Abstract
Mast cells are important as sentinel cells in host defense against bacterial infection. Much of their effectiveness depends upon recruiting other immune cells; however, little is known about the mechanisms of this response. CCL20, also known as macrophage inflammatory protein-3α (MIP-3α), Exodus, and LARC, is a chemokine known to be a potent chemoattractant for immature dendritic cells and T cells. In this study, we examined the human mast cell production of both CCL20 and granulocyte-macrophage colony-stimulating factor (GM-CSF), a critical cytokine for innate immune responses in the lung, in response to Pseudomonas aeruginosa. Reverse transcription-PCR and Western blot analysis demonstrated that the human mast cells (HMC-1) express CCL20 mRNA and are able to produce a significant amount (32.4 ng/ml) of CCL20 protein following stimulation by calcium ionophore and phorbol myristate acetate. Importantly, P. aeruginosa potently stimulated CCL20 production in human cord blood-derived mast cells (CBMC), with production peaking at 6 h after stimulation. This time course of expression was distinct from that of GM-CSF, which peaked after 24 to 48 h. Significant CCL20 production did not occur following immunoglobulin E-mediated activation of CBMC under conditions which induced a substantial GM-CSF response. Interestingly, the CCL20 response of mast cells to P. aeruginosa was relatively resistant to inhibition by the corticosteroid dexamethasone, interleukin-10, or cyclosporine, while GM-CSF production was potently inhibited. However, P. aeruginosa-induced CCL20 production was blocked by the protein kinase C (PKC) inhibitor Ro 31-8220 and a PKC pseudosubstrate. These results support a role for human mast cells in the initiation of immune responses to P. aeruginosa infection.
CCL20, or macrophage inflammatory protein-3α (MIP-3α), also known as Exodus and LARC (67), is a type CC chemokine with important roles in the initiation of immune responses. The CCL20 receptor CCR6 is expressed on dendritic cells (4, 19, 31), memory T cells (32), and NK cells (3). CCR6 has an important role in mediating dendritic cell localization and lymphocyte homeostasis in mucosal tissues, as demonstrated in CCR6 knockout mice (9). CCL20 expression by macrophages, eosinophils (57), dendritic cells, keratinocytes, monocytes, fibroblasts, endothelial cells (4, 23, 25, 26), and several lymphoma and carcinoma cell lines (47) has been described. Moreover, the production of CCL20 is induced by inflammatory stimuli such as lipopolysaccharide (LPS) or tumor necrosis factor alpha (TNF-α) in endothelial cells and monocytes (26, 47). Increased amounts of CCL20 mRNA were found in inflamed tissues (11). In mice, treatment with bacterial LPS stimulates CCL20 mRNA expression in intestinal and lymphoid tissues (60). More recently, treatment of intestinal epithelial cells with either flagellin (54) or TNF-α (21) has been shown to induce CCL20 production, in the latter case via a nonstandard NF-κB binding site on the CCL20 promoter (21). It has been hypothesized that through the local production of CCL20, immune effector cells such as immature dendritic cells would be attracted to the site of inflammation to encounter the invading pathogens (11, 54). However, the nature of the specific bacterial pathogens and resident cell populations required for CCL20 production are poorly defined.
Mast cells are resident tissue cells largely located in areas that interface with the external environment, such as the airways and intestinal mucosa. Mast cells are recognized as critical sentinel cells in host defense against bacterial infection through the recruitment and activation of other immune cells (13, 17, 37). However, little is known about the mechanisms involved. Although many of the effects of mast cells can be explained in terms of their production of a variety of potent cytokine and chemokine mediators, knowledge of mast cell cytokine and chemokine production in the context of bacterial infection is extremely limited.
The gram-negative rod Pseudomonas aeruginosa is a ubiquitous opportunistic pathogen. It causes acute lung disease with high mortality in patients with hospital-acquired pneumonia (46). P. aeruginosa is the predominant pathogen in cystic fibrosis (CF) and colonizes almost all CF patients at some time during the disease process (28, 29). Pulmonary infection caused by P. aeruginosa is characterized by massive inflammatory cell infiltration and is associated with increased production of a number of proinflammatory cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-1 (IL-1), IL-6, IL-8, and TNF-α (10, 43, 58, 62).
GM-CSF is a multifunctional cytokine critical for dendritic cell maturation, neutrophil survival, and T-cell proliferation (41, 52, 61). GM-CSF has been shown to be essential for several P. aeruginosa-induced responses (6, 12, 39, 59). For example, P. aeruginosa exotoxin A-induced T-cell proliferation is dependent on GM-CSF (12). Neutrophils and macrophages derived from GM-CSF-treated mice demonstrated significantly greater killing capacity against P. aeruginosa than those derived from controls (6). Moreover, recombinant GM-CSF significantly improves animal survival after P. aeruginosa infection (6, 39, 59). However, the cellular source of GM-CSF during P. aeruginosa infection remains unclear. Despite intensive studies, the mechanisms of P. aeruginosa-mediated pathogenesis remain incompletely defined. A potential role for mast cells in P. aeruginosa-induced GM-CSF production has not been previously addressed.
In this study, we have demonstrated that P. aeruginosa can provide a direct signal to stimulate CCL20 and GM-CSF production by human mast cells. Interestingly, P. aeruginosa-induced GM-CSF, but not CCL20, can be regulated by IL-10, dexamethasone, or cyclosporine, suggesting that disparate mechanisms are involved in GM-CSF and CCL20 production by mast cells. These findings suggest a novel role for resident mast cells in P. aeruginosa-induced inflammation through the secretion of CCL20 and GM-CSF.
MATERIALS AND METHODS
Reagents.
Human recombinant CCL20 and affinity-purified rabbit anti-human CCL20 antibodies were purchased from Pepro-Tech Inc. (Rocky Hill, N.J.). Biotinylated goat anti-human CCL20 was purchased from R & D Systems (Minneapolis, Minn.).
Bacteria, source, and treatment.
P. aeruginosa strain 8821 (a kind gift from A. Chakrabarty, University of Illinois, Chicago, Ill.) is a mucoid strain isolated from a CF patient (27). P. aeruginosa was cultured in Luria-Bertani broth and harvested when the culture reached an optical density at 640 nm (OD640) of 2 (early stationary phase). Bacteria were washed in phosphate buffer, and their density was adjusted to an OD of 1 before treatment with gentamicin (100 μg/ml) for 2 h. Mast cells were typically treated with P. aeruginosa at a mast cell-to-bacteria ratio of 1:50. Legionella pneumophila Lpl-Svir is a streptomycin-resistant Philadelphia I strain obtained from P. Hoffman (Dalhousie University) (24). Lpl-Svir was kept as a frozen stock at −70°C and was made from an infected culture of HeLa cells. Routinely, an aliquot of the frozen stock was grown on buffered charcoal-yeast extract agar (44) for 3 to 5 days at 37°C in a humid incubator. A bacterial suspension adjusted to an OD620 of 1 (approximately 109 bacteria/ml) was then made in phosphate-buffered saline for infection purposes.
Mast cells.
Highly purified cord blood-derived mast cells (CBMC) were obtained by long-term culture of cord blood progenitor cells, as previously described (35, 49). Briefly, heparinized cord blood, obtained after informed consent, was centrifuged over a Ficoll-Paque separating solution (Seromed, Berlin, Germany). Mononuclear cells, including the progenitors, were cultured at 37°C in a humidified atmosphere containing 5% CO2 at a starting density of 106 cells/ml in RPMI 1640 medium supplemented with l-glutamine, penicillin, streptomycin, 10% fetal calf serum (all from Life Technologies, Grand Island, N.Y.), 1% (wt/vol) bovine serum albumin (BSA) (Sigma, St. Louis, Mo.), 50 μM 2-mercaptoethanol (Sigma), 100 ng of human recombinant stem cell factor (Pepto-Tech)/ml, and 20% CCL-204 (American Type Culture Collection) normal human skin fibroblast supernatant as a source of IL-6. The medium was renewed every 7 days. The percentage of mast cells in the cultures was assessed by toluidine blue staining (pH 1.0) of cytocentrifuge preparations. After more than 8 weeks in culture, mature mast cells were identified by their morphological features and the presence of metachromatic granules and were used in our study. Only preparations confirmed to contain >95% pure mast cells were employed in these experiments.
The human mast cell line HMC-1 5C6 (64), a more differentiated subclone of its parental line, HMC-1, was grown in Iscove's medium (Life Technologies) supplemented with 10% fetal bovine serum (Life Technologies). After confluent growth, the adherent cells were harvested by repeated pipetting and washed in fresh medium before experimentation.
β-Hexosaminidase release assay.
Human CBMC were preincubated with P. aeruginosa (mast cell-to-bacteria ratio, 1:50) for 1 h at 37°C. β-Hexosaminidase was measured in both supernatant and pellet fractions by using a previously reported method (51). Briefly, 50 μl of each sample was incubated with 50 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide (Sigma) dissolved in 0.1 M citrate buffer, pH 5, in a 96-well microtiter plate at 37°C for 1 h. The reaction was stopped with 200 μl of 0.1 M carbonate buffer/well, pH 10.5. The plate was read at 405 nm in an enzyme-linked immunosorbent assay (ELISA) reader.
Reverse transcription-PCR (RT-PCR).
Total RNA was extracted from HMC-1 5C6 by using TRIzol reagent (Life Technologies) in accordance with the manufacturer's instructions. Reverse transcription with MMLV transcriptase (Life Technologies) and PCR with Taq DNA polymerase (Life Technologies) were performed in accordance with the manufacturer's protocols, with some modifications (33). The primers used were as follows: (i) for CCL20, sense, 5′-CTG CTT TGA TGT CAG TGC TGC-3′, and antisense, 5′-TCA CCC AAG TCT GTT TTG G-3′, and (ii) for β2 microglobulin (β2M), sense, 5′-CCA GCA GAG AAT GGA AAG TC-3′, and anti-sense, 5′-GAT GCT GCT TAC ATG TCT CG-3′. The PCR products for CCL20 and β2M were 216 and 269 bp, respectively. Thirty-five cycles were used (95°C for 45 s, 56°C for 45 s, and 72°C for 2 min). Products were run on a 2% agarose gel and stained with ethidium bromide.
Western blot analysis.
HMC-1 5C6 cells (106 cells/ml) were incubated with calcium ionophore A23187 (10−6 M) and phorbol myristate acetate (PMA) (10−7 M) for 24 h. Cell-free supernatants were boiled for 8 min in a sodium dodecyl sulfate sample buffer. After sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, the separated proteins were transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, Calif.). After nonspecific binding sites were blocked with 3% BSA in phosphate-buffered saline, the blots were incubated with anti-CCL20 antibodies at 4°C overnight. After three washes, the blots were incubated with peroxidase-conjugated secondary antibody for 1 h. Prestained rainbow molecular weight standards (Bio-Rad Laboratories) were used as markers. Recombinant human CCL20 was used as a positive control. Visualization of the horseradish peroxidase was done by using Western blot chemiluminescence reagent (DuPont, Boston, Mass.) in accordance with the manufacturer's protocol.
CCL20 and GM-CSF ELISAs.
CCL20 and GM-CSF levels in the supernatants or pellets were measured by using an in-house ELISA. Briefly, 96-well plates were coated with anti-human CCL20 (Pepro-Tech) or anti-human GM-CSF antibodies (Genzyme) at 1 μg/ml for 16 to 20 h at 4°C. Nonspecific binding to the plates was blocked by using a 1% BSA-0.1% Tween-20 solution in phosphate-buffered saline for 1 h at 37°C. The samples and a total of 50 μl of CCL20 (human rMIP-3α; Pepro-Tech) or GM-CSF (human rGM-CSF; R&D Systems) per well were added to the plate and incubated for 18 to 20 h at 4°C. Biotinylated anti-human CCL20 (R&D Systems) or anti-human GM-CSF (Endogen) (0.2 μg/ml) was added to each well and incubated for 1 h at 37°C. After the wells were washed, 50 μl of a 1/2,000 dilution of streptavidin-alkaline phosphatase (Life Technologies) was added to each well in accordance with the manufacturer's instructions. The minimal detectable level was 10 pg/ml for CCL20 and 3 pg/ml for GM-CSF with this system.
RESULTS
Human mast cells express CCL20 mRNA and protein.
Given the importance of CCL20 in immune responses and given that the issue of whether mast cells can produce this chemokine has not been described previously, we used RT-PCR analysis to examine the possibility that human mast cells express CCL20 mRNA. CCL20 primers were designed to detect an mRNA sequence from positions 71 to 286 and yield a 216-bp PCR product. As shown in Fig. 1a, CCL20 mRNA was detected in human mast cell line HMC-1 5C6. No PCR products were detected when reverse transcription was omitted (data not shown).
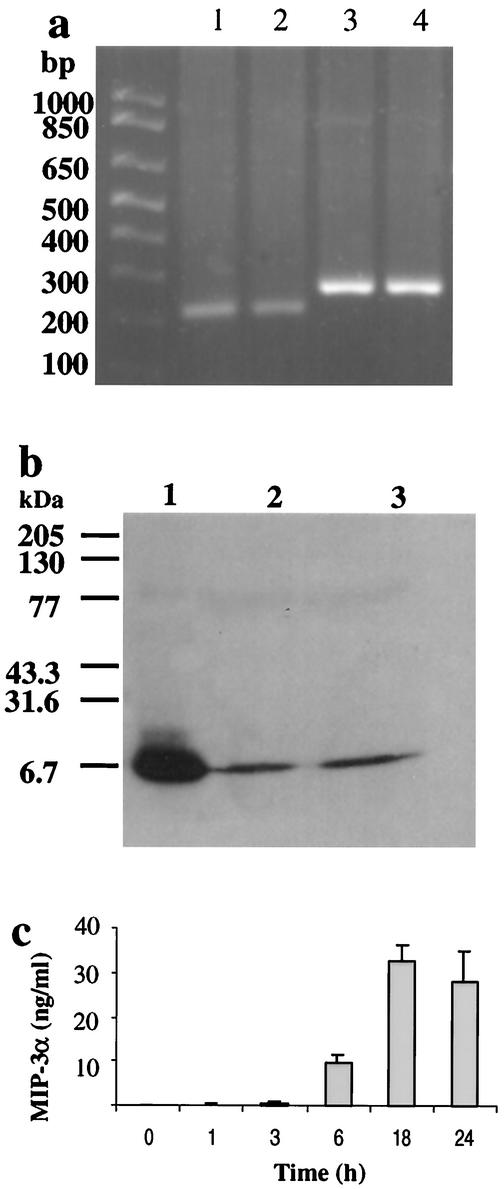
FIG. 1.
mRNA expression and protein secretion of CCL20 by mast cells. (a) RNA samples from HMC-1 cells were analyzed by RT-PCR for CCL20, yielding PCR products of 216 and 269 bp for CCL20 and β2M, respectively. Note the prominent single products at the appropriate sizes. Lanes 1 and 2, CCL20; lanes 3 and 4, β2M. (b) Supernatants from HMC-1 cells stimulated with A23187 (10−6 M) and PMA (10−7 M) for 24 h were analyzed for CCL20 by Western blotting. Lane 1, human recombinant CCL20; lanes 2 and 3, supernatants from A23187- and PMA-treated HMC-1 cells from two independent experiments. (c) CCL20 production by human mast cells. HMC-1 cells were stimulated with A23187 (10−6 M) and PMA (10−7 M) for 1 to 24 h. Cell-free supernatants were used to test CCL20 levels by ELISA. Results are the means ± standard errors of the means for three experiments.
To determine whether human mast cells produce CCL20 protein, supernatants from HMC-1 cells that had been stimulated for 24 h with calcium ionophore A23187 (10−6 M) and PMA (10−7 M) were analyzed by Western blotting with anti-CCL20 antibodies. Human recombinant CCL20 was used as a positive control. The data in Fig. 1b demonstrate that substantial CCL20 production was detected in HMC-1 supernatants following stimulation with A23187 and PMA. This indicates that mast cells express and secrete CCL20 protein after activation. Thus, human mast cells have the capacity to express CCL20 mRNA and secrete CCL20 protein.
To determine the magnitude and kinetics of CCL20 production by human mast cells, we used commercially available anti-CCL20 antibodies to establish an ELISA using human recombinant CCL20 as a standard. After stimulation using HMC-1 5C6 with A23187 and PMA for times between 1 and 24 h, cell-free supernatants were collected and assayed for CCL20 by ELISA. Significant CCL20 production could be detected following 6 h of stimulation (Fig. 1c). After 24 h of stimulation with 10−6 M A23187 and 10−7 M PMA, human mast cells produce 16.1 to 32.4 ng of CCL20 per ml. Production of CCL20 by HMC-1 cells was both time course and dose dependent. Given that mast cells have the capacity to synthesize and store cytokines in granules (18), we compared CCL20 levels in the supernatants and cell pellets. HMC-1 cells with or without stimulation with A23187 and PMA were disrupted by sonication to release cell-associated cytokines. Our results indicated that without stimulation, CCL20 levels in the supernatants and pellet were 15.9 ± 3.7 and 6.8 ± 4.6 pg/ml, respectively (n = 3), suggesting there was little preformed CCL20 stored in HMC-1 5C6. After 24 h of stimulation with PMA and A23187, CCL20 levels in the supernatants and pellet were 2,178 ± 809 and 338 ± 136 pg/ml, respectively (n = 3), indicating that this cytokine is readily secreted after synthesis.
P. aeruginosa stimulates CCL20 and GM-CSF production by human mast cells.
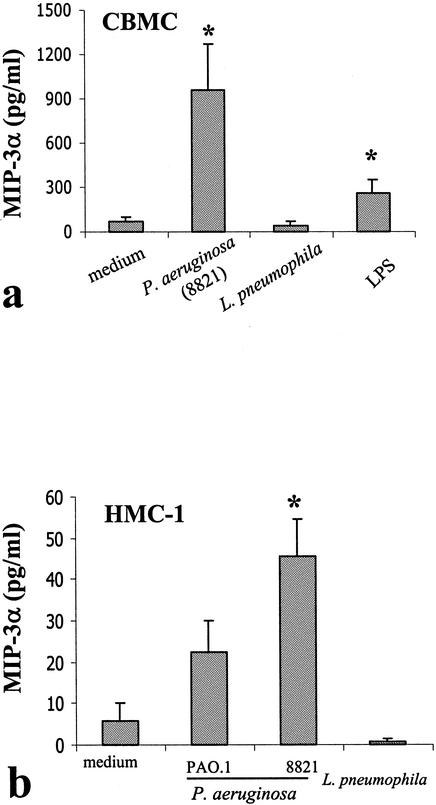
To examine whether mast cells produce CCL20 in response to bacterial stimulation, the CF-associated mucoid P. aeruginosa 8821 was used to stimulate human CBMC. As shown in Fig. 2, this P. aeruginosa strain significantly stimulates CCL20 production by both CBMC and HMC-1. To determine if the mast cell CCL20 response is specific to P. aeruginosa, we employed the bacterium Legionella pneumophila as a control stimulus. Our results indicated that both CBMC and HMC-1 did not respond to L. pneumophila to produce CCL20 (Fig. 2a and b). Interestingly, the CF-associated mucoid strain 8821 was significantly more potent than the nonmucoid P. aeruginosa strain PAO.1 in stimulating CCL20 production from HMC-1 cells (Fig. 2b). Similarly, in CBMC, strain 8821 also elicited a stronger response (CCL20 production, 603.9 pg/ml; GM-CSF production, 325.3 pg/ml) than strain PAO.1 (CCL20 production, 373.3 pg/ml; GM-CSF production, 226.7 pg/ml). The baseline levels for CCL20 and GM-CSF were 157 and 1.9 pg/ml, respectively. It is also notable that the P. aeruginosa-activated CBMC produced approximately 20-fold more CCL20 on a per-cell basis than similarly activated HMC-1 cells. It has been reported that HMC-1 cells express a distinct profile of receptors on their surfaces relative to the profile of in vivo-derived mast cells (2) and possess an abnormal signaling mechanism due to a mutation on c-kit on their surface (16). Such differences may contribute to the distinct responses between HMC-1 cells and CBMC.
FIG. 2.
P. aeruginosa but not L. pneumophila stimulated CCL20 production by human CBMC (a) and the human mast cell line HMC-1 (b). Human mast cells (106 cells/ml) were stimulated with P. aeruginosa (CF-associated mucoid strain 8821 and nonmucoid strain PAO.1), L. pneumophila (cell-to-bacteria ratio, 1/50), or LPS (from P. aeruginosa serotype 10; Sigma) (10 μg/ml) for 24 h. CCL20 levels in cell-free supernatants were determined by ELISA. Results are means ± standard errors of the means from eight individual donors (a) and from four independent experiments (b).
The ability of P. aeruginosa (8821) to induce CBMC degranulation was examined by using cells from five separate donors over a 30-min incubation period. A small but significant (P < 0.01) response was observed with β-hexosaminidase used as a readout (mean degranulation values ± standard errors of the means [n = 5] for control- and P. aeruginosa-treated cells, 10.98 ± 1.8% and 15.18 ± 2.14%, respectively).
Given that various bacterial LPS have been used to stimulate other cell types, such as monocytes, to produce CCL20 (26, 47), we examined whether LPS from P. aeruginosa was able to significantly induce CCL20 production by human mast cells. Human CBMC were incubated with P. aeruginosa-derived LPS at a high concentration (10 μg/ml) for 24 h. CCL20 levels in cell-free supernatants were determined. A small but significant (P < 0.05) level of CCL20 production was observed after LPS stimulation (Fig. 2a). However, even when this high concentration was employed, LPS-mediated CCL20 production was much lower than that induced by P. aeruginosa strain 8821, suggesting that LPS may not be the major component inducing CCL20 production by mast cells.
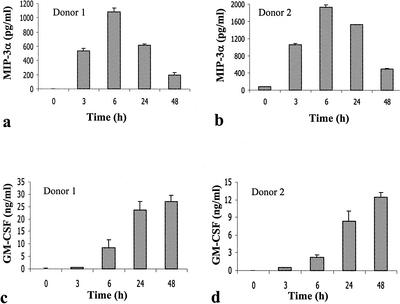
Time course studies demonstrated that significant CCL20 production was observed at 3 h and peaked at 6 h following P. aeruginosa stimulation, a time course of production similar to that of TNF-α following antigen stimulation in other mast cell populations (33). This time course of CCL20 production is distinct from that of GM-CSF (Fig. 3), which peaked at 24 to 48 h after P. aeruginosa stimulation. Given that CCL20 production peaked earlier than that of GM-CSF, we tested whether P. aeruginosa-induced GM-CSF production is dependent on CCL20. CBMC were treated with anti-CCL20 antibodies (22 μg/ml) (R&D Systems) during the course of P. aeruginosa (8821) stimulation for 24 h. There is no difference in GM-CSF production between CBMC treated with anti-CCL20 antibodies together with P. aeruginosa (199.6 ± 12.4 pg/ml) and CBMC treated with P. aeruginosa alone (182.9 ± 12.9 pg/ml). GM-CSF levels were undetectable in both unstimulated CBMC and CMBC treated with anti-CCL20 antibodies alone. This result suggests that P. aeruginosa-induced GM-CSF production by CBMC is independent of CCL20 production.
FIG. 3.
Distinct time course of CCL20 (a and b) and GM-CSF (c and d) production by human CBMC as a result of CF-associated P. aeruginosa (8821) stimulation. CBMC (106 cells/ml) were stimulated with P. aeruginosa (8821) (cell-to-bacteria ratio, 1/50) for various times. CCL20 and GM-CSF levels in cell-free supernatants were determined by ELISA. Mean values ± standard errors of the means of the results from triplicate assays are shown for each donor.
IgE-mediated CBMC activation does not induce a CCL20 response.
Immunoglobulin E (IgE)-mediated human mast cell activation, such as that which occurs during the initiation of allergic responses, is known to induce the expression of a variety of cytokines and chemokines. In order to examine the ability of such activation to induce CCL20 expression, CBMC were sensitized with 10 ng of human myeloma IgE (Chemicon)/ml for 48 h, washed repeatedly, and then activated with rabbit anti-human IgE (Dako, Mississuagua, Ontario, Canada) under conditions similar to those used for bacterial activation of the cells. After 24 h, the supernatants were harvested and assayed for CCL20 and GM-CSF content. IgE-mediated activation induced a significant GM-CSF response. However, CCL20 levels in the supernatants were not significantly elevated compared with levels in the medium control (Table 1). Supernatants from anti-IgE-activated mast cells contained significantly (P < 0.05) less CCL20 than supernatants from cells treated with control rabbit IgG. This apparent decrease in CCL20 production did not appear to be due to degradation of CCL20 within supernatants from anti-IgE-activated mast cells. Experiments in which a recombinant CCL20 standard was incubated with such supernatants for 24 h at 37°C (n = 3) demonstrated that over 75% of the CCL20 added could be detected by ELISA after incubation (data not shown).
TABLE 1.
Effects of IgE-dependent activation on mast cell production of CCL20 and GM-CSFa
| Sample | Supernatant chemokine or cytokine content (pg/ml)
|
|
|---|---|---|
| CCL20 | GM-CSF | |
| Medium | 103 ± 49 | 368 ± 32 |
| Rabbit anti-IgE | 1.8 ± 0.3b | 771 ± 146b |
| Control rabbit-IgG | 136 ± 95 | 195 ± 28 |
Values are means ± standard errors of the means. n = 5 or 6 per group. Groups were compared by a paired Student's t test.
Significant increase (P < 0.01) compared with the medium control group.
Effects of dexamethasone, IL-10, and cyclosporine on P. aeruginosa-induced CCL20 and GM-CSF production by human mast cells.
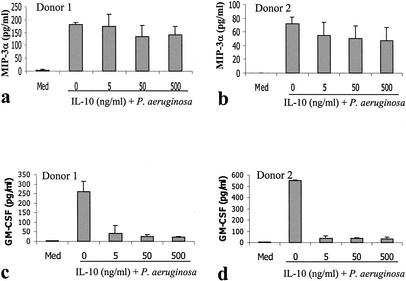
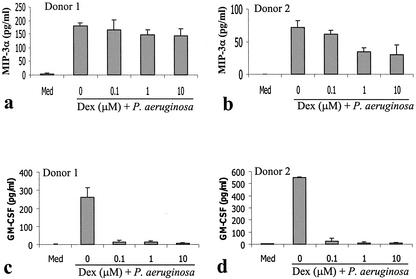
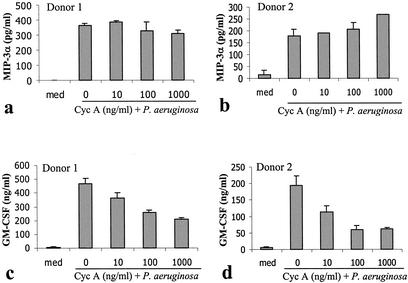
A number of agents have been demonstrated to inhibit the production of inflammatory cytokines by mast cells in response to either IgE-mediated activation or bacterial products. These include the anti-inflammatory cytokine IL-10 (33, 38, 48), the corticosteroid dexamethasone (36, 50, 66), and the calcineurin inhibitor cyclosporine (45, 63, 65). The ability of these agents to alter the mast cell CCL20 and GM-CSF responses to P. aeruginosa was examined. Following IL-10 treatment (5 ng/ml) (Fig. 4), the GM-CSF response was reduced by over 80%. In contrast, the CCL20 response was reduced by less than 30% even when treated with 500 ng of IL-10/ml. The CCL20 response was similarly resistant to corticosteroid and cyclosporine treatment (Fig. 5 and 6). Following corticosteroid treatment, the GM-CSF response to P. aeruginosa was reduced by over 95%, while the majority of the CCL20 response remained. Cyclosporine had a less dramatic effect in inhibiting GM-CSF production but was still able to inhibit the GM-CSF response by over 50% at high doses (1 μg/ml). In contrast, cyclosporine consistently failed to inhibit the CCL20 response.
FIG. 4.
P. aeruginosa-induced production of CCL20 (a and b) by human mast cells is not inhibited by IL-10, but that of GM-CSF (c and d) is inhibited. Human CBMC (5 × 105 cells/ml) were stimulated with P. aeruginosa strain 8821 (mast cell-to-bacteria ratio, 50:1) in the presence or absence of various concentrations (5, 50, and 500 ng/ml) of IL-10 for 24 h. Cell-free supernatants were used to determine cytokine levels by ELISA. Mean results from triplicate assays are shown for each of two representative cell donors.
FIG. 5.
Differential effects of dexamethasone (Dex) on P. aeruginosa-induced CCL20 (a and b) and GM-CSF (c and d) production by human mast cells. Human CBMC (5 × 105 cells/ml) were treated with P. aeruginosa strain 8821 (mast cell-to-bacteria ratio, 50:1) in the absence (0 μM) or presence of various concentrations (0.1, 1, and 10 μM) of Dex for 24 h. Cell-free supernatants were used to determine cytokine levels by ELISA. Mean results from triplicate assays are shown for each of two representative cell donors.
FIG. 6.
Cyclosporine does not block P. aeruginosa-induced production of CCL20 (a and b) by human mast cells but blocks that of GM-CSF (c and d). Human CBMC (5 × 105 cells/ml) were treated with P. aeruginosa strain 8821 (mast cell-to-bacteria ratio, 50:1) in the absence (0 ng/ml) or presence of various concentrations (10, 100, and 1,000 ng/ml) of cyclosporine for 24 h. Cell-free supernatants were used to determine cytokine levels by ELISA. Mean ELISA results from triplicate well assays are shown for each of two representative donors.
Protein kinase C is involved in CCL20 production by human mast cells.
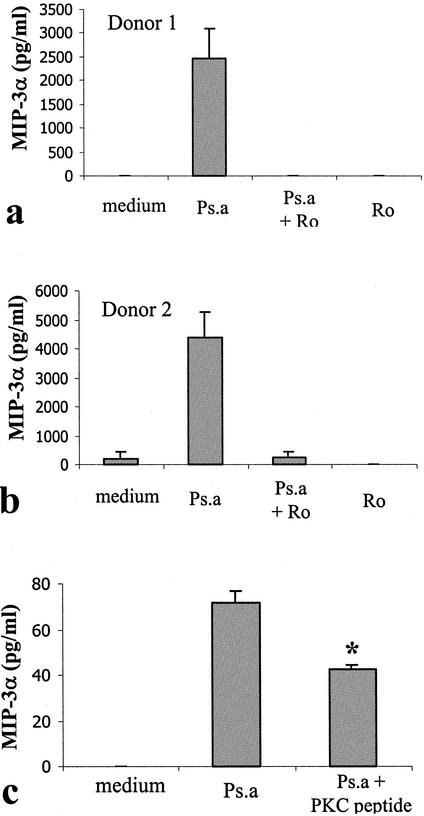
To investigate the role of protein kinase C (PKC) in CCL20 production by human mast cells, PKC inhibitors Ro 31-8220 and a PKC pseudosubstrate (PKC-peptide) were used. Concentrations of 10 and 20 μM for Ro 31-8220 and PKC-peptide, respectively, were used in our recent study and demonstrated inhibitory effects on mast cells (7). Thus, these concentrations were selected in this study. CBMC were treated with Ro 31-8220 at a concentration of 10 μM during the course of P. aeruginosa stimulation. P. aeruginosa-induced CCL20 production was completely inhibited by Ro 31-8220 treatment (Fig. 7). Moreover, treatment of CBMC with a PKC peptide (20 μM), a sequence derived from PKCα and PKCβ, significantly inhibited P. aeruginosa-induced CCL20 production (Fig. 7). Interestingly, P. aeruginosa-induced GM-CSF production (567.4 ± 86.3 pg/ml) was also completely inhibited by Ro 31-8220 (0 ± 0 pg/ml [undetectable]), suggesting that PKC may likely be an early signaling molecule involved in P. aeruginosa-induced mast cell activation. Neither the PKC peptide nor the Ro 31-8220 significantly altered cell viability as assessed by trypan blue exclusion.
FIG. 7.
PKC inhibitors block P. aeruginosa-induced CCL20 production by human mast cells. (a and b) CBMC (5 × 105 cells/ml) were treated with P. aeruginosa strain 8821 (Ps.a) for 24 h in the presence (10 μM) or absence of Ro 31-8220 (Ro). Results are the mean values from triplicate wells from representative donor responses. (c) HMC-1 5C6 cells were treated with a cell-permeable PKC pseudosubstrate, a sequence derived from PKCα and PKCβ (PKC peptide; 20 μM). Cell-free supernatants were used to determine CCL20 proteins by ELISA. Values are the means ± standard errors of the means for four similar experiments.
DISCUSSION
Innate responses usually precede, and are necessary for, the establishment of adaptive immunity (40). An important aspect of such an integrated response is the recruitment of dendritic cells and T cells to sites of infection. Critical roles for mast cells in innate immunity against bacterial infection have previously been demonstrated in mice (13, 17, 37). Through a mechanism dependent upon TNF-α, mast cells recruit neutrophils to the local tissue to eradicate the invading bacteria. In this study, we have demonstrated, for the first time, that human mast cells produce CCL20, a chemokine essential for efficient recruitment of immature dendritic cells and T cells to mucosal sites (9). These data suggest that P. aeruginosa-induced CCL20 production by resident tissue mast cells may serve as part of a mechanism for the induction of adaptive response through dendritic cell-mediated mechanisms. This concept is supported by the finding that in other cell types, CCL20 expression can be induced by bacterial LPS (60) and is increased in the inflamed tissues (11). CCL20 production occurs under conditions that also activate human mast cells to produce GM-CSF. GM-CSF has been repeatedly shown to have a critical role in innate immune responses, especially in the lung (6, 12, 39, 41, 52, 59, 61).
Mast cells produce distinct mediator profiles in response to different stimuli, through differential activation of surface receptors (30, 33). In this study, we found that CCL20 production by human mast cells is specifically induced by P. aeruginosa but not by L. pneumophila. Moreover, activation of mast cells through the IgE receptor, FcɛRI, under conditions which induced significant degranulation and GM-CSF expression, did not lead to CCL20 production. In contrast, IgE-mediated activation of mast cells appeared to profoundly inhibit the baseline levels of production of CCL20. The mechanism of this inhibition is unclear. The multiple anti-IgE-induced mediators which have autocrine effects on mast cell functions may be relevant. These data suggest that mast cell production of CCL20 is tightly regulated and initiated in response to specific pathogen-associated signals. CCL20 is one example of a mast cell product that is not expressed following IgE-mediated mast cell activation; another such molecule is gamma interferon, an important activator of T cells and antigen-presenting cells (20). In keeping with reports for rodent mast cells (5, 15), there was evidence of a low level of degranulation of CBMC in response to P. aeruginosa activation, as assessed with β-hexosaminidase release. However, we do not know whether CCL20 production was linked to this degranulation event. Time course analysis of CCL20 production, as well as direct analysis of the CCL20 content of CBMC pellets, suggests that either no or very low (undetectable) amounts of CCL20 are stored within human mast cells (data not shown).
P. aeruginosa is a common cause of acute and chronic pneumonia in humans. Pulmonary infection caused by P. aeruginosa is a major factor leading to the morbidity and mortality associated with CF. Lung infection with P. aeruginosa in CF is associated with acute and chronic inflammation accompanied by disturbed cytokine and chemokine regulation. Mast cells are abundant in the airways (500 to 4,000 cells/mm [1], occupying approximately 1.6 to 2.1% of the area of the alveolar wall [14]) and are able to produce multiple cytokines and chemokines. However, little is known about the roles of mast cells in P. aeruginosa-mediated pathogenesis. Increased mast cell numbers in P. aeruginosa-infected lung (55, 56) and evidence of mast cell activation in the CF airway (22) suggest a role for mast cells in P. aeruginosa infection. We have examined the responses of mast cells to CF-associated P. aeruginosa and demonstrated significant CCL20 and GM-CSF production by both human primary cultured CBMC and the human mast cell line HMC-1. GM-CSF production by mast cells, in this context, may be important for maintaining the neutrophilic infiltration that is typical of chronic disease and for macrophage differentiation (53), while the immune activation resulting from continued recruitment of dendritic cells to sites of infection may perpetuate the chronic inflammatory state. It is notable that while GM-CSF production by P. aeruginosa-activated human mast cells was readily inhibited by corticosteroids, IL-10, or cyclosporine, CCL20 production was highly resistant to modulation by any of these agents. These data suggest that distinct mechanisms are involved in the P. aeruginosa-induced production of CCL20 and GM-CSF.
P. aeruginosa-mediated CCL20 production is a relatively early event which peaks at 6 h, a time course of production which is distinct both from that of GM-CSF, which peaked after 24 to 48 h following P. aeruginosa stimulation, and from the CBMC IL-6 response to this pathogen, which we have reported to follow a similar pattern (7). These data suggest that CCL20 production is unlikely to be due to a mast cell response to an intermediate cytokine or chemokine signal and precedes the major production of GM-CSF and IL-6.
Understanding the mechanisms by which mast cell function is regulated is a key step towards the design of a therapeutic approach to modulate mast cell activities. PKC is involved in several mast cell activities, such as degranulation (8, 42) or IL-6 production, but not in others, such as antigen-induced IL-10 expression (42). We have reported previously that internalization of bacterial E. coli by human mast cells involves PKC (34) and that P. aeruginosa-induced IL-6 production by mast cells requires PKC (7). In this study, we attempted to test whether PKC is involved in CCL20 production in human mast cells. Activation of mast cells by the PKC activator PMA mediated significant production of CCL20. PKC inhibitor Ro 31-8220 completely inhibited P. aeruginosa-induced CCL20 production. Moreover, a PKC inhibitor peptide significantly blocked CCL20 production by CBMC. Thus, PKC is probably directly involved in CCL20 expression and provides a potential target for the modulation of CCL20 production by human mast cells. This is in keeping with our published evidence of a critical role for PKC in the induction of other mast cell cytokine responses, such as IL-6 production, by P. aeruginosa (7).
In summary, we have demonstrated that human mast cells are able to produce CCL20 mRNA and protein, an important chemokine involved in dendritic cell-associated adaptive immunity. Production of CCL20 by mast cells was specifically induced by P. aeruginosa but not by L. pneumophila or by aggregation of IgE receptors. In contrast to the GM-CSF response to P. aeruginosa, CCL20 production was relatively resistant to the inhibitory effects of corticosteroids, IL-10, or cyclosporine. However, PKC appears to have a role in CCL20 production by human mast cells. These findings suggest a novel role for mast cells in the initiation of host defense against P. aeruginosa infection and in the perpetuation of inflammatory responses at mast cell-rich sites such as the airways.
Acknowledgments
We thank Yi-Song Wei, Ula Kadela-Stolarz, and Elizabeth Garduno for their excellent technical assistance in the culture of cord blood-derived mast cells. We are grateful for the provision of P. aeruginosa strains from Ananda Chakrabarty at the University of Illinois College of Medicine, Chicago, Illinois.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canadian Cystic Fibrosis Foundation, the Nova Scotia Health Research Foundation, and the IWK Health Center to T.J.L., a grant from the Natural Sciences and Engineering and Research Council of Canada to R.G., and a grant from the CIHR to J.S.M.
Editor: B. B. Finlay
REFERENCES
- 1.Abraham, S. N., and R. Malaviya. 1997. Mast cells in infection and immunity. Infect. Immun. 65:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agis, H., W. Fureder, H. C. Bankl, M. Kundi, W. R. Sperr, M. Willheim, G. Boltz-Nitulescu, J. H. Butterfield, K. Kishi, K. Lechner, and P. Valent. 1996. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunology 87:535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aoukaty, A., B. Rolstad, A. Giaid, and A. A. Maghazachi. 1998. MIP-3alpha, MIP-3beta and fractalkine induce the locomotion and the mobilization of intracellular calcium, and activate the heterotrimeric G proteins in human natural killer cells. Immunology 95:618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba, M., T. Imai, M. Nishimura, M. Kakizaki, S. Takagi, K. Hieshima, H. Nomiyama, and O. Yoshie. 1997. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 272:14893-14898. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann, U., J. Scheffer, M. Koller, W. Schonfeld, G. Erbs, F. E. Muller, and W. Konig. 1989. Induction of inflammatory mediators (histamine and leukotrienes) from rat peritoneal mast cells and human granulocytes by Pseudomonas aeruginosa strains from burn patients. Infect. Immun. 57:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez, L. E., J. C. Martinelli, R. Gascon, M. Wu, and L. S. Young. 1990. Protection against gram-negative bacteremia in neutropenic mice with recombinant granulocyte-macrophage colony-stimulating factor. Cytokine 2:287-293. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau, R. T., R. Garduno, and T. J. Lin. 2002. Protein phosphatase 2A and protein kinase C alpha are physically associated and are involved in Pseudomonas aeruginosa-induced interleukin 6 production by mast cells. J. Biol. Chem. 277:5322-5329. [DOI] [PubMed] [Google Scholar]
- 8.Chang, E. Y., Z. Szallasi, P. Acs, V. Raizada, P. C. Wolfe, C. Fewtrell, P. M. Blumberg, and J. Rivera. 1997. Functional effects of overexpression of protein kinase C-alpha, -beta, -delta, -epsilon, and -eta in the mast cell line RBL-2H3. J. Immunol. 159:2624-2632. [PubMed] [Google Scholar]
- 9.Cook, D. N., D. M. Prosser, R. Forster, J. Zhang, N. A. Kuklin, S. J. Abbondanzo, X. D. Niu, S. C. Chen, D. J. Manfra, M. T. Wiekowski, L. M. Sullivan, S. R. Smith, H. B. Greenberg, S. K. Narula, M. Lipp, and S. A. Lira. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495-503. [DOI] [PubMed] [Google Scholar]
- 10.Cripps, A. W., M. L. Dunkley, R. L. Clancy, and J. Kyd. 1995. Pulmonary immunity to Pseudomonas aeruginosa. Immunol. Cell. Biol. 73:418-424. [DOI] [PubMed] [Google Scholar]
- 11.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon, D. M., and M. L. Misfeldt. 1995. GM-CSF is required for the Pseudomonas exotoxin A-induced proliferation of immature T cells in athymic mice. Cell. Immunol. 160:65-70. [DOI] [PubMed] [Google Scholar]
- 13.Echtenacher, B., D. N. Mannel, and L. Hultner. 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381:75-77. [DOI] [PubMed] [Google Scholar]
- 14.Fox, B., T. B. Bull, and A. Guz. 1981. Mast cells in the human alveolar wall: an electronmicroscopic study. J. Clin. Pathol. 34:1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedl, P., B. Konig, and W. Konig. 1992. Effects of mucoid and non-mucoid Pseudomonas aeruginosa isolates from cystic fibrosis patients on inflammatory mediator release from human polymorphonuclear granulocytes and rat mast cells. Immunology 76:86-94. [PMC free article] [PubMed] [Google Scholar]
- 16.Furitsu, T., T. Tsujimura, T. Tono, H. Ikeda, H. Kitayama, U. Koshimizu, H. Sugahara, J. H. Butterfield, L. K. Ashman, Y. Kanayama, et al. 1993. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Investig. 92:1736-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli, S. J., M. Maurer, and C. S. Lantz. 1999. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 11:53-59. [DOI] [PubMed] [Google Scholar]
- 18.Gordon, J. R., and S. J. Galli. 1990. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 346:274-276. [DOI] [PubMed] [Google Scholar]
- 19.Greaves, D. R., W. Wang, D. J. Dairaghi, M. C. Dieu, B. Saint-Vis, K. Franz-Bacon, D. Rossi, C. Caux, T. McClanahan, S. Gordon, A. Zlotnik, and T. J. Schall. 1997. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3 alpha and is highly expressed in human dendritic cells. J. Exp. Med. 186:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, A. A., I. Leal-Berumen, K. Croitoru, and J. S. Marshall. 1996. Rat peritoneal mast cells produce IFN-gamma following IL-12 treatment but not in response to IgE-mediated activation. J. Immunol. 157:2123-2128. [PubMed] [Google Scholar]
- 21.Harant, H., S. A. Eldershaw, and I. J. Lindley. 2001. Human macrophage inflammatory protein-3alpha/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-alpha via a non-standard NF-kappaB site. FEBS Lett. 509:439-445. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, W. R., Jr., and E. Y. Chi. 1992. Degranulation of cystic fibrosis nasal polyp mast cells. J. Pathol. 166:395-404. [DOI] [PubMed] [Google Scholar]
- 23.Hieshima, K., T. Imai, G. Opdenakker, J. Van Damme, J. Kusuda, H. Tei, Y. Sakaki, K. Takatsuki, R. Miura, O. Yoshie, and H. Nomiyama. 1997. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J. Biol. Chem. 272:5846-5853. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman, P. S., C. A. Butler, and F. D. Quinn. 1989. Cloning and temperature-dependent expression in Escherichia coli of a Legionella pneumophila gene coding for a genus-common 60-kilodalton antigen. Infect. Immun 57:1731-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homey, B., M. C. Dieu-Nosjean, A. Wiesenborn, C. Massacrier, J. J. Pin, E. Oldham, D. Catron, M. E. Buchanan, A. Muller, R. deWaal Malefyt, G. Deng, R. Orozco, T. Ruzicka, P. Lehmann, S. Lebecque, C. Caux, and A. Zlotnik. 2000. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J. Immunol. 164:6621-6632. [DOI] [PubMed] [Google Scholar]
- 26.Hromas, R., P. W. Gray, D. Chantry, R. Godiska, M. Krathwohl, K. Fife, G. I. Bell, J. Takeda, S. Aronica, M. Gordon, S. Cooper, H. E. Broxmeyer, and M. J. Klemsz. 1997. Cloning and characterization of exodus, a novel beta-chemokine. Blood 89:3315-3322. [PubMed] [Google Scholar]
- 27.Kamath, S., V. Kapatral, and A. M. Chakrabarty. 1998. Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol. Microbiol. 30:933-941. [DOI] [PubMed] [Google Scholar]
- 28.Kerem, E. 1997. The role of Pseudomonas aeruginosa in the pathogenesis of lung disease in cystic fibrosis: more questions than answers. Pediatr. Pulmonol. Suppl. 16:265-266. [DOI] [PubMed] [Google Scholar]
- 29.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 30.Leal-Berumen, I., P. Conlon, and J. S. Marshall. 1994. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J. Immunol. 152:5468-5476. [PubMed] [Google Scholar]
- 31.Liao, F., R. Alderson, J. Su, S. J. Ullrich, B. L. Kreider, and J. M. Farber. 1997. STRL22 is a receptor for the CC chemokine MIP-3alpha. Biochem. Biophys. Res. Commun. 236:212-217. [DOI] [PubMed] [Google Scholar]
- 32.Liao, F., R. L. Rabin, C. S. Smith, G. Sharma, T. B. Nutman, and J. M. Farber. 1999. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J. Immunol. 162:186-194. [PubMed] [Google Scholar]
- 33.Lin, T. J., and A. D. Befus. 1997. Differential regulation of mast cell function by IL-10 and stem cell factor. J. Immunol. 159:4015-4023. [PubMed] [Google Scholar]
- 34.Lin, T. J., Z. Gao, M. Arock, and S. N. Abraham. 1999. Internalization of FimH+ Escherichia coli by the human mast cell line (HMC-1 5C6) involves protein kinase C. J. Leukoc. Biol. 66:1031-1038. [DOI] [PubMed] [Google Scholar]
- 35.Lin, T. J., T. B. Issekutz, and J. S. Marshall. 2000. Human mast cells transmigrate through human umbilical vein endothelial monolayers and selectively produce IL-8 in response to stromal cell-derived factor-1 alpha. J. Immunol. 165:211-220. [DOI] [PubMed] [Google Scholar]
- 36.Lippert, U., S. Kruger-Krasagakes, A. Moller, U. Kiessling, and B. M. Czarnetzki. 1995. Pharmacological modulation of IL-6 and IL-8 secretion by the H1-antagonist decarboethoxy-loratadine and dexamethasone by human mast and basophilic cell lines. Exp. Dermatol. 4:272-276. [DOI] [PubMed] [Google Scholar]
- 37.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 38.Marshall, J. S., I. Leal-Berumen, L. Nielsen, M. Glibetic, and M. Jordana. 1996. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J. Clin. Investig. 97:1122-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayer, P., E. Schutze, C. Lam, F. Kricek, and E. Liehl. 1991. Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice. J. Infect. Dis. 163:584-590. [DOI] [PubMed] [Google Scholar]
- 40.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 41.Mellstedt, H., J. Fagerberg, J. E. Frodin, L. Henriksson, A. L. Hjelm-Skoog, M. Liljefors, P. Ragnhammar, J. Shetye, and A. Osterborg. 1999. Augmentation of the immune response with granulocyte-macrophage colony-stimulating factor and other hematopoietic growth factors. Curr. Opin. Hematol. 6:169-175. [DOI] [PubMed] [Google Scholar]
- 42.Nechushtan, H., M. Leitges, C. Cohen, G. Kay, and E. Razin. 2000. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cβ. Blood 95:1752-1757. [PubMed] [Google Scholar]
- 43.Osika, E., J. M. Cavaillon, K. Chadelat, M. Boule, C. Fitting, G. Tournier, and A. Clement. 1999. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur. Respir. J. 14:339-346. [DOI] [PubMed] [Google Scholar]
- 44.Pasculle, A. W., J. C. Feeley, R. J. Gibson, L. G. Cordes, R. L. Myerowitz, C. M. Patton, G. W. Gorman, C. L. Carmack, J. W. Ezzell, and J. N. Dowling. 1980. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J. Infect. Dis. 141:727-732. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen, C., H. Permin, P. Stahl Skov, S. Norn, M. Svenson, H. Mosbech, and K. Bendtzen. 1985. Inhibitory effect of cyclosporin A on histamine release from human leukocytes and rat mast cells. Allergy 40:103-107. [DOI] [PubMed] [Google Scholar]
- 46.Pennington, J. E. 1990. Pseudomonas aeruginosa. Vaccines and immunotherapy. Infect. Dis. Clin. North Am. 4:259-270. [PubMed] [Google Scholar]
- 47.Rossi, D. L., A. P. Vicari, K. Franz-Bacon, T. K. McClanahan, and A. Zlotnik. 1997. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3α and MIP-3β. J. Immunol. 158:1033-1036. [PubMed] [Google Scholar]
- 48.Royer, B., S. Varadaradjalou, P. Saas, J. J. Guillosson, J. P. Kantelip, and M. Arock. 2001. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin. Exp. Allergy 31:694-704. [DOI] [PubMed] [Google Scholar]
- 49.Saito, H., M. Ebisawa, H. Tachimoto, M. Shichijo, K. Fukagawa, K. Matsumoto, Y. Iikura, T. Awaji, G. Tsujimoto, M. Yanagida, H. Uzumaki, G. Takahashi, K. Tsuji, and T. Nakahata. 1996. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J. Immunol. 157:343-350. [PubMed] [Google Scholar]
- 50.Schleimer, R. P., E. S. Schulman, D. W. MacGlashan, Jr., S. P. Peters, E. C. Hayes, G. K. Adams III, L. M. Lichtenstein, and N. F. Adkinson, Jr. 1983. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J. Clin. Investig. 71:1830-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz, L. B., K. F. Austen, and S. I. Wasserman. 1979. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J. Immunol. 123:1445-1450. [PubMed] [Google Scholar]
- 52.Shannon, M. F., S. R. Himes, and L. S. Coles. 1995. GM-CSF and IL-2 share common control mechanisms in response to costimulatory signals in T cells. J. Leukoc. Biol. 57:767-773. [DOI] [PubMed] [Google Scholar]
- 53.Shibata, Y., P. Y. Berclaz, Z. C. Chroneos, M. Yoshida, J. A. Whitsett, and B. C. Trapnell. 2001. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15:557-667. [DOI] [PubMed] [Google Scholar]
- 54.Sierro, F., B. Dubois, A. Coste, D. Kaiserlian, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 98:13722-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song, Z., H. K. Johansen, V. Faber, C. Moser, A. Kharazmi, J. Rygaard, and N. Hoiby. 1997. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob. Agents Chemother. 41:961-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song, Z. J., H. K. Johansen, V. Faber, and N. Hoiby. 1997. Ginseng treatment enhances bacterial clearance and decreases lung pathology in athymic rats with chronic P. aeruginosa pneumonia. APMIS 105:438-444. [PubMed] [Google Scholar]
- 57.Sullivan, S. K., D. A. McGrath, F. Liao, S. A. Boehme, J. M. Farber, and K. B. Bacon. 1999. MIP-3alpha induces human eosinophil migration and activation of the mitogen-activated protein kinases (p42/p44 MAPK). J. Leukoc. Biol. 66:674-682. [DOI] [PubMed] [Google Scholar]
- 58.Tam, M., G. J. Snipes, and M. M. Stevenson. 1999. Characterization of chronic bronchopulmonary Pseudomonas aeruginosa infection in resistant and susceptible inbred mouse strains. Am. J. Respir. Cell. Mol. Biol. 20:710-719. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka, T., S. Okamura, K. Okada, A. Suga, N. Shimono, N. Ohhara, Y. Hirota, Y. Sawae, and Y. Niho. 1989. Protective effect of recombinant murine granulocyte-macrophage colony-stimulating factor against Pseudomonas aeruginosa infection in leukocytopenic mice. Infect. Immun. 57:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka, Y., T. Imai, M. Baba, I. Ishikawa, M. Uehira, H. Nomiyama, and O. Yoshie. 1999. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur. J. Immunol. 29:633-642. [DOI] [PubMed] [Google Scholar]
- 61.Tarr, P. E. 1996. Granulocyte-macrophage colony-stimulating factor and the immune system. Med. Oncol. 13:133-140. [DOI] [PubMed] [Google Scholar]
- 62.van Heeckeren, A. M., J. Tscheikuna, R. W. Walenga, M. W. Konstan, P. B. Davis, B. Erokwu, M. A. Haxhiu, and T. W. Ferkol. 2000. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 161:271-279. [DOI] [PubMed] [Google Scholar]
- 63.Warbrick, E. V., A. L. Thomas, and C. M. Williams. 1997. The effects of cyclosporin A, dexamethasone and other immunomodulatory drugs on induced expression of IL-3, IL-4 and IL-8 mRNA in a human mast cell line. Toxicology 116:211-218. [DOI] [PubMed] [Google Scholar]
- 64.Weber, S., M. Babina, S. Kruger-Krasagakes, A. Grutzkau, and B. M. Henz. 1996. A subclone (5C6) of the human mast cell line HMC-1 represents a more differentiated phenotype than the original cell line. Arch. Dermatol. Res. 288:778-782. [DOI] [PubMed] [Google Scholar]
- 65.Wershil, B. K., G. T. Furuta, J. A. Lavigne, A. R. Choudhury, Z. S. Wang, and S. J. Galli. 1995. Dexamethasone and cyclosporin A suppress mast cell-leukocyte cytokine cascades by multiple mechanisms. Int. Arch. Allergy Immunol. 107:323-324. [DOI] [PubMed] [Google Scholar]
- 66.Wershil, B. K., G. T. Furuta, J. A. Lavigne, A. R. Choudhury, Z. S. Wang, and S. J. Galli. 1995. Dexamethasone or cyclosporin A suppress mast cell-leukocyte cytokine cascades. Multiple mechanisms of inhibition of IgE- and mast cell-dependent cutaneous inflammation in the mouse. J. Immunol. 154:1391-1398. [PubMed] [Google Scholar]
- 67.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]