Abstract
Molecular interaction between host mucosal surfaces and outer membrane components of microbes is crucial in the infection process. The outer membrane of pathogenic Neisseria contains surface molecules such as pili, PilC, and Opa and a monolayer of lipooligosaccharide (LOS), all of which are involved in the interaction with host cells. Pili mediate the initial attachment to human epithelial cells, which is followed by tight contact between bacteria and the eucaryotic cells, leading to bacterial invasion. To further examine the basis for bacterium-host cell contact, we constructed an LOS-deficient Neisseria meningitidis serogroup C mutant. LOS deficiency was without exception accompanied by altered colony opacity and morphology, which most likely represented an “on” switch for Opa540 expression, and by reduced levels of the iron-regulated proteins FetA and FbpA. We show here that LOS is essential for pilus-associated adherence but dispensable for fiber formation and twitching motility. The absence of attachment to epithelial cells could not be attributed to altered levels of piliation or defects in the pilus adhesion phenotype. Further, LOS mutants do not invade host cells and have lost the natural competence for genetic transformation.
The mucosal epithelial cell barrier is the first physical defense encountered by bacteria upon contact with the human host. Immediately upon adherence of bacteria, the epithelial cells initiate a nonspecific or innate immune response by production of proinflammatory factors. The inflammatory response to bacterial infections plays an important role in detection and elimination of invading microorganisms. Various components of the bacterial cell wall such as peptidoglycan, lipoteichoic acid, lipoproteins, and lipopolysaccharide (LPS) are capable of activating the proinflammatory reaction. For gram-negative bacteria, LPS is the dominant trigger of the systemic inflammatory response. Recently, Toll-like receptors (TLRs) have been implicated in host responses to bacterial pathogens. Specifically, TLR4 mediates LPS responses whereas TLR2 plays a broader role in the recognition of a variety of bacteria and bacterial antigens (41). The host inflammatory response in meningococcal sepsis is generally believed to be induced by lipooligosaccharide (LOS).
LOS of Neisseria meningitidis (meningococcus) is an endotoxin that is structurally distinct from LPS of enteric gram-negative bacteria (21, 33). Unlike most enteric LPSs, meningococcal LOS lacks O-antigen and possesses relatively short polysaccharides, only two to five sugar residues, attached to the meningococcal LOS inner core. LOS is an amphipathic molecule that consists of a hydrophilic carbohydrate portion and a hydrophobic lipid A portion that anchors the LOS to the outer membrane. Endotoxic shock is mediated by the lipid A portion of LOS and is characterized by activation of macrophages and production of a diverse array of cytokines, including those that act as chemoattractants for other leukocytes. Meningococcal septic shock is a direct result of the overstimulation of this response and is characterized by hypotension, organ failure, and death. The severity of the disease correlates with the concentrations of circulating LOS and proinflammatory cytokines.
Escherichia coli mutants defective in early steps of lipid A biosynthesis are not viable, indicating that LPS is essential for bacterial growth and survival. However, conditionally lethal mutants have been reported despite the fact that intact LPS is important in maintaining the permeability barrier of the outer membrane (35). Those mutants show a compensatory rearrangement of the outer leaflet with replacement of LPS by glycerophospolipids. This creates patches in the outer membrane, allowing diffusion of hydrophobic solutes which, in turn, make those mutants extremely susceptible to hydrophobic antibiotics (49). In contrast to E. coli, N. meningitidis strains with mutations in genes encoding enzymes for early steps of lipid A biosynthesis are viable. Recently, viable LOS-deficient N. meningitidis mutants derived from the serogroup B strain H44/76 were isolated (46) and have been intensively studied for proinflammatory activity, immunogenicity, and outer membrane composition (6, 17, 34, 45, 47-48).
Bacterial attachment to the host mucosa is a first and essential step in the process of development of disease caused by pathogenic Neisseria. Colonization of target cells is modeled in two steps: an initial attachment mediated by type IV pili followed by an intimate contact between bacterial membrane components and host cell surface receptors that can lead to uptake of the bacterium and invasion of host cells. The type IV pili of pathogenic Neisseria are essential during the initial stage of infection. These structures are built up of thousands of major pilus subunits, PilE proteins, and a few copies of pilus-associated proteins, such as PilC and PilV (19, 39, 49). Adherence to epithelial cells is dependent on expression of PilC and PilV and is modulated by sequence variation of PilE (20, 28, 37). Pili have been shown to recognize cellular receptor CD46 on the surfaces of human cells (22-24).
Colonization of epithelial cells by pathogenic Neisseria is followed by cellular invasion. The opacity proteins (Opa) are a family of invasion-associated outer membrane proteins that bind to CEACAM and heparan sulfate proteoglycan receptors on human cells (1). Several recent studies have shown that LOS of Neisseria gonorrhoeae is also important for invasion into epithelial cells. In the absence of detectable Opa proteins, the lacto-N-neotetraose of LOS is required for invasion of, but not for adherence to, ME180 cells by N. gonorrhoeae (43). Minor et al. (27) showed the importance of the proximal glucose residue of the α-oligosaccharide chain of LOS for efficient invasion of gonococci into the host mucosa. Further, Harvey et al. (13) demonstrated that gonococcal LOS binds specifically to the asialoglycoprotein receptor (ASGP-R) on sperm cells, leading to the hypothesis that LOS could also mediate adherence.
To examine the role of LOS in meningococcal adhesion to target cells, LOS-deficient N. meningitidis FAM20 serogroup C lpxA mutants were generated. The lpxA gene encodes an enzyme responsible of the first step of the lipid A biosynthesis pathway, adding the O-linked 3-OH fatty acid to UDP-N-acetylglucosamine. lpxA mutants switched on Opa540 expression, suggesting that Opa540 might be required to maintain integrity of the outer membrane in the absence of LOS. LOS-deficient meningococci retained piliation levels and pilus morphology indistinguishable from those for the wild type. However, adherence to and invasion into epithelial cells by lpxA mutants were severely reduced. Further, LOS-deficient mutants lost natural competence for DNA transformation.
MATERIALS AND METHODS
Bacterial strains.
E. coli DH5α was used for cloning and manipulation of the meningococcal lpxA gene. Neisseria strains were grown at 37°C in a 5% CO2 atmosphere on Difco GCB agar or GC broth containing Kellogg's supplement (25). Bacterial growth was determined by measuring optical density at 620 nm (OD620) as a function of time. N. meningitidis FAM20 (P+ PilC1+ PilC2+) belongs to serogroup C (36). Meningococcal lpxA-deficient mutants were generated by transformation of FAM20 with a plasmid containing the lpxA gene disrupted with a kanamycin resistance gene. An internal fragment encompassing positions 5 to 587 of the lpxA gene was amplified by PCR using the following primers: lpxA02m (5′-GCCGTCTGAAGCCCGGCGTACTCATCATC-3′) and lpX03 (5′-TGCCTTCGCTG TTGAGCCCC-3′). Primer lpxA02m contains a DNA uptake sequence (underlined) at the 5′ end, which is required for efficient DNA transformation of Neisseria (12). The fragment was cloned into the SmaI site of pSK+ to produce pAB12. The kanamycin resistance gene from pUCKan was inserted into a unique EcoRI site (position 272) of the lpxA gene to produce pAB20. The wild-type meningococcal strain FAM20 was transformed with linearized pAB20, and colonies were selected on plates with 50 μg of kanamycin/ml. Nine independent transformants (L1 to L9) were restreaked on GCB plates with kanamycin to get pure clones and to check for resistance stability. The pilE gene was amplified by PCR and sequenced by using primers 5′-GATGCCGCAAATTTCCAATC-3′ and 5′-GGTTTGACCCGGTCGTGA-3′. Twitching motility was assessed by the slide culture method (5), in which cells are inoculated onto GC agar slices on microscope slides, covered with a coverslip, and visualized at the periphery of colonies under a Leica phase-contrast microscope.
Cell line and growth conditions.
ME180 (ATCC HTB33), an epithelium-like human cell line from a cervical carcinoma, was maintained in McCoy's 5A medium supplemented with 10% inactivated fetal bovine serum (FBS) and 2 mM l-glutamine. The cell line was maintained at 37°C and 5% CO2 and occasionally grown in penicillin-streptomycin-containing medium to prevent contaminations. All experiments were performed without FBS, antibiotics, and l-glutamine. Media and growth supplements were purchased from Life Technologies. Cell culture materials were purchased from Costar.
LOS and outer membrane preparations.
Bacteria were grown on GCB plates for 18 h at 37°C in 5% CO2. Bacteria from one plate were harvested into 600 ml of Tris-EDTA buffer containing 0.5% sodium dodecyl sulfate (SDS) and 12.5 mg of proteinase K/ml and incubated for 12 h at 65°C. Ten-microliter aliquots of each sample lysate were mixed with sample buffer and used for electrophoresis on a 16.5% Tricine SDS-polyacrylamide gel electrophoresis (PAGE) gel. LOS was visualized by silver staining.
Outer membranes were prepared either by lithium acetate (LiAc) purification or by sarcosyl purification as previously described (20). Briefly, for LiAc purification meningococci from one GCB plate were suspended in 1 ml of LiAc buffer (0.2 M lithium chloride, 0.1 M sodium acetate, 0.01 M EDTA, pH 5.8). The suspension was passaged 15 times through a 22-gauge needle to shear off the outer membrane blebs and centrifuged for 1 min at 12,000 × g. The supernatant was centrifuged for 2 h at 100,000 × g at 4°C to pellet the outer membrane. The pellet was resuspended in 100 μl of distilled water. For sarcosyl purification, meningococci from one GCB plate were suspended in 1 ml of phosphate-buffered saline (PBS), sonicated, and centrifuged for 15 min at 5,000 × g. The supernatant was centrifuged for 1 h at 100,000 × g and 4°C. After resuspension of the pellet in 0.2% sarcosyl, the sample was recentrifuged for 1 h at 100,000 × g at 4°C in order to collect the outer membrane blebs. Finally, the pellet was resuspended in 200 μl of distilled water. Total-protein concentrations were determined by using the Bio-Rad protein assay and bovine serum albumin standards according to the manufacturer's instructions. Pili were purified as previously described (19, 50).
Immunoblotting.
Outer membrane preparations were heated to either 37 or 95°C, electrophoresed on a 15% Tris-glycine SDS-PAGE gel, and transferred to a polyvinylidene difluoride Immobilon membrane (Millipore) by using a Bio-Rad semidry transfer system. Membranes were incubated overnight at 4°C with 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20. Membranes were immunoblotted with mouse monoclonal Opa antibodies (1:3,000), washed, and incubated with horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin G (1:10,000). After being washed, the blots were developed by using the ECL system (Perkin-Elmer). Opa-specific monoclonal antibodies (H22.2, H21.1, 4B12/C11, and 7-24-D9) were a kind gift from Mark Achtman (Max Planck Institut für Molekular Genetik, Berlin, Germany) and J. Cannon (University of North Carolina, Chapel Hill). 4B12/C11 recognizes all Opa proteins, H21.1 recognizes Opa1700, H22.2 recognizes both Opa540 and Opa1800, and 7-24-D9 recognizes Opa540 (15).
For N-terminal sequence determination, proteins were electrophoresed on SDS-PAGE gels and transferred to a polyvinylidene difluoride Immobilon filter (Millipore) by electroblotting. The proteins were stained and sequenced as previously described (19).
Assays of adhesion to and invasion of cultured cell lines.
Cells were grown to a confluent monolayer in 24-well tissue culture plates. After the cell layer was washed, N. meningitidis wild-type FAM20 and lpxA-deficient mutant (OD600 = 0.1) cells were added and incubated with ME180 cells for 90 min at 37°C in 5% CO2 to allow the bacteria to adhere. The wells were extensively washed in PBS until no unbound bacteria were seen in the microscope. The infected cells were treated with 1% saponin for 5 min, serially diluted, spread onto GCB plates, and incubated overnight at 37°C and 5% CO2. Adherence was quantified by counting CFU the next day.
Prior to invasion assays the cell layer was carefully washed. N. meningitidis FAM20 and lpxA-deficient mutant cells were added at an OD600 of 0.1 to ME180 cells for 6 h at 37°C in 5% CO2 to allow the bacteria to invade the cells. The wells were extensively washed in PBS until no unbound bacteria were seen. The cells were treated 2 h with gentamicin (200 μg/ml) to kill extracellular bacteria. The infected cells were treated with 1% saponin for 5 min, serially diluted, spread onto GCB plates, and incubated overnight at 37°C and 5% CO2. CFU were counted the next day. The sensitivity of the lpxA mutant to saponin was examined. No difference in saponin sensitivity between the wild type and the mutant was observed.
TEM.
For transmission electron microscopy (TEM), strains of N. meningitidis were grown overnight on GCB agar plates, suspended in Tris-Mg buffer (10 mM Tris-HCl [pH 7.4], 10 mM MgCl2), and overlaid on 200-mesh carbon-coated copper grids for 5 min. The grids were washed with water and negatively stained with 1% sodium silicotungstate and examined in a Philip 100× microscope.
DNA transformation.
Meningococci were grown on plates for 18 h, collected with cotton swabs, and suspended to a density of 108 bacteria per ml in GC liquid medium. Twenty microliters of bacterial cells was added to 200 μl of GC liquid medium containing 1 μg of pABJ04 (19), which when recombined into the meningococcal chromosome confers resistance to chloramphenicol. After 30 min at 37°C, the transformation mixtures were diluted into 2 ml of GC liquid with Kellogg's supplement and then incubated for 5 h at 37°C in 5% CO2. The transformation mixtures were diluted and plated on GCB medium with 10 μg of chloramphenicol/ml to select transformants and on GCB plates to determine CFU.
RESULTS
Construction of an LOS-deficient N. meningitidis serogroup C mutant.
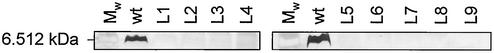
LOS of N. gonorrhoeae has been carefully studied and is involved in bacterial invasion of epithelial cells (13, 27, 43). However, little is know about the interaction of meningococcal LOS with host target cells. To study the role of meningococcal LOS in adherence and invasion of epithelial cells, we constructed an lpxA mutant version of the N. meningitidis serogroup C strain FAM20. The lpxA gene was insertionally inactivated by introduction of a kanamycin cassette. PCR amplification and Southern blotting confirmed insertion of a 1.4-kb fragment into the lpxA genes of nine independent mutants (data not shown). To further verify the genetic inactivation of lpxA, LOS was extracted from lysates of whole cells grown on GC agar plates and analyzed by Tricine SDS-PAGE. As expected, LOS was not detected in the lpxA mutants, whereas wild-type FAM20 expressed a single LOS band of ∼6.5 kDa (Fig. 1). These data show that the lpxA mutants were completely deficient in LOS biosynthesis.
FIG. 1.
SDS-PAGE analysis of LOS expressed by N. meningitidis FAM20 (wt) and its lpxA mutants (L1 to L9). Proteinase K-digested whole-cell lysates were separated on Tricine polyacrylamide gels and silver stained. Mw, molecular weight marker.
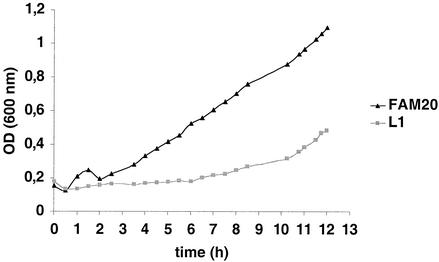
The serogroup C strain FAM20 lpxA mutants show reduced growth rate.
All lpxA mutants produced smaller colonies than wild-type bacteria, indicating either reduced growth rate or smaller bacterial size. Electron microscopy indicated that the retardation was not due to the smaller size of the bacteria (data not shown). Consequently, the growth rates of the lpxA mutants were compared with that of the wild type by measuring the OD during 13 h of culture. As shown in Fig. 2, the generation time of the lpxA mutant L1 was clearly reduced compared to that of FAM20. These data are in accordance with those of Steeghs et al. (46), who showed that the doubling time for lpxA-deficient N. meningitidis H44/76, a serogroup B strain, was 50% longer than that for the wild-type strain.
FIG. 2.
Growth curves of the wild-type FAM20 and the lpxA mutant L1. Bacteria were grown in GC medium with Kellogg's supplement. The same growth retardation was observed for mutants L2 to L9 (data not shown).
Inactivation of lpxA is accompanied by altered Opa expression.
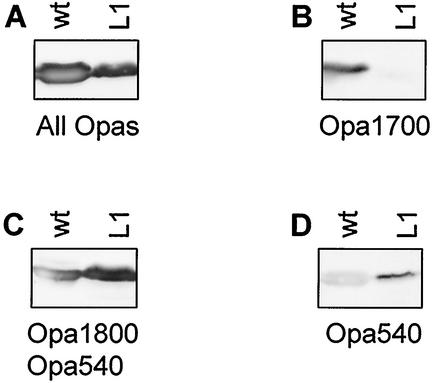
In addition to smaller colony size, the lpxA mutants exhibited altered colony color when observed under a binocular microscope. Colonies of the wild-type strain were smooth and weakly opaque, whereas all mutants had a strong opaque color, indicating alteration in Opa expression. All attempts to select or screen for weakly opaque lpxA mutants failed, suggesting a strong correlation between survival of the mutants and expression of the phenotype of strong opacity. We investigated the Opa repertoire of FAM20 and its lpxA mutants by using a polyclonal antiserum that recognizes all Opa proteins. We also used monoclonal antibodies H21.1, H22.2, and 7-24-D9, which distinguish between Opa1700, Opa1800, and Opa540. FAM20 and the lpxA mutants presented differences in expression pattern when analyzed by immunoblotting with Opa antibodies. The wild-type strain expressed two Opa proteins, Opa1800 and Opa1700, whereas the mutants fell into two classes of Opa repertoires (Fig. 3 and Table 1). One class of mutants expressed Opa1800 and Opa540 and therefore must have turned on Opa540 and turned off Opa1700. The other class of mutants expressed Opa1800, Opa1700, and Opa540, i.e., this class of mutants apparently turned on Opa540 but retained the Opa proteins of the wild type. Our results show that colony morphology and Opa expression in the lpxA mutants are clearly altered. Furthermore, our data suggest that there is a strong correlation between survival of the mutant strains and expression of Opa540.
FIG. 3.
Western immunoblots of outer membranes of FAM20 (wt) and its lpxA mutant L1 incubated with anti-Opa monoclonal antibody (MAb) 4B12/C11 recognizing all Opas (A), anti-Opa MAb H21.1, recognizing Opa1700 (B), anti-Opa MAb H22.2, recognizing Opa1800 and Opa540 (C), and anti-Opa MAb 7-24-D9, recognizing Opa540 (D). Mutant L1 lost Opa1700 expression and gained Opa540 expression. A faint band reacting with Opa540 antibodies was often, but not always, observed in the wild type and could represent cross-reaction of the antibody with Opa1700 and Opa1800 or a minor population of wild-type bacteria that spontaneously turned on Opa540 by phase variation. The criteria for turn-on of Opa540 in L1 are the colony color difference between the wild type (weakly opaque) and L1 (strongly opaque and yellowish) and the very distinct Opa540 signal in the L1 mutant.
TABLE 1.
Genotype and phenotype of N. meningitidis strains used in this study
Outer membrane proteins in the LOS-deficient mutant.
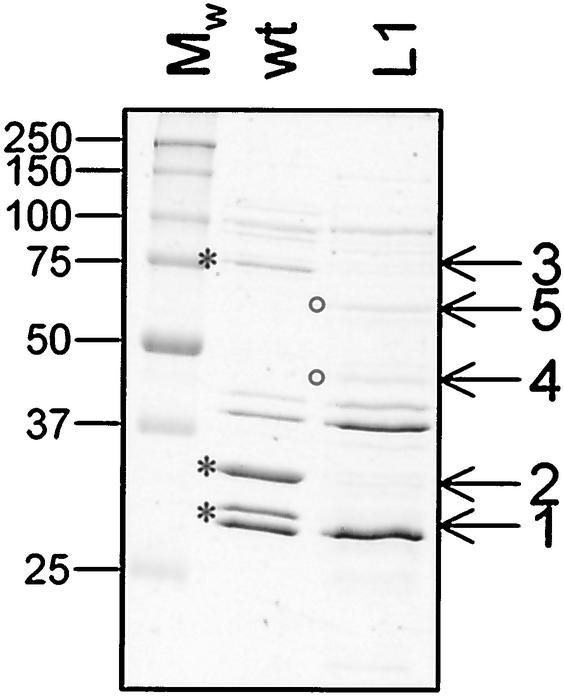
Steeghs et al. (47) showed that expression of iron limitation-inducible cell surface-exposed lipoproteins, such as LbpB and TbpB, in the lpxA mutant of the serogroup B strain H44/76 was greatly reduced. In contrast, no difference in levels of expression of other outer membrane-associated proteins was found. To identify changes to the outer membrane in the serogroup C strain FAM20 lpxA mutant, we purified outer membranes using both sarcosyl extraction and LiAc extraction for outer membrane blebs. The lpxA mutant expressed reduced amounts of three major outer membrane proteins and increased amounts of two proteins as detected by SDS-PAGE analysis (Fig. 4). Proteins of interest were subjected to amino-terminal sequencing and subsequently identified in the National Center for Biotechnology Information GenBank database (Table 2). Expression of iron-regulated proteins FbpA and FetA (formerly designated FrpB) was reduced in the lpxA mutant. The third protein with reduced expression in the lpxA mutant was identified as Opa1700, supporting the finding that the opacity phenotype was altered. Comigration of Opa540 and Opa1800 made it impossible to detect changes in expression of these two Opa proteins by SDS-PAGE. In addition, PorA protein expression was slightly reduced in the LOS mutant (Fig. 4 and data not shown), a phenomenon previously described as an effect of proteolytic degradation of PorA due to improper localization in the outer membrane (47). The two proteins with increased amounts in the lpxA mutant were identified as GroEL and glutamate dehydrogenase and probably represent a general response to biological stress conditions. In conclusion, these results show that inactivation of lpxA leads to alterations of the outer membrane protein composition.
FIG. 4.
Analysis of outer membrane proteins expressed by N. meningitidis FAM20 (wt) and its lpxA mutant L1. Outer membrane proteins were purified by the LiAc method for outer membrane bleb purification and separated by SDS-PAGE. Five proteins with altered expression levels in the lpxA mutant were subjected to amino-terminal sequencing. Asterisks, proteins with reduced levels in the LOS mutants; circles, proteins with increased amounts in the mutants. Identical results were obtained with outer membranes prepared by sarcosyl extraction. The same profiles were observed with the other eight mutants (L2 to L9; data not shown). Mw, molecular weight marker.
TABLE 2.
Proteins with altered expression level in the LOS mutant
| Protein no.a | N-terminal sequence | Designation |
|---|---|---|
| 1 | ASEDGSRSPYTVQAD | Opa1700 |
| 2 | DITVYNGQHKEAAQA | FbpA |
| 3 | AENNAKVVLDTITVK | FetA (FrpB) |
| 4 | HLNTLFANLK | Glutamate dehydrogenase |
| 5 | AAKDVQFGNEVRGKM | GroEL |
Protein numbers are indicated in Fig. 4. Proteins 1 to 3 are expressed in reduced amounts and proteins 4 and 5 are expressed in increased amounts in the LOS mutant relative to expression in the wild type.
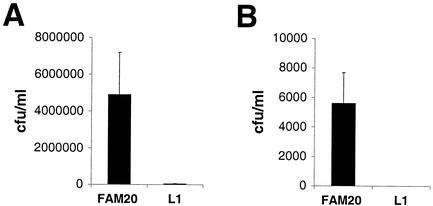
LOS-deficient N. meningitidis is unable to interact with ME180 epithelial cells.
Assays of adherence to the epithelial cell line ME180 showed that the lpxA mutant was severely impaired, with the level of adherence being reduced 50-fold relative to that for the wild-type strain (Fig. 5A). Similarly, the mutant did not invade epithelial cells as determined by gentamicin treatment assays (Fig. 5B). To exclude the possibility that the impaired adherence was due to PilE antigenic variation, the pilE gene in the mutant was sequenced and found to be unaltered (data not shown).
FIG. 5.
LOS-deficient N. meningitidis fails to interact with human epithelial cells. (A) FAM20 and its lpxA-deficient mutant L1 were allowed to adhere to confluent layers of ME180 cells for 90 min. The cell layers were washed, treated with saponin, and spread on GC plates. (B) Invasion into ME180 cells of FAM20 and its lpxA-deficient mutant L1 was assessed for 6 h. Extracellular bacteria were killed by gentamicin, and the cell layers were treated with saponin and spread on GC plates. Mean values and standards deviations of three independent experiments are shown. Identical results were obtained with mutants L2 to L9 (data not shown).
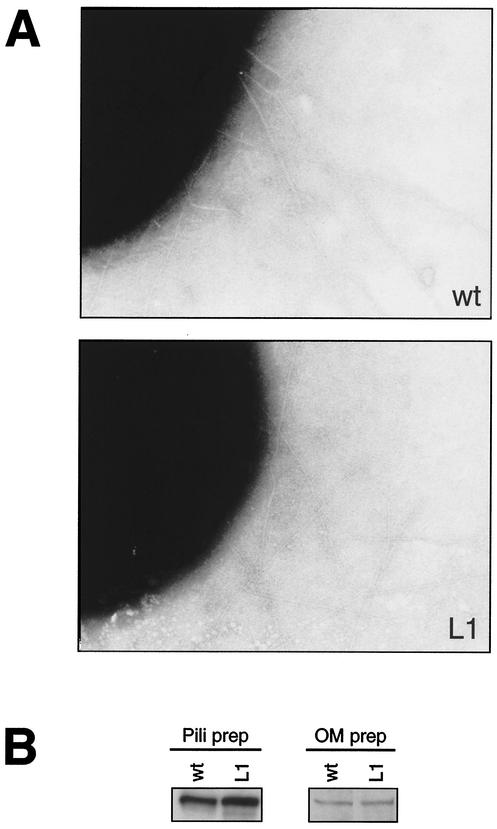
The simplest explanation for the nonadherence phenotype would be a defect in type IV pilus formation. Examination by TEM demonstrated that the lpxA mutants retained piliation levels indistinguishable from that of the wild type (Fig. 6A). Piliation levels were confirmed by immunoblotting with meningococcal pilus antiserum in whole-cell lysates and sheared fractions. No difference in piliation between the wild type and the mutant was observed (data not shown). Another possible explanation for the lack of attachment of the mutant to human epithelial cells would be that LOS is required for PilC stability or localization. Consequently, we examined PilC expression in outer membrane preparations and in purified pili by immunoblotting with PilC antiserum but could not detect any differences between the wild type and the lpxA mutant (Fig. 6B and Table 1). Our data show that, despite the expression of pili and PilC, the LOS-deficient mutant was unable to adhere to host epithelial cells. The adherence defect could be the result of expression of nonadherent pili, i.e., pili unable to attach to host target cells. To address this issue, crude pili isolated by the shearing of bacteria were added to ME180 epithelial cells and detected with fluorescein isothiocyanate-conjugated pilus antibodies. Pili isolated from the lpxA mutant and the wild type bound to the cells, indicating that the pili still possessed cell-binding capacity by themselves (data not shown). However, pili from the lpxA mutant seemed to attach at slightly lower levels.
FIG. 6.
(A) Transmission electron micrographs showing piliation of meningococcal strain FAM20 (wt) and its LOS-deficient mutant L1. (B) Immunoblotting of purified pili and outer membrane (OM) preparations by using PilC-specific antiserum. The same results were obtained with mutants L2 to L9 (data not shown).
LOS-deficient N. meningitidis is not naturally competent for DNA transformation.
Expression of type IV pili by Neisseria is essential for natural genetic transformation at the level of sequence-specific uptake of DNA (10). Although the pilus morphology of the lpxA mutant was indistinguishable from that of the wild type, the mutant had dramatically reduced competence for DNA transformation (Table 1). Further, the LOS mutant was examined microscopically for the expression of twitching motility. Zones of spreading at the periphery of colonies of both wild-type and mutant strains were readily detected. As negative controls, nonpiliated mutants were assayed in parallel; no evidence of cell movement was found. These results, taken together, show that the lpxA mutant has lost natural competence for DNA transformation but retains twitching motility.
DISCUSSION
Attachment of microbes to host epithelial cells represents the first step in the pathogenesis of infection, with the target specificity being defined by precise adhesin-receptor interactions. The ability of most gram-negative bacteria to colonize host mucosa requires the expression of pili or fimbriae (44). The purpose of these proteinaceous filaments appears to be presentation of adhesive molecules capable of binding to specific epithelial cell receptors. Since pili extend several millimeters from the bacterial cell surface, the effective physical range of interaction allows the pathogen to maintain dense surface structures such as polysaccharide, capsules, and LPS, which enable the bacteria to evade immune responses.
In this work we generated N. meningitidis serogroup C LOS-deficient mutants and analyzed their pilus-associated characteristics, including their ability to interact with human target cells. The lpxA mutants were viable, did not express any LOS, and showed reduced growth rate, which is in agreement with the results of Steeghs et al. (46). Our lpxA mutants expressed and assembled most outer membrane proteins similarly to the wild type. However, expression of the iron-regulated proteins FbpA and FetA in the mutant was reduced. In addition, mutant colonies displayed an altered opacity relative to the wild type, which encouraged us to further analyze the Opa repertoire of the mutants and the wild type. The finding that the lpxA mutants without exception turned on expression Opa540 and that mutants with wild-type opacity morphology could never be isolated strongly suggested a correlation between bacterial survival and expression of Opa540.
Purified outer membranes from mutants contained reduced amounts of the iron-regulated proteins FbpA and FetA (formerly designated FrpB) as well as less PorA. FbpA is a surface-exposed 37-kDa outer membrane protein that plays a role in transporting iron from the TbpAB complex to FbpB. FetA is a 77-kDa outer membrane protein involved in iron uptake (32). It functions as a ferric enterobactin receptor that can take part in transporting siderophores into the cell and is nonessential for iron acquisition (2, 3). It is therefore unlikely that a reduced amount of this protein in the outer membrane would affect viability, growth, or adherence properties of the lpxA mutants. In contrast, the presence of reduced amounts of PorA in the outer membrane is a possible explanation for reduced growth rate. Porins are essential for organism survival since they modulate the exchange of ions between the bacteria and the surrounding environment (26). Further, Por is involved in invasion but not in adherence (11). Expression of GroEL and glutamate dehydrogenase was greater in the mutant than in the wild type. GroEL is a stress-inducible chaperone and belongs to the heat shock protein family Hsp60 (29, 30, 53). Bacterial Hsps are conserved proteins that play important roles in protein folding and assembly and in the translocation of proteins between compartments. Under stress, Hsp synthesis is drastically increased, representing an essential mechanism for cell survival. Hsp60 in E. coli has been localized exclusively to the cytoplasm; however, in other microorganisms such as Clostridium difficile, Haemophilus ducreyi, Helicobacter pylori, Legionella pneumophila, and Salmonella enterica serovar Typhimurium Hsps are either associated with the membrane or secreted (7, 8, 14, 16). A recent study demonstrated that alterations in GroEL levels resulted in diminished survival of stressed Haemophilus ducreyi (31). The large amount of GroEL-like protein in the lpxA-deficient mutants could represent an essential factor for the survival of bacteria or could represent stress-induced expression of GroEL.
Absence of LOS in the meningococcal outer membrane is likely to affect membrane integrity, with phospholipids as the candidates of choice to replace LOS in order to maintain the bilayer structure. It has been demonstrated for E. coli that unbalanced membrane phospholipid composition could affect transcriptional expression of certain genes (18). It is not known whether the expression of FetA, FbpA, PorA, or GroEL is affected at the transcriptional or translational level as a consequence of changes in membrane phospholipid composition in the lpxA-deficient mutant.
Initial attachment of bacteria to epithelial cells requires pili and PilC. Although piliation and PilC expression in the wild type were indistinguishable from those in the LOS mutant, the mutant was greatly impaired for adherence to and invasion into epithelial cells. The possibility that PilC is not properly inserted in the lpxA mutant membrane or pili cannot be excluded. In addition, the lpxA mutant was not naturally competent for DNA transformation. Several components have been implicated as essential for natural transformation in Neisseria, among which are pili, PilT, PilC, ComL, ComP, ComE, and Dca (4, 9, 38, 40, 42, 51, 52). Although most of the studies of DNA transformation have been performed with N. gonorrhoeae, many of the gene products have counterparts in meningococci, with the exception of Dca, which has been found only in gonococcal strains. It is possible that lack of LOS in the outer membrane alters the localization and structural formation of proteins involved in the transformation process, either by impeding the proper insertion of the membrane proteins into the membrane or by affecting the general charge of the bacterial surface, preventing the first necessary step of DNA binding. It is also possible that LOS is itself essential for natural competence. LOS deficiency may perturb flexibility and fluidity of the basic membrane structure, which modifies the physicochemistry properties of pili, which in turn lose their transformation competence and ability to mediate bacterial adherence to target cells. It is possible that adhesion-associated proteins such as PilV may not be incorporated or may not be allowed to perform their functions in formation of a functional pilus. However, pilV mutation does not affect DNA transformation (50).
Taken together, we have shown that a completely LOS-deficient N. meningitidis serogroup C strain is unable to adhere to human cells and that this phenomenon is linked to disrupted competence for natural DNA transformation. Further, absence of LOS strongly selected for expression of Opa540, which may assist in maintaining integrity of the membrane, thereby promoting bacterial survival.
Acknowledgments
We thank Jane Cannon and Mark Achtman for kindly providing polyclonal and monoclonal antibodies (H21.1, H22.2, 7-24-D9, and 4B12/C11) against Opa proteins, Lenore Johansson for electron microscopy, and Vendela Asp for reading the manuscript.
This work was supported by grants from the Swedish Medical Research Council (Dnr 10846), Swedish Cancer Society, Swedish Society for Medicine, Claes Groschinskys Stiftelse, Åke Wibergs Stiftelse, Magnus Bergvalls Stiftelse, Strategic Foundation (I&V program), Karolinska Institutet Research grants, and a grant from Aventis Pasteur. B.A. was supported by a postdoctoral fellowship from Stiftelsen Wenner Grenska Samfundet and grants from Karolinska Institutet.
Editor: V. J. DiRita
REFERENCES
- 1.Billker, O., A. Popp, S. D. Gray-Owen, and T. F. Meyer. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8:258-260. [DOI] [PubMed] [Google Scholar]
- 2.Carson, S. D., P. E. Klebba, S. M. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson, S. D., B. Stone, M. Beucher, J. Fu, and P. F. Sparling. 2000. Phase variation of the gonococcal siderophore receptor FetA. Mol. Microbiol. 36:585-593. [DOI] [PubMed] [Google Scholar]
- 4.Chen, I., and E. C. Gotschlich. 2001. ComE, a competence protein from Neisseria gonorrhoeae with DNA-binding activity. J. Bacteriol. 183:3160-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darzins, A. 1993. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric single-domain response regulator CheY. J. Bacteriol. 175:5934-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon, G. L. J., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipooligosaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ensgraber, M., and M. Loos. 1992. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect. Immun. 60:3072-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisk, A., C. A. Ison, and T. Lagergård. 1998. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect. Immun. 66:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fussenegger, M., D. Facius, J. Meier, and T. F. Meyer. 1996. A novel peptidoglycan-linked lipoprotein (ComL) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol. Microbiol. 19:1095-1105. [DOI] [PubMed] [Google Scholar]
- 10.Fussenegger, M., T. Rudel, R. Barten, R. Ryll, and T. F. Meyer. 1997. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae. Gene 192:125-134. [DOI] [PubMed] [Google Scholar]
- 11.Gorby, G. L., A. F. Ehrhardt, M. A. Apicella, and C. Elkins. 2001. Invasion of human fallopian tube epithelium by Escherichia coli expressing combinations of a gonococcal porin, opacity-associated protein, and chimeric lipo-oligosaccharide. J. Infect. Dis. 184:460-472. [DOI] [PubMed] [Google Scholar]
- 12.Graves, J. F., G. D. Biswas, and P. F. Sparling. 1982. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J. Bacteriol. 152:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey, H. A., N. Porat, C. A. Campbell, M. Jennings, B. W. Gibson, N. Phillips, M. A. Apicella, and M. S. Blake. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 36:1059-1070. [DOI] [PubMed] [Google Scholar]
- 14.Hennequin, C., F. Porcheray, A. J. Waligora-Dupriet, A. Collignon, M. Barc, P. Bourlioux, and T. Karjalainen. 2001. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147:87-96. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs, M. M., B. Malorny, P. Prasad, G. Morelli, B. Kusecek, J. E. Heckels, J. G. Cannon, and M. Achtman. 1998. Recombinational reassortment among opa genes from ET-37 complex Neisseria meningitidis isolates of diverse geographical origins. Microbiology 144:157-166. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, P. S., and R. A. Garduno. 1999. Surface-associated heat shock proteins of Legionella pneumophila and Helicobacter pylori: roles in pathogenesis and immunity. Infect. Dis. Obstet. Gynecol. 7:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipooligosaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through toll-like receptor 2. Infect. Immun. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue, K., H. Matsuzaki, K. Matsumoto, and I. Shibuya. 1997. Unbalanced membrane phospholipid compositions affect transcriptional expression of certain regulatory genes in Escherichia coli. J. Bacteriol. 179:2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson, A.-B., G. Nyberg, and S. Normark. 1991. Phase variation of gonococcal pili by frameshift mutation in pilC. EMBO J. 10:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonsson, A.-B., D. Ilver, P. Falk, J. Pepose, and S. Normark. 1994. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 13:403-416. [DOI] [PubMed] [Google Scholar]
- 21.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 22.Källström, H., M. K. Liszewski, J. P. Atkinson, and A.-B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus-receptor for pathogenic Neisseria. Mol. Microbiol. 12:639-647. [DOI] [PubMed] [Google Scholar]
- 23.Källström, H., M. S. Islam, P.-O. Berggren, and A.-B. Jonsson. 1998. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 273:21777-21782. [DOI] [PubMed] [Google Scholar]
- 24.Källström, H., D. B. Gill, B. Albiger, M. K. Liszewski, J. P. Atkinson, and A.-B. Jonsson. 2001. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell. Microbiol. 3:133-143. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg, D. S., I. R. Cohen, L. C. Norins, A. L. Schroeter, and G. Reising. 1968. Neisseria gonorrhoeae. II. Clonal variation and pathogenicity during 35 month in vitro. J. Bacteriol. 96:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massari, P., and L. M. Wetzler. 2000. Effect of neisserial porins on host immune response. J. Immunol. Immunopharmacol. 20:49-53. [Google Scholar]
- 27.Minor, S. Y., A. Banerjee, and E. C. Gotschlich. 2000. Effect of α-oligosaccharide phenotype of Neisseria gonorrhoeae strain MS11 on invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. Infect. Immun. 68:6526-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassif, X., J.-L. Beretti, J. Lowy, P. Stenberg, P. O'Gaora, J. Pfeifer, S. Normark, and M. So. 1994. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc. Natl. Acad. Sci. USA 91:3769-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pannekoek, Y., I. G. Schuurman., J. Dankert, and J. P. van Putten. 1993. Immunogenicity of the meningococcal stress protein MSP63 during natural infection. Clin. Exp. Immunol. 3:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannekoek, Y., J. Dankert, and J. P. van Putten. 1995. Construction of recombinant neisserial Hsp60 proteins and mapping of antigenic domains. Mol. Microbiol. 15:277-285. [DOI] [PubMed] [Google Scholar]
- 31.Parsons, L. M., R. J. Limberger, and M. Shayegani. 1997. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect. Immun. 65:2413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22:139-180. [DOI] [PubMed] [Google Scholar]
- 34.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipooligosaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 35.Raetz, C. R. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 36.Rahman, M., H. Källström, S. Normark, and A.-B. Jonsson. 1997. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol. Microbiol. 25:11-25. [DOI] [PubMed] [Google Scholar]
- 37.Rudel, T., H.-J. Boxberger, and T. F. Meyer. 1995. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol. Microbiol. 17:1057-1071. [DOI] [PubMed] [Google Scholar]
- 38.Rudel, T., D. Facius, R. Barten, I. Scheuerpflug, E. Nonnenmacher, and T. F. Meyer. 1995. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 92:7986-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudel, T., I. Scheurerpflug, and T. F. Meyer. 1995. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 373:357-359. [DOI] [PubMed] [Google Scholar]
- 40.Ryll, R. R., T. Rudel, I. Scheuerpflug, R. Barten, and T. F. Meyer. 1997. PilC of Neisseria meningitidis is involved in class II pilus formation and restores pilus assembly, natural transformation competence and adherence to epithelial cells in PilC-deficient gonococci. Mol. Microbiol. 23:879-892. [DOI] [PubMed] [Google Scholar]
- 41.Sieling, P. A., and R. L. Modlin. 2002. Toll-like receptors: mammalian “taste receptors” for a smorgasbord of microbial invaders. Curr. Opin. Microbiol. 5:70-75. [DOI] [PubMed] [Google Scholar]
- 42.Snyder, L. A. S., N. J. Saunders, and W. M. Shafer. 2001. A putatively phase variable gene (dca) required for natural competence in Neisseria gonorrhoeae but not Neisseria meningitidis is located within the division cell wall (dcw) gene cluster. J. Bacteriol. 183:1233-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song, W., L. Ma, R. Chen, and D. C. Stein. 2000. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J. Exp. Med. 191:949-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LOS and non-LOS to proinflammatory cytokine response. J. Leukoc. Biol. 70:283-288. [PubMed] [Google Scholar]
- 46.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitidis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 47.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipooligosaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20:6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. van der Ley, M. van Deuren, R. E. Callard, and N. Klein. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaara, M., and M. Nurminen. 1999. Outer membrane permeability barrier in Escherichia coli mutants that are defective in the late acyltransferases of lipid A biosynthesis. Antimicrob. Agents Chemother. 43:1459-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winther-Larsen, H. C., F. T. Hegge, M. Wolfgang, S. F. Hayes, J. P. van Putten, and M. Koomey. 2001. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. USA 98:15276-15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfgang, M., J. P. van Putten, S. F. Hayes, and M. Koomey. 1999. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol. Microbiol. 31:1345-1357. [DOI] [PubMed] [Google Scholar]
- 52.Wolfgang, M., P. Lauer, H. S. Park, L. Brossay, J. Hebert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321-330. [DOI] [PubMed] [Google Scholar]
- 53.Woods, M. L., II, R. Bonfiglioli, Z. A. McGee, and C. Georgopoulos. 1990. Synthesis of a select group of proteins by Neisseria gonorrhoeae in response to thermal stress. Infect. Immun. 58:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]