Abstract
Staphylococcus aureus achieves resistance to defensins and similar cationic antimicrobial peptides (CAMPs) by modifying anionic membrane lipids via MprF with l-lysine, which leads to repulsion of these host defense molecules. S. aureus ΔmprF, which lacks the modification, was very efficiently killed by neutrophil defensins and CAMP-producing leukocytes, even when oxygen-dependent killing was disrupted, but was as susceptible as wild-type bacteria to inactivation by myeloperoxidase or human monocytes lacking defensins. These results demonstrate the impact and specificity of MprF-mediated CAMP resistance and underscore the role of defensin-like peptides in innate host defense.
The first leukocytes appearing at a site of infection are the neutrophils, which elaborate a great number of antimicrobial activities that participate in the killing of ingested microbes. In addition to the oxygen-dependent respiratory burst compounds, neutrophils produce bacteriolytic enzymes and cationic antimicrobial peptides (CAMPs) (1). The various types of CAMPs attack the bacterial cytoplasmic membrane and cause leakage of the bacterial cell and breakdown of membrane potential (14). Neutrophils contain particularly large amounts of CAMPs, such as defensin HNP1-3 or cathelicidin LL-37 (4, 5); these molecules are lacking in monocyte granules. Staphylococcus aureus, a major bacterial pathogen, is highly resistant to defensins and other antimicrobial peptides (9). Several studies have shown that CAMP resistance mechanisms contribute to bacterial virulence and are prerequisites for the colonization of CAMP-shielded body surfaces and tissues (8). We have recently described two types of S. aureus mutants, dltABCD (11) and mprF (10, 13), that are highly susceptible to human neutrophil defensins and other CAMPs. The two defense systems inactivated in these mutants modify negatively charged cell envelope components with amino acids, thereby leading to introduction of positive charges and repulsion of CAMP molecules (8). Teichoic acids, staphylococcal cell wall polymers composed of glycerol- or ribitol-phosphate repeating units, are esterified with d-alanine by the gene products of the dltABCD operon (11). The novel membrane protein MprF is necessary for synthesis of lysyl-phosphatidylglycerol (L-PG), an unusual derivative of phosphatidylglycerol modified with l-lysine (10). While the other S. aureus phospholipids are anionic, L-PG is the only phospholipid with a positive net charge; its absence leads to a profound alteration of the electrostatic properties of the membrane surface. The dltABCD and the mprF mutants are more susceptible to neutrophil killing, and their virulence in mice is attenuated (2, 10).
In vitro killing of S. aureus by defensins and myeloperoxidase.
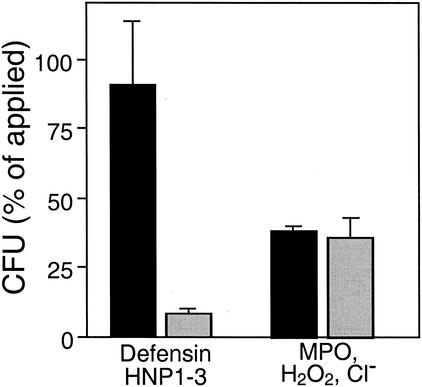
We have recently demonstrated that the human neutrophil defensin HNP1-3 inhibits the growth of S. aureus ΔmprF but has no effect on the wild type (10). In order to determine whether the lack of L-PG also leads to increased killing by defensins, bacterial cells grown in Mueller-Hinton broth to the mid-exponential phase were resuspended in potassium phosphate buffer (10 mM, pH 7.5) containing 0.0005% human serum albumin (HSA) and 3 × 105 CFU/ml were incubated with 100 μg of HNP1-3/ml (a gift from H. Kalbacher, University of Tübingen) at 37°C as described recently (2). Briefly, samples of 10 μl each were shaken and killing was stopped after 60 min by 25-fold dilution in ice-cold potassium phosphate buffer. Viable bacteria were counted 24 h after plating of appropriate dilutions on LB agar (1% Tryptone, 0.5% yeast extract, 0.5% NaCl, 1.5% agar). The mutant was very effectively inactivated by HNP1-3, whereas the wild type was not affected (Fig. 1), which demonstrates that the lack of L-PG leads not only to inhibition but also to efficient killing of staphylococci.
FIG. 1.
Inactivation of S. aureus by isolated neutrophil defensins and by MPO. The numbers of CFU of viable wild-type (black bars) and ΔmprF mutant (gray bars) bacteria after incubation with defensin HNP1-3 or MPO are expressed as percentages of the initial counts (means and standard errors of four counts from a representative experiment).
Neutrophils use oxygen-dependent killing mechanisms in addition to defensins. In order to analyze whether the high susceptibility of the mprF mutant to neutrophil killing is in part caused by increased sensitivity to toxic products of the respiratory burst, the killing of wild-type and mprF cells by myeloperoxidase (MPO) was compared. This experiment was carried out as described above for HNP1-3-mediated killing, except that the bacteria were resuspended in phosphate-buffered saline with 0.0005% HSA and incubated with 0.05 U of MPO (Calbiochem, La Jolla, Calif.) plus 10 μM H2O2 for 45 min (2). NaCl (154 mM) from the phosphate-buffered saline buffer was present in the samples to permit generation of toxic chlorinating compounds. Wild-type and mprF mutant bacteria were equally susceptible to MPO-mediated killing (Fig. 1), which indicated that the presence of L-PG does not affect the susceptibility to oxygen-dependent antimicrobial mechanisms. This is in contrast to the defensin-sensitive S. aureus dltA mutant, which is more susceptible to MPO-mediated inactivation than is the wild type (2).
Neutrophil killing of S. aureus upon inhibition of the respiratory burst.
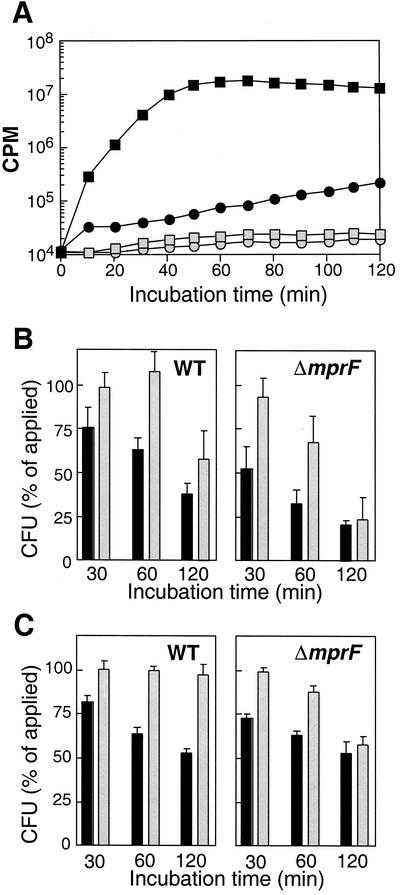
To confirm that the increased killing of S. aureus ΔmprF by neutrophils is due to oxygen-independent mechanisms, inactivation of S. aureus wild-type and ΔmprF mutant bacteria was compared in the presence of diphenyleneiodonium (DPI), which blocks the NADPH oxidase, thereby preventing the formation of the MPO substrate, H2O2 (3, 7), as described earlier in detail (2). Briefly, neutrophils were purified from peripheral blood of healthy human volunteers by using Ficoll-Histopaque gradients. Bacteria were grown as described above for in vitro killing studies, washed twice in Hanks balanced salt solution containing 0.05% HSA, and opsonized with normal human serum (4%) for 10 min at 37°C. Prewarmed bacterial and leukocyte suspensions were mixed to final concentrations of 8.5 × 104 CFU/ml and 2.5 × 106 leukocytes/ml. DPI (20 μM) was added immediately before the bacteria and neutrophils were mixed. Samples (50 μl) were shaken at 37°C, and incubation was stopped after 30, 60, and 120 min by the addition of 2 ml of ice-cold distilled water to disrupt the leukocytes. Appropriate sample volumes were spread onto LB agar plates, and colonies were counted after 24 h of incubation at 37°C. Inhibition of the respiratory burst was confirmed by monitoring luminol-enhanced chemiluminescence of neutrophils in response to opsonized S. aureus as described recently (2). Addition of DPI caused a drop in chemiluminescence of several orders of magnitude, confirming the complete inhibition of NADPH oxidase activity (Fig. 2A); DPI did not affect bacterial viability (data not shown). S. aureus wild-type and ΔmprF mutant bacteria have been shown to be ingested with similar efficiencies (10). Inhibition of the respiratory burst abolished killing of S. aureus wild-type bacteria completely for at least 1 h (Fig. 2B). After 2 h, wild-type bacteria were killed to some extent in the presence of DPI but the efficiency was still considerably lower than that in samples without DPI. In contrast, inhibition of NADPH oxidase only led to some reduction in the killing of S. aureus ΔmprF after 1 h. After 2 h, no significant difference was observed in the killing of S. aureus ΔmprF in the presence or absence of DPI. Thus, oxygen-dependent killing is the most important mechanism for inactivation of S. aureus wild-type bacteria whereas it has only some impact on inactivation of the defensin-susceptible ΔmprF mutant.
FIG. 2.
Inhibition of the respiratory burst (A) and its impact on killing of S. aureus by human neutrophils (B) or monocytes (C). (A) The respiratory burst of neutrophils was monitored by measuring luminol-enhanced chemiluminescence in the presence (open symbols) or absence (black symbols) of the NADPH oxidase inhibitor DPI. Neutrophils were not challenged (circles) or were challenged with opsonized S. aureus (squares) The results of a representative experiment are shown. (B and C) The numbers of viable opsonized wild-type (WT) and ΔmprF mutant CFU after 30, 60, or 120 min of incubation with human neutrophils (B) or monocytes (C) in the absence (black bars) or presence (gray bars) of DPI are expressed as percentages of the initial counts. The values shown are the means and standard errors of at least three independent experiments.
L-PG does not affect susceptibility to killing by normal monocytes.
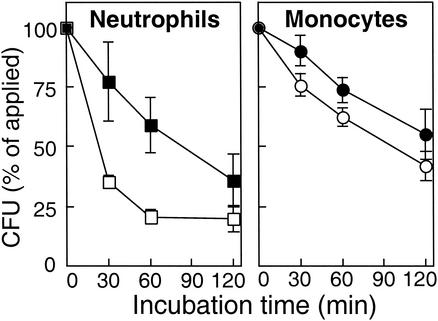
Since neutrophils and monocytes, the major antistaphylococcal phagocytes, differ in the presence of defensins and cathelicidins yet are similar in using oxygen-dependent mechanisms, the killing kinetics of S. aureus wild-type and ΔmprF mutant bacteria by these two types of phagocytes were compared. Neutrophils were prepared as described above, and monocytes were purified with a Monocyte Isolation Kit (Dynal Biotech, Oslo, Norway) (2). Defensin-producing neutrophils inactivated the mutant bacteria considerably faster than wild-type bacteria, while monocytes, which lack defensins, showed no significant differences in the killing kinetics of wild-type and ΔmprF mutant bacteria (Fig. 3). Inhibition of the respiratory burst led to complete abrogation of monocyte killing of S. aureus wild-type bacteria (Fig. 2C), which is in accordance with a major role of oxygen-dependent mechanisms in monocyte antimicrobial activity. Inactivation of S. aureus ΔmprF was strongly reduced in the presence of DPI as well. Interestingly, some killing was observed after 60 min, indicating that human monocytes produce oxygen-independent antimicrobial substances with activity against the mprF mutant that remain to be identified. However, the activity of these compounds appears not to be additive in the oxygen-dependent killing of S. aureus ΔmprF.
FIG. 3.
Killing of S. aureus strains by human phagocytes. The numbers of viable opsonized S. aureus wild-type (black symbols) and ΔmprF mutant (open symbols) CFU after incubation with human neutrophils or monocytes are expressed as percentages of the initial counts. The means and standard deviations of two (neutrophils) or three (monocytes) representative experiments are shown.
Concluding comments.
The existence of redundant defensin genes and differences in CAMP expression in mice and humans have been major obstacles in the analysis of the role of CAMPs and of CAMP resistance by using knockout mice. However, in a recent publication, a mouse strain deficient in cathelicidin production was described (6) that provides important possibilities for future research. Along these lines, we generated defensin-susceptible bacterial mutants of the defensin-resistant bacterium S. aureus. Here it was demonstrated that the lack of L-PG leads not only to inhibition of growth by CAMPs but also to increased killing by defensin HNP1-3 and increased inactivation by neutrophils, even when the oxygen-dependent mechanisms are blocked. These results further support the notion that CAMPs have an important role in preventing microbial infections if the invading microbes are CAMP susceptible. Moreover, the results demonstrate the importance of bacterial mechanisms conferring CAMP resistance for bacterial virulence. The MprF-mediated changes in the staphylococcal cell envelope seem to be rather specific for resistance to oxygen-independent killing, as demonstrated by the equal susceptibility of S. aureus wild-type and ΔmprF mutant bacteria to MPO-generated toxic substances and to killing by monocytes that do not produce defensins and cathelicidins.
The existence of at least two CAMP resistance mechanisms in S. aureus, encoded by the dltABCD and mprF loci, raises the question of whether these systems play different roles in evasion of the host defense. It is important to note that the dltA mutant is more susceptible to MPO-mediated killing (2), whereas the mprF mutant is not. In contrast to S. aureus ΔmprF, which has a highly negatively charged membrane surface compared to the wild type but an unaltered cell wall surface, S. aureus ΔdltA has a more anionic cell envelope (11, 12). Compared to the CAMP molecules, which are usually smaller than 4 kDa, MPO is much larger (131 kDa) and can probably not diffuse through the cell wall and interact directly with the cytoplasmic membrane. However, MPO can probably be better bound by the cell envelope of the dltA mutant, as demonstrated recently with other cationic proteins, such as cytochrome c (11), allowing the production of toxic products in close proximity to the essential components of the bacterial cell. On the other hand, it is noteworthy that the mprF mutant is more susceptible to HNP1-3-mediated killing than the dltA mutant. This is in agreement with the better ability of CAMPs to attack the cytoplasmic membrane of bacteria whose membrane lacks any positively charged lipids.
Acknowledgments
We thank Kok P. M. van Kessel, Friedrich Götz, and L. Vincent Collins for support and helpful discussions; Erik Heezius and Marion Faigle for excellent technical assistance; and Karen A. Brune for critically reading the manuscript.
This work was supported by an EMBO fellowship (ASTF 9521) and grants from the German Research Council (Research Group 449/T2) to A.P. and by the German Ministry of Education and Research (Fö, 01KS9602) and the Interdisciplinary Center of Clinical Research Tübingen (IZKF) to B.N.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Cohen, M. S. 1994. Molecular events in the activation of human neutrophils for microbial killing. Clin. Infect. Dis. 18(Suppl. 2):S170-S179. [DOI] [PubMed] [Google Scholar]
- 2.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. M. van Kessel, J. A. G. van Strijp, F. Götz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to neutrophil killing and are virulence-attenuated in mice. J. Infect. Dis. 186: 214-219. [DOI] [PubMed] [Google Scholar]
- 3.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1996. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 64:3512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehrer, R. I., and T. Ganz. 2002. Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 9:18-22. [DOI] [PubMed] [Google Scholar]
- 5.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 6.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell, V. B., D. G. Tew, O. T. G. Jones, and P. J. England. 1993. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 9.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 10.Peschel, A., R. W. Jack, M. Otto, V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. van Kessel, and J. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Götz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 12.Peschel, A., C. Vuong, M. Otto, and F. Götz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolysins. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staubitz, P., A. Peschel, W. F. Nieuwenhuizen, M. Otto, F. Götz, G. Jung, and R. W. Jack. 2001. Structure-function relationships in the tryptophan-rich, antimicrobial peptide indolicidin. J. Pept. Sci. 7:552-564. [DOI] [PubMed] [Google Scholar]
- 14.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]