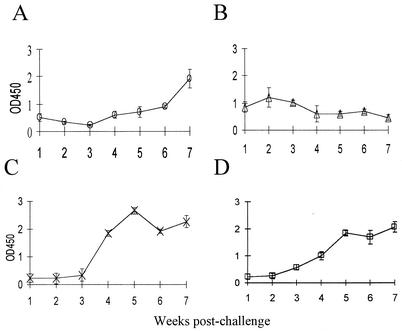
We next examined the isotypes of serum antibody responses induced by experimental challenge with C. fetus subsp. fetus 23D. There was a low-level and short-lived IgM serum antibody response to challenge with the wild-type strain. The levels of anti-SLP IgM antibodies peaked at 2 weeks after challenge and then declined rapidly (Fig. 3). However, the IgM antibody level after 2 weeks was not significantly higher than the level after 1 or 7 weeks. In contrast, the levels of specific IgG1 and IgG2 antibodies were significantly (P < 0.001) greater from week 4 until at least week 7 postchallenge. A significant serum IgA antibody response (P < 0.05) was not detected until 6 weeks after challenge. These results were consistent with the presence of a systemic infection resulting from the subcutaneous challenge.
FIG. 3.
Serum IgA (A), IgM (B), IgG1 (C), and IgG2 (D) anti-SLP antibody levels in six pregnant ewes challenged with C. fetus subsp. fetus 23D until 7 weeks after challenge. The error bars indicate standard errors. OD450, optical density at 450 nm.
Mucosal antibody responses.
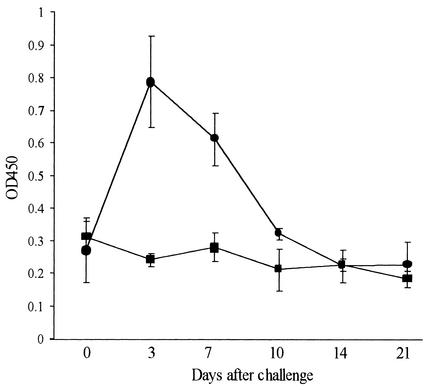
To determine whether mucosal responses were induced by subcutaneous or oral challenge with C. fetus subsp. fetus 23D, specific antibodies in feces, milk, and urine were investigated. Anti-SLP IgA antibodies were detected in the feces of all five animals challenged orally with strain 23D by 7 days postchallenge (P < 0.01), but the levels declined to prechallenge levels within 14 days (Fig. 4). In contrast, there was no significant increase over the 3-week monitoring period in specific fecal IgA levels in the six animals that were subcutaneously challenged with 23D. These results suggest that a detectable ovine mucosal IgA antibody response was induced at the intestinal surface following oral challenge with C. fetus subsp. fetus but not following subcutaneous challenge with C. fetus subsp. fetus.
FIG. 4.
Anti-SLP IgA antibodies detected in fecal extracts from pregnant ewes challenged orally (n = 5) (•) or subcutaneously (n = 6) (▪) with C. fetus subsp. fetus 23D. The error bars indicate standard errors. OD450, optical density at 450 nm.
Mucosal anti-SLP IgA antibodies were also detected postmortem in the bile and urine of animals challenged subcutaneously with 23D. In these animals, the mean specific IgA antibody levels (optical density at 450 nm) were 1.4 for bile samples and 0.67 for urine samples and were significantly higher than the mean levels in animals challenged with mutant 23D:502, which were 0.3 (P < 0.05) and 0.45 (P < 0.05) for bile and urine, respectively. The colostrum and milk of ewes are additional fluids in which mucosal immune responses can be detected. In ruminant milk the primary antibody is IgG rather IgA (25); therefore, anti-SLP IgG antibodies were investigated in the milk whey of subcutaneously challenged ewes after successful lambing. In ewes challenged with strain 23D, at 1 to 3 days after parturition the mean antibody level (optical density at 450 nm) in milk was 1.7. This level decreased to 0.7 by 2 weeks, to 0.65 by 6 weeks, and to 0.5 by 8 weeks after parturition. In contrast, in four ewes subcutaneously challenged with mutant 23D:502, the mean IgG antibody levels in milk were consistently significantly lower (P < 0.001) throughout the monitoring period (0.04, 0.07, 0.03, and 0.04 at 1 to 3 days and 2, 6, and 8 weeks, respectively).
Overall, these results indicate that subcutaneous challenge with C. fetus subsp. fetus induced detectable antibody responses throughout most of the common mucosa-associated lymphatic tissue but that responses at the gastrointestinal surface were detectable only when the challenge was oral.
Ovine immune protection studies.
The protective effect of prior exposure to C. fetus subsp. fetus for ewes subsequently challenged during pregnancy was determined. All six ewes vaccinated twice by subcutaneous challenge with strain 23D and then challenged at day 105 of gestation with the homologous strain lambed normally (Table 1). However, C. fetus subsp. fetus was recovered from the placentas of two (33%) of the six animals. In these ewes there were initial increases in the anti-SLP IgG serum antibody levels over the 4 weeks following the first vaccination dose (Fig. 5). The antibody levels then decreased slowly over the next 15 weeks. Following the challenge at day 105 of gestation further increases in the antibody levels were detected. One (20%) of the five ewes challenge vaccinated with SLP-negative mutant 23D:502 before the final challenge during pregnancy with wild-type strain 23D aborted, and organisms were recovered from the placentas of two (40%) of these animals. Similar results were obtained when control ewes were injected with FBP broth instead of 23D:502 (Table 1). In each of these two control groups, which received a prior vaccination challenge with FBP broth or SLP-negative deletion mutant 23D:502, there was abortion in only one animal (20%). This was an unexpectedly low level of abortion compared to the level previously reported (13). The reason for this finding is unknown, but the results may be a reflection of undetected prior exposure of the ewes. A transient, low-level, nonsignificant (P > 0.05) increase in the serum anti-SLP antibody levels was detected in the ewes challenge vaccinated with 23D:502 but not in the ewes challenge vaccinated with FBP broth (Fig. 5). There was a substantial increase in antibody levels after subsequent challenge of these pregnant ewes with strain 23D (Fig. 5). Although because of the unexpectedly low levels of abortion there was no statistically significant difference (P = 0.454, as determined by Fisher's exact test) between the outcome of challenge in the unvaccinated controls and the outcome of challenge in the vaccinated controls, the only abortions detected were in the groups of ewes which were not given a vaccine containing SLP. These results suggest that prior vaccination challenge of ewes with SLP-positive strains subsequently protected animals from abortion when they were challenged during pregnancy. This protection appeared to be associated with increased circulating antibody responses directed against the SLP.
TABLE 1.
Effect of previous challenge of nonpregnant ewes with C.fetus susbsp. fetus 23D or its mutants on challenge during pregnancy
| Vaccination challenge of prepregnant ewesa | Challenge at day 105 of gestation | No. of sheep | No. of normal parturitions | No. of abortionsb | No. of culture-positive placentas |
|---|---|---|---|---|---|
| 23D | 23D | 6 | 6 | 0 | 2 |
| FBP broth | 23D | 5 | 4 | 1 | 2 |
| 23D:502 | 23D | 5 | 4 | 1 | 3 |
| 23D:600(2) | 23D:600(2) | 6 | 6 | 0 | 2 |
| 23D:600(2) | 23D:600(4) | 6 | 6 | 0 | 1 |
All ewes were challenged at zero time and day 42.
Abortion caused by C. fetus subsp. fetus was confirmed by culture of organisms from products of parturition.
FIG. 5.
Serum anti-SLP antibodies during immune protection studies. Ewes received primary (zero time) and secondary (day 42) challenges with C. fetus subsp. fetus strains as follows: broth and 23D (•), 23D and 23D:502 (×), 23D and 23D (□), 23D:600(2) and 23D:600(2) (♦), and 23D:600(2) and 23D:600(4) (▵). The error bars indicate standard errors. OD450, optical density at 450 nm.
To determine whether the immune responses were induced in the presence of SLP switching, 12 ewes were vaccinated twice by subcutaneous challenge with mutant 23D:600(2), which expressed only a fixed 97-kDa SLP. All ewes which were then challenged either with 23D:600(2) or with 23D:600(4), a mutant expressing a fixed 127-kDa SLP, lambed normally, even though organisms were recovered from the placentas of 3 of the 12 animals (Table 1). Once again, because of the unexpected low abortion rates in the unvaccinated controls, the differences were not statistically significant. Nevertheless, none of the 12 animals in the groups vaccinated with the mutants aborted, whereas 2 of 10 animals given control vaccines aborted. Between vaccination challenges 1 and 2 there was an initial increase and then a slow decrease in the levels of circulating anti-SLP antibodies similar to the results observed with wild-type strain 23D (Fig. 5). Following challenge of the pregnant ewes with 23D:600(2) and 23D:600(4) the serum anti-SLP antibody levels increased significantly (P < 0.001), but there was not a significant difference (P > 0.05) in the levels of the antibody responses to the homologous and heterologous challenges (Fig. 5).
Overall, these findings suggest that that prior exposure to C. fetus subsp. fetus provides protection against abortion, although not necessarily against placental infection, following a subsequent challenge of pregnant animals. This protection is associated with induction of a systemic IgG antibody response directed against the SLP. These results support the hypothesis that circulating anti-SLP antibodies may protect against the diseases resulting from C. fetus subsp. fetus infection. There is no evidence to suggest that this protection is dependent on the ability of the SLP to vary.
Epitope mapping of the N-terminal conserved region of the SLP.
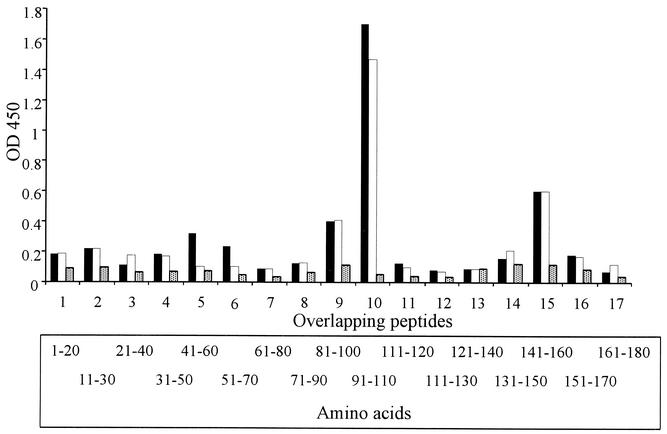
Previous experimental studies have indicated that antibody responses to C. fetus subsp. fetus provide some protection against abortion (15). As the SLP is a major antigen of C. fetus subsp. fetus, it seem likely that immune responses directed against this antigen are associated with this protection. This possibility is supported by our experimental data. However, field isolates express multiple SLPs, which suggests that this effect may be independent of S-layer switching. This suggestion is also supported by our experimental observations. We, therefore, hypothesized that any protective epitopes should be present in conserved regions of the SLPs. A conserved region consisting of 184 amino acids was identified at the N termini of all the type A SLP amino acid sequences that have been described (3). Based on this sequence, 20-mer peptides that overlapped by 10 amino acids were synthesized and used in an ELISA to identify antigenic peptides. The specificities of anti-SLP IgG antibodies from two rabbit antisera were consistent. Both antisera bound more strongly to peptide 10 (amino acids 91 to 110) than the prechallenge sera bound (Fig. 6). Antibodies from both antisera also bound, although less strongly, to peptides 15 (amino acids 141 to 160) and 9 (amino acids 81 to 100).
FIG. 6.
IgG antibodies bound to overlapping synthetic peptides derived from the 184-amino-acid conserved region of the SLPs. The antibodies were detected in antisera from two rabbits (solid bars, rabbit 1; open bars, rabbit 2) that were hyperimmunized with 97-kDa SLP antigen of C. fetus subsp. fetus 23D and in the pooled prebleed sera (stippled bars). OD450, optical density at 450 nm.
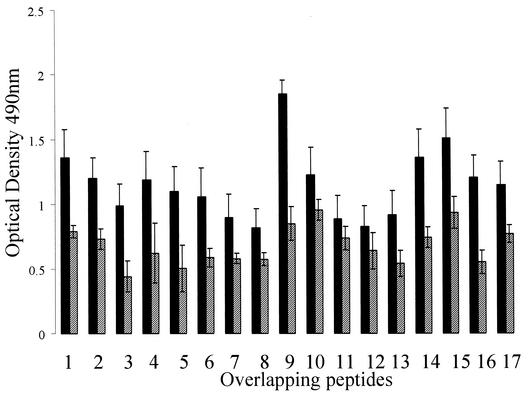
IgG antibodies present in sera from six sheep experimentally challenged with either strain 23D (n = 2), strain 23D:600(2) (n = 2), or strain 23D:600(4) (n = 2) bound to all peptides tested. As the N-terminal region was conserved in each of these strains, the data for all ewes were pooled and are shown in Fig. 7. Prechallenge sera from these animals also demonstrated some binding of IgG to all the peptides. Only peptide 9 (amino acids 81 to 100) bound significantly more IgG from the sera of challenged animals than from the sera of prechallenged animals.
FIG. 7.
Mean levels of serum IgG antibodies bound to overlapping synthetic peptides derived from the 184-amino-acid conserved region of the SLPs. The data were derived from six ewes before (cross-hatched bars) and after (solid bars) challenge with C. fetus subsp. fetus 23D or its mutants. The error bars indicate standard errors.
DISCUSSION
The role of SLPs in the pathogenesis of C. fetus subsp. fetus ovine infections presents a conundrum. Previous studies have clearly demonstrated that SLP expression is essential for the organism to survive systemic infection and induce ovine abortion (13). This is apparently independent of the type of SLP expressed or the need for S-layer switching. The role in vivo of SLP switching has not been defined yet, but SLP switching is believed to enable the organism to avoid host immune responses to a specific SLP, which may be highly antigenic (4). However, this role may be questioned since the options for antigenic diversity appear to be restricted to only nine antigenic forms and in naturally acquired ovine infections prior infection associated with abortion appears to confer protection against subsequent challenge (1, 15).
To investigate this problem, an ELISA was developed to detect ovine antibody responses directed against the SLPs. The results confirmed that the S layer was highly antigenic during experimental challenges of sheep with C. fetus subsp. fetus strains. Antibodies directed against the SLPs of all isotypes tested were induced and sustained for many weeks. These antibodies were detected in serum and in mucosal fluids, such as fluids from the intestine (bile), and in urine and colostrum. However, there was evidence of a delay of up to 1 week in the detectable serum antibody response when the challenge organism was able to switch SLP expression. This evidence is the only evidence currently available that supports the hypothesis that antigenic variation in the SLP enables the organism, in some as-yet-undefined way, to evade the initial host immune responses. This would potentially allow rapid establishment of infection, providing an obvious benefit to the pathogen. These observations are obviously dependent on comparisons of the outcomes of experimental infections with wild-type strains and recA mutants. Because RecA is involved in DNA repair mechanisms, the recA mutants may have other defects in addition to an inability to vary the SLP. However, no other differences detectable by SDS-PAGE or immunblotting have been reported, and the mutants are fully pathogenic, as previously demonstrated (13). This is in contrast to C. fetus 23B, a spontaneous mutant, which has a 9-kb deletion in the sapA promoter region. This mutant exhibits several other differences in polypeptide expression, as determined by SDS-PAGE and immunoblot analysis (13).
The role of the anti-SLP antibodies in protective immunity was investigated by using a vaccination and challenge model of ovine abortion. In this model, ewes were vaccinated subcutaneously with live wild-type C. fetus subsp. fetus strain 23D, a broth control, or an SLP-negative mutant. Subsequently the vaccinated ewes were challenged with the 23D wild-type strain. Unfortunately, due to an unexpectedly low level of abortion in control groups, our experimental data failed to demonstrate vaccine-induced protection at a statistically significant level. Nevertheless, animals in the control challenge-vaccinated groups (2 of 10 ewes), but not animals in the groups vaccinated with the SLP-containing wild type (none of 18 ewes), aborted, and the difference in these results approaches significance (P = 0.119, as determined by Fisher's exact test). These results support previous experimental studies (16) and observations based on field data (1, 15), in which animals associated with campylobacter abortion outbreaks were protected from subsequent abortion. As in our study significant differences in the antibody responses to the SLPs were detectable prior to the experimental challenge, it seems likely that anti-SLP antibodies contributed to this potential protection. This assumption is supported by the reduced lethality of experimental C. fetus subsp. fetus infections in mice passively immunized with anti-SLP antibodies (20). Other surface antigens of C. fetus subsp. fetus also may confer immune protection. Certainly, small but rising antibody levels were detected by ELISA in sheep challenged with 23D:502. The probable explanation for this observation is the presence of low-level contamination of the ELISA antigen with flagellin. Flagellin is a highly antigenic protein in other Campylobacter species (18), and this antigen also may confer some immune protection against disease.
Whether the anti-SLP antibody responses are cross-reactive in terms of potential protection is debatable. In the field C. fetus subsp. fetus strains from ovine abortions express multiple SLPs (12). Although there is evidence that protection is serotype specific (16), there is no evidence of restricted protection due to SLP expression. In an attempt to investigate this question, sheep were vaccinated with 23D:600(2) expressing a 97-kDa SLP and then challenged with 23D:600(4) expressing a 127-kDa SLP. There was no difference between the outcome of this challenge and the outcome observed with a homologous vaccinated control (Table 1). Although, as before, the level of abortion in the broth-vaccinated control was insufficient to provide statistically significant observations, the absence of abortion in the heterologous challenge experiment suggests that immune protection is independent of SLP switching. Thus, despite the association between antigenic variation of the SLP and a delay in the host immune response, the eventual outcomes of challenges with strains that can and cannot vary antigenically are the same in that all strains that produce an SLP can induce abortion (13) and in all infected hosts there is an immune response which appears to be associated with subsequent potential protection. Thus, the use of antigenic variation by the pathogen to avoid host antibodies appears have only a transitory effect.
In the experimental model which we used, the route of challenge was subcutaneous rather than the more natural oral route. It is possible that at the intestinal mucosa surface immune avoidance, as a consequence of antigenic variation, could be more effective, enabling longer periods for establishment of colonization. The role of the SLP in colonization at the intestinal surface is not known yet, but it is clear from our studies that intestinal challenge with strain 23D induces a significant antibody response, as detected in feces. This response occurs within 3 weeks, but whether the response time can be reduced in the absence of antigen switching is unknown. Evidence from natural abortion outbreaks support this hypothesis; however, there is very little information available on the efficiency or duration of gut colonization within an infected flock. Chronic persistence of gut infection would be an obvious advantage for the organism as the opportunities for spread both within and between flocks increase with duration of infection.
The possibility that immune protection is independent of SLP switching, as suggested by field observations (12) and our experimental data, indicates that the protective immune responses are due to conserved or cross-reacting epitopes within the SLPs. Alignment of all three previously published SLP sequences indicated the presence of a 184-amino-acid sequence at the N terminus which was highly conserved. This region of the SLP has been shown to enable binding of the proteins to lipopolysaccharide (9). It might be expected that such a close interaction with the lipopolysaccharide may obscure any antigenic regions, preventing antibody induction and/or binding. To investigate this, synthetic 20-mer peptides were produced, and the conserved region epitope was mapped by ELISA. Rabbit antisera directed against purified SLPs clearly detected two major epitopes, peptides 9 and 10 (amino acids 81 to 110) and peptide 15 (amino acids 141 to 160). Interestingly, the pooled data obtained from the sera from six ewes challenged with strain 23D or its fixed SLP mutants also revealed the presence of peptide 9. If this epitope is shown to induce the observed protective immune responses, it may be possible to develop a simple synthetic vaccine which could be used to protect sheep from campylobacter-associated abortion blooms. Such a vaccine would be of considerable value in those regions of the world where lamb is a major food source.
The results of this study indicate that the role of the SLP in the outcome of C. fetus subsp. fetus infections is complex. The normal source or route of transmission of C. fetus subsp. fetus infection in sheep, and probably humans, is fecal-oral. Previous studies indicated that the SLP is probably essential for bacterial translocation across the intestinal mucosa and systemic survival in sheep but is not responsible for the abortion outcome (13). It is thought that the role of the SLP is to prevent the binding of C3b, thus providing a barrier against innate host immunity (5). This would allow initial systemic infection. Nevertheless, as is evident from the present study, the SLP is highly immunogenic. Systemic infection induces substantial serum antibody responses of all isotypes for at least weeks. Moreover, anti-SLP antibodies are detectable at common mucosal surfaces like mammary and urogenital tissues, and even transient intestinal colonization induces mucosal antibody responses directed against these antigens. These observations suggest that there are widespread host immune responses to antigen challenge. In a normal host such rapid and comprehensive responses directed against a major bacterial surface antigen would presumably limit the progress of the infection, possibly restricting the organism to the intestine, where disease outcomes are mild or negligible. However, in immunocompromised hosts, including pregnant ewes, such responses are limited (23) so that extraintestinal colonization can occur. Such events are encouraged by the expression of multiple SLPs. The data presented here suggest that the initial immune responses are directed against immunodominant epitopes in the antigenically diverse regions of the SLPs. These epitopes may act as a diversion for the host immune response, potentially enabling the organism to establish itself within a protected host niche. However, this is only a temporary situation, and within a short time the host detects epitopes within the conserved region. It is the latter immune responses which appear to be protective, preventing disease after subsequent challenges.
Acknowledgments
This work was supported mainly by the Wellcome Trust, United Kingdom, (0535115/2/98) and in part by the Department for Environment, Food and Rural Affairs, Great Britain, and by RO1 AI24145 from the National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Anonymous. 1999. Veterinary investigation data analysis (VIDA). Veterinary Laboratories Agency, Surrey, United Kingdom.
- 2.Blaser, M. J. 1998. Campylobacter fetus—emerging infection and model system for bacterial pathogenesis at mucosal surfaces. Clin. Infect Dis. 27:256-258. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J., and E. C. Gotschlich. 1990. Surface array protein of Campylobacter fetus. Cloning and gene structure. J. Biol. Chem. 265:14529-14535. [PubMed] [Google Scholar]
- 4.Blaser, M. J., and Z. Pei. 1993. Pathogenesis of Campylobacter fetus infections: critical role of high-molecular-weight S-layer proteins in virulence. J. Infect. Dis. 167:372-377. [DOI] [PubMed] [Google Scholar]
- 5.Blaser, M. J., P. F. Smith, J. E. Repine, and K. A. Joiner. 1988. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J. Clin. Investig. 81:1434-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, M. J., E. Wang, M. K. Tummuru, R. Washburn, S. Fujimoto, and A. Labigne. 1994. High-frequency S-layer protein variation in Campylobacter fetus revealed by sapA mutagenesis. Mol. Microbiol. 14:453-462. [DOI] [PubMed] [Google Scholar]
- 7.Dubreuil, J. D., S. M. Logan, S. Cubbage, D. N. Eidhin, W. D. McCubbin, C. M. Kay, T. J. Beveridge, F. G. Ferris, and T. J. Trust. 1988. Structural and biochemical analyses of a surface array protein of Campylobacter fetus. J. Bacteriol. 170:4165-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin, J., and M. J. Blaser. 1997. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol. Microbiol. 26:433-440. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin, J., M. K. Tummuru, and M. J. Blaser. 1995. A lipopolysaccharide-binding domain of the Campylobacter fetus S-layer protein resides within the conserved N terminus of a family of silent and divergent homologs. J. Bacteriol. 177:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita, M., S. Fujimoto, T. Morooka, and K. Amako. 1995. Analysis of strains of Campylobacter fetus by pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:1676-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia, M. M., C. L. Lutze-Wallace, A. S. Denes, M. D. Eaglesome, E. Holst, and M. J. Blaser. 1995. Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J. Bacteriol. 177:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grogono-Thomas, R. 2000. The role of surface (S)-layer proteins in ovine Campylobacter fetus subsp. fetus infections. Ph.D. thesis. University of London, London, United Kingdom.
- 13.Grogono-Thomas, R., J. Dworkin, M. J. Blaser, and D. G. Newell. 2000. Roles of the surface layer proteins of Campylobacter fetus subsp. fetus in ovine abortion. Infect. Immun. 68:1687-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy, E. C., D. Doyle, K. Burda, L. B. Corbeil, and A. J. Winter. 1975. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect. Immun. 11:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinershagen, W., F. Frank, C. Hulet, and D. Price. 1969. Immunity in ewes resulting from natural exposure to Vibrio fetus. Am. J. Vet. Res. 30:203-206. [PubMed] [Google Scholar]
- 16.Miller, V. A., and R. Jensen. 1961. Experimental immunization against ovine vibriosis. 1. The use of live and formalin-killed Vibrio fetus vaccine. Am. J. Vet. Res. 22:43-46. [PubMed] [Google Scholar]
- 17.Newell, D. G. 1986. Monoclonal antibodies directed against the flagella of Campylobacter jejuni: cross-reacting and serotypic specificity and potential use in diagnosis. J. Hyg. 96:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newell, D. G., H. McBride, and A. D. Pearson. 1984. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J. Gen. Microbiol. 130:1201-1208. [DOI] [PubMed] [Google Scholar]
- 19.Orr, M. 1994. Animal health laboratory network. Review of diagnostic cases. Surveillance 21:3-6. [Google Scholar]
- 20.Pei, Z., and M. J. Blaser. 1990. Pathogenesis of Campylobacter fetus infections. Role of surface array proteins in virulence in a mouse model. J. Clin. Investig. 85:1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei, Z., R. T. D. Ellison, R. V. Lewis, and M. J. Blaser. 1988. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J. Biol. Chem. 263:6416-6420. [PubMed] [Google Scholar]
- 22.Ray, K. C., Z. C. Tu, R. Grogono-Thomas, D. G. Newell, S. A. Thompson, and M. J. Blaser. 2000. Campylobacter fetus sap inversion occurs in the absence of RecA function. Infect. Immun. 68:5663-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds, G., and J. Griffin. 1990. Humoral immunity in the ewes. 2. The effect of pregnancy on the primary and secondary antibody response to protein antigen. Vet. Immunol. Immunopathol. 25:155-166. [DOI] [PubMed] [Google Scholar]
- 24.Skirrow, M. 1994. Diseases due to Campylobacter, Helicobacter and related bacteria. J. Comp. Pathol. 111:113-149. [DOI] [PubMed] [Google Scholar]
- 25.Tizzard, I. R. 2000. Veterinary immunology, an introduction, 6th ed. W.B. Saunders Co., London, United Kingdom.
- 26.Vaz, A. 1994. Some aspects of the immunity of Pasturella mastitis in sheep. Ph.D. thesis. Royal Veterinary College, University of London, London, United Kingdom.
- 27.Wagenaar, J., M. van Bergen, D. Newell, R. Grogono-Thomas, and B. Duim. 2001. Comparative study using amplified fragment length polymorphism fingerprinting, PCR, genotyping, and phenotyping to differentiate Campylobacter fetus strains isolated from animals. J. Clin. Microbiol. 39:2283-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, E., M. M. Garcia, M. S. Blake, Z. Pei, and M. J. Blaser. 1993. Shift in S-layer protein expression responsible for antigenic variation in Campylobacter fetus. J. Bacteriol. 175:4979-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter, A. J., E. C. McCoy, C. S. Fullmer, K. Burda, and P. J. Bier. 1978. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect. Immun. 22:963-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, L. Y., Z. H. Pei, S. Fujimoto, and M. J. Blaser. 1992. Reattachment of surface array proteins to Campylobacter fetus cells. J. Bacteriol. 174:1258-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]