Summary
Recent reviews of the adaptive hypotheses for animal responses to acclimation have highlighted the importance of distinguishing between developmental and adult (non-developmental) phenotypic plasticity. However, little work has been undertaken separating the effects of developmental plasticity from adult acclimation in physiological traits. Therefore, we investigate the relative contributions of these two distinct forms of plasticity to the environmental physiology of adult tsetse flies by exposing developing pupae or adult flies to different temperatures and comparing their responses. We also exposed flies to different temperatures during development and re-exposed them as adults to the same temperatures to investigate possible cumulative effects. Critical thermal maxima were relatively inflexible in response to acclimation temperatures (21, 25, 29 °C) with plasticity type accounting for the majority of the variation (49-67 %, nested ANOVA). By contrast, acclimation had a larger effect on critical thermal minima with treatment temperature accounting for most of the variance (84-92 %). Surprisingly little of the variance in desiccation rate could be explained by plasticity type (30-47 %). The only significant effect of acclimation on standard (resting) metabolic rate of adult flies occurred in response to 21 °C, resulting in treatment temperature, rather than plasticity type, accounting for the majority of the variance (30-76 %). This study demonstrates that the stage at which acclimation takes place has significant, though often different effects on several adult physiological traits in G. pallidipes, and therefore that it is not only important to consider the form of plasticity but also the direction of the response and its significance from a life-history perspective.
Keywords: intra-specific variation, metabolic rate, phenotypic plasticity, water balance, beneficial acclimation hypothesis, climatic stress resistance
Introduction
Recently, interest in the nature and magnitude of non-genetic effects on phenotypic variation has increased. Phenotypic plasticity might not only account for much of the variation among populations (Ayrinhac et al., 2004; Hoffmann et al., 2005b), but it can also significantly affect the nature of evolutionary responses of populations (Price et al., 2003), and their likely survival especially in the face of rapidly changing modern climates (Helmuth et al., 2005; Somero, 2005). Physiologists have long assumed that acclimation (or acclimatization, both are forms of plasticity) to a particular environment enhances performance in that environment (e.g. Hochachka and Somero, 2002; Prosser, 1986) such that acclimation is beneficial (see Leroi et al., 1994). However, in recent years the generality of this beneficial acclimation hypothesis (BAH) has been questioned because most formal tests of the predictions thereof have been negative (Gibert et al., 2001; Gilchrist and Huey, 2001; Leroi et al., 1994; Sibly et al., 1997; Woods, 1999; Woods and Harrison, 2001; Zamudio et al., 1995; but see Nunney and Cheung, 1997; Thomson et al., 2001). Subsequently, Wilson and Franklin (2002) argued that many of these tests have actually addressed the adaptive significance of developmental plasticity rather than of acclimation. That is, the tests have examined the consequences for the adult phenotype of altered rearing environments, rather than the consequences of environmental variation within a given life-stage, and more specifically the adults. Therefore, they should not be considered tests of the beneficial acclimation hypothesis because acclimation is typically defined as a reversible, facultative response to changes in the adult environment (Spicer and Gaston, 1999; Wilson and Franklin, 2002). Given the wide range of conditions under which adaptive phenotypic plasticity is thought to arise (Berrigan and Scheiner, 2004; Scheiner, 1993), these alternative perspectives on beneficial acclimation have proven controversial.
One outcome of the debate is the realization that few studies have sought to examine the effects of acclimation in the rearing stage (developmental plasticity) relative to those in the adult or within a given life-stage (adult acclimation, sometimes also known as phenotypic flexibility, see Piersma and Drent, 2003), and the nature of the interactions between them. The most detailed work to date has been that of Fischer et al. (2003) and Zeilstra and Fischer (2005). In the former study, rearing temperature had a substantial effect on the size of eggs laid by Bicyclus anynana butterflies. However, these effects were largely reversible after adults were held at different temperatures. Thus, the magnitude of the developmental plasticity and adult acclimation effects was similar. Zeilstra and Fischer (2005) used Lycaena tityrus and again found that the effects of developmental plasticity on recovery time from cold shock were largely reversible. They also found that as long as newly eclosed adults were not exposed to cold shock, adult temperatures (i.e. an adult exposure and a rearing exposure to different temperatures) also affected recovery time, though these times tended to be shorter than those for butterflies exposed to cold shock immediately after eclosion (i.e. a rearing exposure only). Few other studies of this nature have been undertaken (though see Spicer and Gaston, 1999 for discussion of early work; see also Tracy and Walsberg, 2001), and therefore their generality is not certain.
In this study we therefore examine the effects of altering treatment temperatures during pupal development (developmental plasticity), during the adult stage (adult acclimation) and during both stages (combined plasticity) on four traits, viz. critical thermal minimum and maximum, water loss rate, and metabolic rate in the tsetse fly Glossina pallidipes Austen. This species was chosen for several reasons. First, variation in the traits of interest across the Insecta is typically partitioned at family and order levels (Chown et al., 2002). Therefore, investigations of non-lepidopteran species are likely to provide a rapid way of determining how general previous findings are likely to be. Second, temperature and water availability are important correlates of the distribution of G. pallidipes and play major roles in influencing its population dynamics directly (Hargrove, 2004; Rogers and Robinson, 2004) and indirectly via metabolic rate (Bursell et al., 1974; Bursell and Taylor, 1980; Terblanche et al., 2004). Investigating the mechanistic bases of the responses of flies to their external environment is therefore a significant link in the causal chain of reasoning from environment to population dynamics. Finally, as a vector of animal disease, G. pallidipes has important effects on animal health, and thus indirect effects upon socio-economic development in Africa (Maudlin et al., 2004). Understanding the likely current and future determinants of its abundance and distribution is therefore of considerable significance for ongoing development of the continent (Maudlin et al., 2004).
Materials and methods
Laboratory conditions and maintenance
Twelve to thirteen-day old Glossina pallidipes Austen (Diptera, Glossinidae) puparia (hereafter pupae) were received from a laboratory colony maintained at the Entomology Unit, FAO/IAEA Agriculture and Biotechnology Laboratory, Seibersdorf, International Atomic Energy Agency, Vienna, Austria. It is unlikely that these experimental flies exhibited inbreeding depression or other deleterious genetic effects of colonization because gene diversities over two mitochondrial loci were within the range of 18 field populations from Ethiopia, East and southern Africa (Krafsur and Wohlford, 1999). It is these pupae that were subjected to an acclimation treatment, or simply maintained close to the original conditions in which they were held at the Seibersdorf laboratory.
On arrival, pupae were immediately placed in Petri dishes (n = 50 per dish) on paper towels which were stored inside two plastic containers with non-airtight lids and transferred to a climate chamber held at 25 °C (mean ± SD: 24.8 ± 1.0). They were retained at this temperature except in the case of the developmental plasticity treatments, in which pupae were moved to incubators set at either 21 °C or 29 °C (see below), but otherwise identical to those described here. Relative humidity (R.H.) inside containers was regulated by means of saturated salt (NaCl) solutions located within each container to give 76 % R.H. (Winston and Bates, 1960). At the first sign of eclosion, pupae were transferred to 10-12 mesh cages (10 cm diameter, n < 50 per cage) and either retained at the original temperature or moved later to a different temperature for an adult acclimation treatment (details below). Cages were stored inside closed, non-airtight plastic containers with relative humidity regulated as above.
The adults were fed using a membrane-tray system (see Gooding et al., 1997) every alternative day (similar to the methods described in Terblanche et al., 2004), and subsequently container locations were randomized within climate chambers. Care was taken to ensure that all treatment groups were handled for the same duration during transfer from the climate chamber to the feeding area, and spent a similar amount of time outside of the climate chambers whilst feeding (∼25 min per group). Temperatures during shipment and acclimation were recorded using Thermocron iButtons (Dallas Semiconductors, Dallas, Texas, USA; sampling rate = 15 min; temperature (°C) ± SD during shipment: 24.2 ± 2.9).
Treatments
Three experimental treatments were undertaken: a developmental plasticity treatment, involving manipulation of the temperatures which pupae experienced, an adult acclimation treatment in which pupae developed at a common temperature, but adults were exposed to different temperatures, and a combined treatment where both pupae and adults were exposed to a given temperature (Fig. 1). In the case of developmental plasticity the following protocol was followed: Pupae received a six day acclimation in climate chambers set to 21 °C (mean ± SD: 20.5 ± 1.0), and 29 °C (28.0 ± 0.2) starting approximately 12-13 days prior to the expected date of emergence (i.e. just over halfway through the pupal stage). Pupae were also kept at 25 °C, though handled in the same way as the other groups, to provide insight into the changes relative to baseline conditions (see Sinclair and Chown, 2005 for rationale). After six days of acclimation, all groups were returned to 25 °C for emergence. This treatment does not constitute a full rearing exposure (as in Fischer et al., 2003), but nonetheless has a marked effect on the pupae (as confirmed in pilot trials). Moreover, it was undertaken because the timecourse of CTMin has been examined in adult G. pallidipes previously. This revealed that a maximum response is typically obtained within five days of exposure, and does not reflect a graded (temperature-dependent) response, but rather demonstrates a distinct temperature threshold (Terblanche et al., submitted ms). Eclosion commenced in the warmest acclimation group c. 7-8 days before the coolest acclimation group. Thus, depending on the experimental temperature in each developmental plasticity group, a total of 12-19 days passed between the end of the acclimation period and the onset of the experimental assessments. For G. pallidipes, ten days at 25 °C is sufficient to completely reverse adult acclimation responses in critical thermal minima after nine days at 19-21 °C (i.e. adult acclimation responses for this trait were reversible) (Terblanche et al., submitted ms). After 30-40% of the flies in an experimental group had begun to eclose, flies were taken through three blood meals, or ‘hunger cycles’ (∼ six days) and used for experimental assessment on approximately the eighth day of the adult stage in a fasted, post-developmental (non-teneral) state. This group was considered the developmental ‘plasticity’ treatment (Fig. 1).
Figure 1.
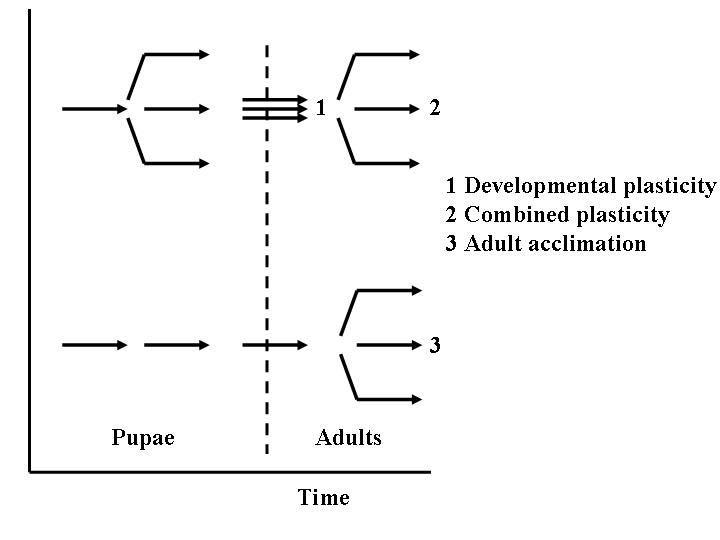
Schematic diagram of the experimental design used to investigate the relative contribution of developmental plasticity, adult acclimation and combined treatment to adult physiological performance in Glossina pallidipes. (Stippled line represents eclosion.)
On the day following the experimental assessment, the remaining flies were fed as usual and were then transferred back to the same temperature received during the pupal stage (e.g. flies which were exposed to 21 °C during the pupal stage were returned to 21 °C) and left to acclimate for a further six days whereupon the same physiological measures were assessed. These flies were labelled the ‘combined plasticity’ treatment (Fig. 1). Preliminary experiments using CTMin in adult G. pallidipes showed that acclimation responses can be fully induced after six days and CTMin does not change further with longer duration of acclimation (up to 12 days) (Terblanche et al., submitted ms).
The final treatment was the adult acclimation treatment (Fig. 1). Pupae left at 25 °C (24.8 ± 1.0) until after eclosion were divided into groups and placed in climate chambers set to 21, 25 and 29 °C. The flies were switched to these cabinets on day six after three hunger cycles (as above), and left at these temperatures for six days before traits were assessed.
Experimental assessments
All experimental assessments were performed on adult flies. In each experiment, care was taken to randomly select flies from as many cages as possible. In cases where the number of flies required was higher than the number of available cages, fly selection was balanced among cages. In addition, all acclimation groups were handled similarly (duration and vigour). Although no cage by treatment effects could be detected in preliminary experiments (Terblanche, unpublished data), the present treatments, in conjunction with frequently randomized cage locations, probably prevented any significant cage by treatment interactions.
Critical thermal limits
An insulated system with eleven double-jacketed isolation chambers was connected to a programmable water bath (LTD 20 with PZ1 programmer, Grant Instruments, Cambridge, UK) which regulated water flow around the chambers. A single fly was placed in each of the ten chambers. A 40 SWG type-T thermocouple was inserted into a control chamber to measure chamber temperatures. The flies were allowed to equilibrate for ten minutes at either 12 or 35 °C before the commencement of the respective minimum and maximum critical thermal limit assessments. Because of their small body mass, the body temperature of flies was considered equivalent to chamber temperature. Moreover, Edney and Barrass (1962) have demonstrated that there is no significant difference between body temperature and ambient temperature across the range of 25-45 °C under high humidity conditions in G. morsitans. After equilibration, the chambers' temperature was decreased or increased at 0.25 °C.min−1. Critical thermal minima (CTMin) were defined as the loss of coordinated muscle function at decreasing temperatures and critical thermal maxima (CTMax) were defined as the onset of muscle spasms at increasing temperatures (Klok and Chown, 1998). These endpoints are readily identifiable for any species once an observer is practiced (Lutterschmidt and Hutchison, 1997). Typically the variance about the endpoints is low, and here a single observer (CJK) undertook all of this work. Moreover, this experimental procedure has been verified using thermolimit respirometry (Klok et al., 2004), is widely used to assess thermal limits (Chown and Nicolson, 2004), and the observer typically was not informed which acclimation treatment was being assessed. Groups of ten flies were assessed at once and the temperature at which these limits were observed was recorded for each individual fly. Preliminary experiments using adult flies found no effect of gender, age or feeding status on critical thermal limits (unpublished data, C.J. Klok and J.S. Terblanche) and we assumed that this was the case in the present study too.
Desiccation rate
To remove possible confounding effects of sex or pregnancy, only male flies were used in desiccation experiments. Flies (n = 16), individually contained in 5 ml cuvettes, were subjected to desiccation in flowing air (< 2.5 % relative humidity) for ten hours at 25.0 (± 1.0) °C in a climate chamber (Labotech, South Africa). Air flow, produced by an aquarium pump, was directed through a scrubbing column containing silica gel and Drierite (Xenia, OH, USA) as desiccants to remove residual water, and then into a mass flow-controller (MFC) to control the air flow rate. The MFC outlet was connected to a Sable Systems (Las Vegas, NV, USA) MF8 airflow manifold. Each outflow channel of the manifold was further split in two so that two cuvettes, each containing a fly, were attached per manifold channel. The air flow rate through each cuvette, tested with a second MFC, was regulated to 100 ml.min-1. Experiments took place during the night (21h00-07h00) because this represents a period of minimal activity in tsetse (Brady, 1988; Kyorku and Brady, 1993). Mass was recorded before and after an experiment on an electronic microbalance (0.1 mg, Avery Berkel FA 304T, EU) and the difference was assumed to be a result of water loss (acknowledging that some mass loss is due to substrate catabolism but that this is negligible relative to the quantity of water loss over the time scales investigated here (Bursell, 1957)). Flies were dried to constant mass (∼50-60 °C for ∼72 hrs) and reweighed to give dry body mass.
Metabolic rate
Metabolic rate was recorded using flow-through respirometry. A calibrated LI-6262 (LiCor; Lincoln, Nebraska) infra-red gas analyzer (IRGA) was connected to a Sable Systems eight channel multiplexer inside a temperature-controlled cabinet (details given in Terblanche et al., 2004). The first seven channels regulated the flow-through respirometry for individual flies and channel eight was used as an empty reference channel for CO2 and H2O baseline measurements. These recordings were performed for fasted, post-development males at 25 °C in each acclimation group. Mass was recorded before and after respirometry recordings as described in the desiccation experiments. Airflow was regulated to 100 ml.min-1 using a MFC and the outside air was scrubbed through sodalime, silica-gel and Drierite columns to remove water and CO2. A Sable Systems AD-1 activity detector was connected to the first cuvette only to compare active and resting gas exchange traces in all flies. Previous studies have shown that in this species activity can be reliably detected from the V.CO2 trace without an activity detection system (Terblanche et al., 2004), which was confirmed here. Sable Systems Datacan V software was used to extract and analyze standard (resting) metabolic rate (SMR) data from 5-8 individuals per treatment group.
Statistical analyses
For all traits, the effect of the treatment (21 or 29 °C) relative to the standard rearing conditions (25 °C) was assessed. One way analyses of variance followed by post-hoc tests for homogeneity were used to compare the three temperature groups in the case of CTMin and CTMax. Because body mass influences both metabolic rate and water loss rate (Addo-Bediako et al., 2001; Addo-Bediako et al., 2002) analyses of covariance were used for these traits. These analyses provided primary insight into the effects of developmental plasticity, adult acclimation, and the combined treatment. However, to assess the relative contributions of plasticity type and treatment temperature to trait variation, two pure Model II (random effects) nested analyses of variance were undertaken (Sokal and Rohlf, 1995: 274). In the first analysis, treatment temperature was nested within plasticity type using data for developmental plasticity and adult acclimation only. In the second analysis, all three plasticity types were assessed nested within treatment temperature. These two nested analyses of variance present complimentary perspectives on the relative importance of plasticity type and treatment temperature for variation in each of the traits. Previous work demonstrated that the effects of developmental plasticity can be altered following adult acclimation for several traits (Fischer et al., 2003; Zeilstra and Fischer, 2005). Therefore we expected equal contributions of plasticity types to total variance. Sample sizes for physiological assessments ranged from 9-20 (CTMax), 12-20 (CTMin), 8-16 (water balance) and 5-8 (metabolic rate) individuals per treatment per temperature.
Results
Differences in CTMax associated with treatment temperature were small irrespective of whether this treatment took place in the pupal or adult stages, and significant in one case of adult acclimation only (Fig. 2). Thus, much of the variance in CTMax was explained by plasticity type (Table 1). The combined plasticity treatment tended to be most similar to the pupal treatment, and treatment temperature effects were small, resulting in plasticity type retaining its importance in the nested ANOVA including all three plasticity types (Table 2). Differences in CTMin among treatment temperatures were much more pronounced (Fig. 3). The effect was largest in the case of adult acclimation and combined plasticity for the 21 °C treatment, but in the 29 °C treatment developmental plasticity (and combined plasticity) had the largest effects. Perhaps more significantly, the effects of the two treatment temperatures were opposite in sign. Thus, in both nested ANOVAs treatment temperature accounted for most of the variance (Tables 1 and 2).
Figure 2.
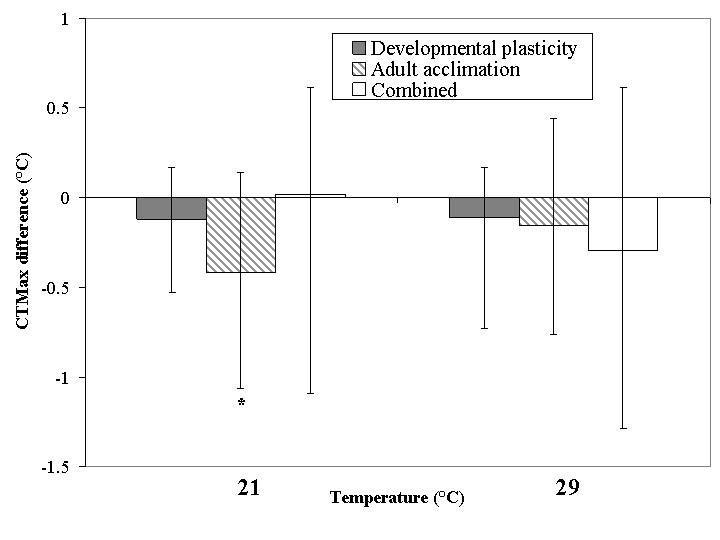
Relative change in mean critical thermal maxima for developmental plasticity, adult acclimation and combined plasticity groups within the acclimation temperature treatments expressed as change from the mean of the 25 °C group. Asterisks indicate a significant effect of the acclimation relative to the 25 °C group by One-way ANOVA (P < 0.05). Lower and upper error bars represent minimum and maximum values respectively.
Table 1.
Summary of nested (hierarchical) analyses of variance testing for an effect of the plasticity type (developmental vs. adult; Effect: Plasticity) and treatment temperature (21, 29 °C; Effect: Acclimation) on physiological traits of climatic stress resistance in adult Glossina pallidipes (Diptera, Glossinidae). Plasticity and Acclimation were categorised as random factors. MS = mean squares, DF = degrees of freedom.
| Physiological trait | Effect | DF | MS | DF error | MS error | F-ratio | P | % variance explained |
|---|---|---|---|---|---|---|---|---|
| Critical Thermal Maxima | ||||||||
| (°C) | Plasticity | 1 | 7.117 | 2.19 | 0.322 | 22.100 | < 0.05 | 66.8 |
| Acclimation | 2 | 0.352 | 55.00 | 0.115 | 3.048 | 0.056 | 4.1 | |
| Error |
29.2 |
|||||||
| Critical Thermal Minima | ||||||||
| (°C) | Plasticity | 1 | 2.408 | 2.00 | 35.975 | 0.067 | 0.820 | 0 |
| Acclimation | 2 | 40.442 | 56.00 | 0.240 | 168.406 | < 0.0001 | 91.8 | |
| Error |
8.2 |
|||||||
| Desiccation Rate1 | ||||||||
| (mg H2O/hr) | Plasticity | 1 | 0.146 | 2.33 | 0.080 | 1.825 | 0.293 | 15.5 |
| Acclimation | 2 | 0.119 | 43.00 | 0.016 | 7.624 | < 0.005 | 30.1 | |
| Error |
54.4 |
|||||||
| Standard Metabolic Rate2 | ||||||||
| (log10 μl CO2/hr) | Plasticity | 1 | 0.066 | 2.08 | 0.087 | 0.757 | 0.473 | 0 |
| Acclimation | 2 | 0.122 | 23.00 | 0.006 | 21.275 | < 0.0001 | 74.6 | |
| Error | 25.4 | |||||||
Covarying for dry body mass (P < 0.05)
Covarying for mean experimental body mass (P < 0.05)
Table 2.
Summary of nested (hierarchical) analyses of variance testing for an effect of treatment temperature (21, 29 °C) (Effect: Acclimation) and experimental plasticity treatment (Effect: Plasticity) on physiological traits of climatic stress resistance in adult Glossina pallidipes (Diptera, Glossinidae). Acclimation and Plasticity were categorised as random factors. MS = mean squares, DF = degrees of freedom.
| Physiological trait | Effect | DF | MS | DF error | MS error | F-ratio | P | % variance explained |
|---|---|---|---|---|---|---|---|---|
| Critical Thermal Maxima | ||||||||
| (°C) | Acclimation | 1 | 0.041 | 3.89 | 2.493 | 0.016 | 0.904 | 0 |
| Plasticity | 4 | 2.077 | 73.00 | 0.164 | 12.665 | < 0.0001 | 48.7 | |
| Error |
51.3 |
|||||||
| Critical Thermal Minima | ||||||||
| (°C) | Acclimation | 1 | 100.352 | 3.90 | 3.688 | 27.213 | < 0.01 | 83.6 |
| Plasticity | 4 | 3.114 | 74.00 | 0.245 | 12.725 | < 0.0001 | 7.9 | |
| Error |
8.5 |
|||||||
| Desiccation Rate1 | ||||||||
| (mg H2O/hr) | Acclimation | 1 | 0.287 | 3.89 | 0.231 | 1.241 | 0.329 | 4.4 |
| Plasticity | 4 | 0.196 | 73.00 | 0.016 | 12.251 | < 0.0001 | 46.7 | |
| Error |
48.9 |
|||||||
| Standard Metabolic Rate2 | ||||||||
| (log10 μl CO2/hr) | Acclimation | 1 | 0.211 | 3.85 | 0.051 | 4.137 | 0.114 | 36.4 |
| Plasticity | 4 | 0.046 | 33.00 | 0.008 | 6.045 | < 0.001 | 29.0 | |
| Error | 34.6 | |||||||
Covarying for dry body mass (P < 0.05)
Covarying for mean experimental body mass (P < 0.05)
Figure 3.
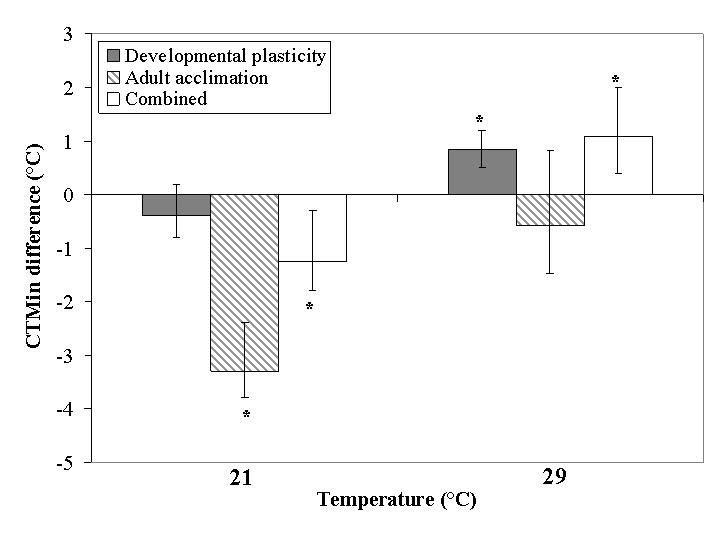
Relative change in mean critical thermal minima for developmental plasticity, adult acclimation and combined plasticity groups within the acclimation temperature treatments expressed as change from the mean of the 25 °C group. Asterisks indicate a significant effect of the acclimation relative to the 25 °C group by One-way ANOVA (P < 0.05). Lower and upper error bars represent minimum and maximum values respectively.
Treatment temperature in the pupal stage had little effect on water loss rate, and this was true for adult acclimation too, with the exception of a strong decline following adult acclimation at a treatment temperature of 29 °C (Fig. 4). The combined plasticity treatment at 29 °C also resulted in a substantial increase in water loss rate. In consequence, plasticity type explained a larger proportion of the variance when when all three plasticity types were included in the nested ANOVA (Table 2). However, surprisingly little of the variance in desiccation rate could be explained by plasticity type when only developmental plasticity and adult acclimation were considered (Table 1).
Figure 4.
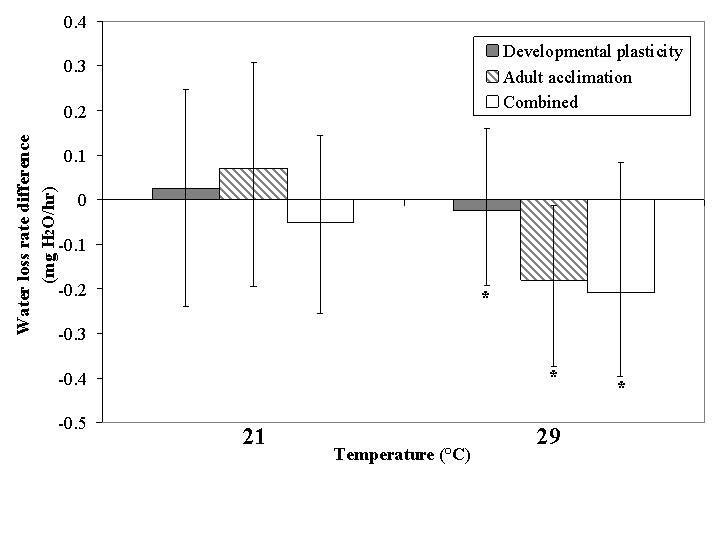
Relative change in mean water loss rate for developmental plasticity, adult acclimation and combined plasticity groups within the acclimation temperature treatments expressed as change from the mean of the 25 °C group. Asterisks indicate a significant effect of the acclimation relative to the 25 °C group by GLM (covariate: dry body mass; P < 0.05). Lower and upper error bars represent minimum and maximum values respectively.
When only developmental plasticity and adult acclimation were considered, the only significant effect was a response of adult metabolic rate to the 21 °C treatment (Fig. 5). In consequence, treatment temperature explained most of the variance (Table 1) among treatment temperature and plasticity type. This increase in metabolic rate following the 21 °C treatment was also found when both adults and pupae were exposed to acclimation, resulting in a more even partitioning of variance between plasticity type and treatment temperature (Table 2).
Figure 5.
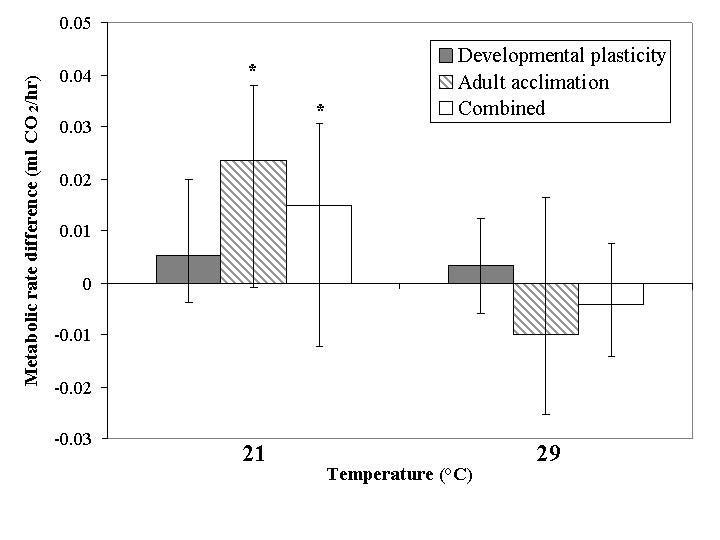
Relative change in mean metabolic rate as indicated by CO2 production for developmental plasticity, adult acclimation and combined plasticity groups within the acclimation temperature treatments expressed as change from the mean of the 25 °C group. Asterisks indicate a significant effect of the acclimation relative to the 25 °C group by GLM (covariate: mean experimental body mass; P < 0.05). Lower and upper error bars represent minimum and maximum values respectively.
Discussion
The effects of treatment temperature on the traits investigated in G. pallidipes here are largely similar to what has been found in other investigations of insects. In the case of thermal tolerances, acclimation effects were more pronounced for CTMin than for CTMax, as has been found in several other studies examining acclimation (Klok and Chown, 2003; Slabber and Chown, 2005) or acclimatization (Hoffmann et al., 2005b) effects on thermal limits. Although a more pronounced acclimation effect on lower than on upper limits is not universal (e.g. the converse has been found the caterpillars of the moth Embryonopsis halticella, (Klok and Chown, 1998)), it does seem to be typical of most ectotherms (Kingsolver and Huey, 1998). In insects, greater variation in lower than in upper thermal tolerances is typical not only of acclimation responses, but also of intra- and interspecific geographic variation in thermal limits, and in the responses of these limits to selection (Addo-Bediako et al., 2000; Chen et al., 1990; Chown, 2001; Gaston and Chown, 1999; Gilchrist et al., 1997; Hercus et al., 2000; Hoffmann et al., 1997; Kimura, 2004; Stanley et al., 1980; Terblanche et al., 2005b).
Increases in metabolic rate of the G. pallidipes adults with adult exposure to low temperature seems to be typical of this group of tsetse because it has also been found in Glossina morsitans. Terblanche et al. (2005a) showed that exposure of adult G. moristans to 29 °C had little effect on metabolic rate relative to adults held at 24 °C, but exposure to 19 °C resulted in a significant increase in metabolic rate across a wide range of test temperatures. Increases of metabolic rate in insects exposed to low temperatures are not uncommon (reviewed in Addo-Bediako et al., 2002; Chown and Gaston, 1999). However, the causes, consequences and likely significance of such whole organismal metabolic upregulation remain controversial and poorly investigated (Chown et al., 2003; Clarke, 2003; Hodkinson, 2003). Why metabolic rate should increase following exposure to a relatively low temperature in tsetse adults is not clear, but might contribute to the absence of this species (and G. morsitans) from low temperature areas. Elevated metabolic rates will result in increased use of lipid reserves, lower tolerance of starvation, and increased pressure for foraging, all of which are likely to enhance the chances of mortality (Rajagopal and Bursell, 1966), likely limiting the ability of the flies to survive in low temperature environments (though a lack of pupal development is also important for restricting flies to warmer areas, for review see Hargrove, 2004; Rogers and Robinson, 2004).
Perhaps more unusual than the responses of the other traits to acclimation was the change in desiccation rate in response to the temperature treatments. Previous studies have shown strong responses of insect desiccation rate to changes in relative humidity (e.g. Gibbs et al., 2003; Hoffmann, 1990). However, responses of water balance parameters to different temperature regimes are more variable. In some cases treatment temperature has elicited either no response in water balance-related traits (Terblanche et al., 2005b), or a response that is expressed in some traits but not others, such as cuticular hydrocarbon profile changes but no alteration of water loss rates in cactophilic Drosophila (Gibbs et al., 1998; see also Cloudsley-Thompson, 1969). In others, pronounced responses in desiccation rate have been found. This is true of D. melanogaster from the east coast of Australia, where in several populations in response to summer acclimation temperatures, individuals showed greater desiccation resistance than flies exposed to constant or winter temperatures (Hoffmann et al., 2005b). Similar declines in desiccation resistance with increasing treatment temperature have also been found for D. takahashii and D. nepalensis (Parkash et al., 2005). Here, we found that when exposed to 29 °C, either as adults or as pupae, adult G. pallidipes had significantly reduced rates of water loss compared to flies held at 21 °C or 25 °C (and similar to results described in Terblanche et al., submitted ms). Neither the ultimate, nor proximate causes of this change have been fully investigated. It seems likely that in the former case a reduction in desiccation rate at high temperatures should increase survival given that high temperatures can be associated with low water availability in the habitats occupied by these flies. Indeed, inter-population comparisons of G. pallidipes in Kenya have shown that populations from drier areas tend to have the lower water loss rates than those from moister areas (Terblanche et al., submitted ms). How the acclimation or inter-population differences are effected mechanistically has not been well studied, but differences in the amount and composition of cuticular lipids might be significant, as has been found in other species (Gibbs et al., 1991; Rourke, 2000). Preliminary studies in this species have indicated that variation in cuticular hydrocarbon profiles and desiccation rates can occur among populations, however desiccation rates in these flies are not correlated with cuticular lipid mass (i.e. quantity) (R. Jurenka, G. Marquez, J. Odera, J. Terblanche, C. Klok, S. Chown, E. Krafsur, unpublished data).
Whilst the extent of the acclimation responses varied among traits, several generalizations can be made about the effects of plasticity type on the traits measured in the adults. Responses to treatment temperature showed an asymmetric effect across all responsive plasticity types, with either the low treatment temperature or the high treatment temperature having a significant effect, but responses to both being found only for CTMin. In consequence, treatment temperature typically explained less than half of the variance in the measured traits except in the case of CTMin where it accounted for more than 80% of the variance. However, the symmetric CTMin response was found only for the combined plasticity treatment, but in this case appeared to be composed of a response to the 21 °C treatment in the adults, and a response to the 29 °C treatment in the pupae, but not vice versa. This finding deserves further exploration. Nonetheless, strong opposing responses to high and low temperature treatments for lower thermal limits have been found in several other studies, though typically these have examined acclimation effects on a single stage only (e.g. Ayrinhac et al., 2004; Hoffmann et al., 2005b; Klok and Chown, 2003; Slabber and Chown, 2005; Terblanche et al., 2005b; Zeilstra and Fischer, 2005). Likewise, asymmetric or threshold effects of treatment temperature have previously been recorded for CTMax (Hoffmann et al., 2005b; Klok and Chown, 1998; Slabber and Chown, 2005), metabolic rate (Hoffmann, 1985; Terblanche et al., 2005a) and desiccation rate (Rourke, 2000).
By contrast, plasticity type usually accounted for substantial proportions of the variation in the response of the traits to acclimation. Developmental plasticity following the 29 °C temperature treatments was significant and irreversible for CTMin and desiccation rate, but the pupal treatment was either reversed or had little effect following the 21 °C treatment for these traits and for both temperature treatments in the other traits. The absence of reversibility in CTMin following exposure to 29 °C is unlike the situation in Lycaena tityrus, in which lower thermal limits that change within the pupal stage are typically reversible in the adults (Zeilstra and Fischer, 2005). No other work has examined the effects of developmental plasticity on desiccation rate in insects, but clearly it is significant. Developmental plasticity is known from a wide variety of other traits (e.g. morphological, behavioural, locomotion performance; Atkinson, 1996; Crill et al., 1996; Nijhout, 2003; Sheeba et al., 2002), and generally is assumed to be fixed in the adult stage (e.g. Gibert et al., 2000; and discussed in Wilson and Franklin, 2002). Although early work assumed that such plasticity would be beneficial, most recent studies have shown that this is rarely the case (for discussion see Huey et al., 1999; Wilson and Franklin, 2002). The present design did not enable us to fully explore this hypothesis, though clearly the responses shown by CTMin and desiccation rate could initially be considered beneficial given that vapour pressure deficit would increase at higher temperatures (for a given quantity of water in the air) (Addo-Bediako et al., 2001), and that a tradeoff between the extent of lower thermal limits and starvation resistance has been found (Hoffmann et al., 2005a).
Adult acclimation effects were most common following low temperature exposures, although for desiccation rate it was only the high temperature exposure that had an effect. Although the time-course of the persistence of changes in physiological tolerances have not yet been investigated, other work on the same species has shown that plasticity in CTMin is reversible within 10 days (Terblanche et al., submitted ms). The generality of these findings for other traits of physiological tolerance in this and other insect species warrants further attention. These findings are in keeping with what has been found in many other studies (reviewed in Spicer and Gaston, 1999; see also Wilson and Franklin, 2002), and confirm the notion that adult acclimation is typically reversible, while developmental plasticity is not (Piersma and Drent, 2003). What was perhaps most significant is that the combined plasticity treatments did not seem to have noticeable additive effects. Rather, the extent of the response following the combined treatments was either quite similar to the pupal or adult response following exposure, or in the case of the response of critical thermal limits to the low temperatures seemed to have a negative effect on tolerance. What the basis for this negative interaction might be is not clear. However, it is known that basal and induced cold tolerance responses are linked in some species (Chown and Nicolson, 2004).
In conclusion, this study has shown that the stage at which acclimation takes place has significant, though often different, effects on several adult traits that are likely to modify environmental effects on populations of G. pallidipes. These show that it is not only important to distinguish between developmental plasticity and adult acclimation (Wilson and Franklin, 2002; Piersma and Drent, 2003) and consider possible interactions among them, but also to consider the direction of the responses and their significance from a life-history perspective (Fischer et al., 2003).
Acknowledgements
We thank Larissa Heyns for laboratory assistance and the IAEA Entomology Unit at Seibersdorf, in particular Andrew Parker, for flies and rearing equipment. We are grateful to Jaco Klok who performed the critical thermal limit assessments. Elliot Krafsur, Ulrike Irlich, Peter le Roux, Elrike Marais, and two anonymous referees provided useful and constructive comments on an earlier version. This work was funded by an NIH grant AI-52456 to E.S. Krafsur.
References:
- Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society of London B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addo-Bediako A, Chown SL, Gaston KJ. Revisiting water loss in insects: a large scale view. Journal of Insect Physiology. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Addo-Bediako A, Chown SL, Gaston KJ. Metabolic cold adaptation in insects: a large-scale perspective. Functional Ecology. 2002;16:332–338. [Google Scholar]
- Atkinson D. Ectotherm life-history responses to developmental temperature. In: Johnston IA, Bennett AF, editors. Animals and temperature. Phenotypic and evolutionary adaptation. Cambridge University Press; Cambridge: 1996. pp. 183–204. [Google Scholar]
- Ayrinhac A, Debat V, Gibert P, Kister A-G, Legout H, Moreteau B, Vergilino R, David JR. Cold adaptation in geographical populations of Drosophila melanogaster: phenotypic plasticity is more important than genetic variability. Functional Ecology. 2004;18:700–706. [Google Scholar]
- Berrigan D, Scheiner SM. Modeling the evolution of phenotypic plasticity. In: De Witt TJ, Scheiner SM, editors. Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford University Press; New York: 2004. [Google Scholar]
- Brady J. Circadian ontogeny in the tsetse fly: A permanent major phase change after the first feed. Journal of Insect Physiology. 1988;34:743–749. [Google Scholar]
- Bursell E. Spiracular control of water loss in the tsetse fly. Proceedings of the Royal Entomological Society of London A. 1957;32:21–29. [Google Scholar]
- Bursell E, Billing KC, Hargrove JW, McCabe CT, Slack E. Metabolism of the bloodmeal in tsetse flies. Acta Tropica. 1974;31:297–320. [PubMed] [Google Scholar]
- Bursell E, Taylor P. An energy budget for Glossina (Diptera: Glossinidae) Bulletin of Entomological Research. 1980;70:187–196. [Google Scholar]
- Chen C-P, Lee Jr RE, Denlinger DL. A comparison of the responses of tropical and temperate flies (Diptera: Sarcophagidae) to cold and heat stress. Journal of Comparative Physiology. 1990;160:543–547. [Google Scholar]
- Chown SL. Physiological variation in insects: hierarchical levels and implications. Journal of Insect Physiology. 2001;47:649–660. doi: 10.1016/s0022-1910(00)00163-3. [DOI] [PubMed] [Google Scholar]
- Chown SL, Addo-Bediako A, Gaston KJ. Physiological variation in insects: large-scale patterns and their implications. Comparative Biochemistry and Physiology B. 2002;131:587–602. doi: 10.1016/s1096-4959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Chown SL, Addo-Bediako A, Gaston KJ. Physiological diversity: listening to the large-scale signal. Functional Ecology. 2003;17:568–572. [Google Scholar]
- Chown SL, Gaston KJ. Exploring links between physiology and ecology at macro scales: the role of respiratory metabolism in insects. Biological Reviews. 1999;74:87–120. [Google Scholar]
- Chown SL, Nicolson SW. Insect Physiological Ecology. Mechanisms and Patterns. Oxford University Press; Oxford: 2004. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends in Ecology and Evolution. 2003;18:573–581. [Google Scholar]
- Cloudsley-Thompson JL. Acclimation, water and temperature relations of the woodlice Metoponorthus pruinosus and Periscyphis jannonei in the Sudan. Journal of Zoology. 1969;158:267–276. [Google Scholar]
- Crill WD, Huey RB, Gilchrist GW. Within- and between-generation effects of temperature on the morphology and physiology of Drosophilia melanogaster. Evolution. 1996;50:1205–1218. doi: 10.1111/j.1558-5646.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- Edney EB, Barrass R. The body temperature of the tsetse fly, Glossina morsitans Westwood (Diptera, Muscidae) Journal of Insect Physiology. 1962;8:469–481. [Google Scholar]
- Fischer K, Eenhorn E, Bot ANM, Brakefield PM, Zwaan BJ. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. London B. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Chown SL. Elevation and climatic tolerance: a test using dung beetles. Oikos. 1999;86:584–590. [Google Scholar]
- Gibbs A, Mousseau TA, Crowe JH. Genetic and acclimatory variation in biophysical properties of insect cuticle lipids. Proceedings of the National Academy of Sciences of the USA. 1991;88:7257–7260. doi: 10.1073/pnas.88.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs AG, Fukuzato F, Matzkin LM. Evolution of water conservation mechanisms in Drosophila. Journal of Experimental Biology. 2003;206:1183–1192. doi: 10.1242/jeb.00233. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Louie AK, Ayala JA. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: is thermal acclimation beneficial? Journal of Experimental Biology. 1998;201:71–80. doi: 10.1242/jeb.201.1.71. [DOI] [PubMed] [Google Scholar]
- Gibert P, Huey RB, Gilchrist GW. Locomotor performance of Drosophila melanogaster: Interactions among developmental and adult temperatures, age, and geography. Evolution. 2001;55:205–209. doi: 10.1111/j.0014-3820.2001.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Gibert P, Moreteau B, David JR. Developmental constraints on an adaptive plasticity: reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evolution & Development. 2000;2:249–260. doi: 10.1046/j.1525-142x.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB. Parental and developmental temperature effects on the thermal dependence of fitness in Drosophila melanogaster. Evolution. 2001;55:209–214. doi: 10.1111/j.0014-3820.2001.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist GW, Huey RB, Partridge L. Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiological Zoology. 1997;70:403–414. doi: 10.1086/515853. [DOI] [PubMed] [Google Scholar]
- Gooding RH, Feldmann U, Robinson AS. Care and maintenance of tsetse colonies. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors. Chapman & Hall; London: 1997. pp. 41–55. [Google Scholar]
- Hargrove JW. Tsetse population dynamics. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiases. CABI Publishing; Wallingford: 2004. pp. 113–138. [Google Scholar]
- Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Annual Review of Physiology. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- Hercus MJ, Berrigan D, Blows MW, Magiafoglou A, Hoffmann AA. Resistance to temperature extremes between and within life cycle stages in Drosophila serrata, D. birchii and their hybrids: intraspecific and interspecific comparisons. Biological Journal of the Linnean Society. 2000;71:403–416. [Google Scholar]
- Hochachka PW, Somero GN. Oxford University Press; New York: 2002. Biochemical Adaptation. Mechanism and Process in Physiological Evolution. [Google Scholar]
- Hodkinson ID. Metabolic cold adaptation in arthropods: a smaller-scale perspective. Functional Ecology. 2003;17:562–567. [Google Scholar]
- Hoffmann AA. Acclimation for desiccation resistance in Drosophila melanogaster and the association between acclimation responses and genetic variation. Journal of Insect Physiology. 1990;36:885–891. [Google Scholar]
- Hoffmann AA, Dagher H, Hercus M, Berrigan D. Comparing different measures of heat resistance in selected lines of Drosophila melanogaster. Journal of Insect Physiology. 1997;43:393–405. doi: 10.1016/s0022-1910(96)00108-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hallas R, Anderson AR, Telonis-Scott M. Evidence for a robust sex-specific trade-off between cold resistance and starvation resistance in Drosophila melanogaster. Journal of Evolutionary Biology. 2005a;18:804–810. doi: 10.1111/j.1420-9101.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Shirrifs J, Scott M. Relative importance of plastic vs genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Functional Ecology. 2005b;19:222–227. [Google Scholar]
- Hoffmann KH. Metabolic and enzyme adaptation to temperature. In: Hoffmann KH, editor. Environmental Physiology and Biochemistry of Insects. Springer-Verlag; Berlin: 1985. pp. 1–32. [Google Scholar]
- Huey RB, Berrigan D, Gilchrist GW, Herron JC. Testing the adaptive significance of acclimation: a strong inference approach. American Zoologist. 1999;39:323–336. [Google Scholar]
- Kimura MT. Cold and heat tolerance of drosopholid flies with reference to their latitudinal distributions. Oecologia. 2004;140:442–449. doi: 10.1007/s00442-004-1605-4. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Huey RB. Evolutionary analyses of morphological and physiological plasticity in thermally variable environments. American Zoologist. 1998;38:545–560. [Google Scholar]
- Klok CJ, Chown SL. Interactions between desiccation resistance, host-plant contact and the thermal biology of a leaf-dwelling sub-Antarctic caterpillar, Embryonopsis halticella (Lepidoptera: Yponomeutidae) Journal of Insect Physiology. 1998;44:615–628. doi: 10.1016/s0022-1910(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Klok CJ, Chown SL. Resistance to temperature extremes in sub-Antarctic weevils: interspecific variation, population differentiation and acclimation. Biological Journal of the Linnean Society. 2003;78:401–414. [Google Scholar]
- Klok CJ, Sinclair BJ, Chown SL. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J. exp. Biol. 2004;207:2361–2370. doi: 10.1242/jeb.01023. [DOI] [PubMed] [Google Scholar]
- Krafsur ES, Wohlford DL. Breeding structure of Glossina pallidipes populations evaluated by mitochondrial variation. Journal of Heredity. 1999;90:635–642. doi: 10.1093/jhered/90.6.635. [DOI] [PubMed] [Google Scholar]
- Kyorku C, Brady J. A free-running bimodal circadian rhythm in the tsetse fly Glossina longipennis. Journal of Insect Physiology. 1993;40:63–67. [Google Scholar]
- Leroi AM, Bennett AF, Lenski RE. Temperature acclimation and competitive fitness: an experimental test of the Beneficial Acclimation Assumption. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1917–1921. doi: 10.1073/pnas.91.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterschmidt WI, Hutchison VH. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Canadian Journal of Zoology. 1997;75:1553–1560. [Google Scholar]
- Maudlin I, Holmes PH, Miles MA. CABI Publishing; Wallingford: 2004. The Trypanosomiases. [Google Scholar]
- Nijhout HF. Development and evolution of adaptive polyphenisms. Evolution & Development. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Nunney L, Cheung W. The effect of temperature on body size and fecundity in female Drosophila melanogaster: evidence for adaptive plasticity. Evolution. 1997;51:1529–1535. doi: 10.1111/j.1558-5646.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Parkash R, Tyagi PK, Sharma I, Rajpurohit S. Adaptations to environmental stress in altitudinal populations of two Drosophila species. Physiological Entomology. 2005 doi: 10.1111/j.1365-3032.2005.00470.x. [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends in Ecology & Evolution. 2003;18:228–233. [Google Scholar]
- Price TD, Qvarnström A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser CL. John Wiley & Sons; New York: 1986. Adaptational Biology. Molecules to Organisms. [Google Scholar]
- Rajagopal PK, Bursell E. The respiratory metabolism of resting tsetse flies. Journal of Insect Physiology. 1966;12:287–297. [Google Scholar]
- Rogers DJ, Robinson TP. Tsetse distribution. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiases. CABI Publishing; Wallingford: 2004. pp. 139–180. [Google Scholar]
- Rourke BC. Geographic and altitudinal variation in water balance and metabolic rate in a California grasshopper, Melanoplus sanguinipes. Journal of Experimental Biology. 2000;203:2699–2712. doi: 10.1242/jeb.203.17.2699. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. Genetics and evolution of phenotypic plasticity. Annual Review of Ecology and Systematics. 1993;24:35–68. [Google Scholar]
- Sheeba V, Chandrashekaran MK, Joshi A, Sharma VK. Developmental plasticity of the locomotor activity rhythm of Drosophila melanogaster. Journal of Insect Physiology. 2002;48:25–32. doi: 10.1016/s0022-1910(01)00139-1. [DOI] [PubMed] [Google Scholar]
- Sibly RM, Winokus L, Smith RH. Interpopulation variation in phenotypic plasticity in the speckled wood butterfly, Pararge aegeria. Oikos. 1997;78:323–330. [Google Scholar]
- Sinclair BJ, Chown SL. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. Journal of Experimental Biology. 2005;208:869–879. doi: 10.1242/jeb.01455. [DOI] [PubMed] [Google Scholar]
- Slabber S, Chown SL. Differential responses of thermal tolerance to acclimation in the sub-Antarctic rove beetle Halmaeusa atriceps. Physiological Entomology. 2005;30:195–204. [Google Scholar]
- Somero GN. Linking biogeography to physiology: Evolutionary and acclimatory adjustements of thermal limits. Frontiers in Zoology. 2005;2 doi: 10.1186/1742-9994-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. The Principles and Practice of Statistics in Biological Research. 3rd W.H. Freeman; New York: 2005. Biometry. [Google Scholar]
- Spicer JI, Gaston KJ. Blackwell Science; Oxford: 1999. Physiological Diversity and its Ecological Implications. [Google Scholar]
- Stanley SM, Parsons PA, Spence GA, Weber L. Resistance of species of the Drosophila melanogaster subgroup to environmental extremes. Australian Journal of Zoology. 1980;28:413–421. [Google Scholar]
- Terblanche JS, Klok CJ, Chown SL. Metabolic rate variation in Glossina pallidipes (Diptera : Glossinidae): gender, ageing and repeatability. Journal of Insect Physiology. 2004;50:419–428. doi: 10.1016/j.jinsphys.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Terblanche JS, Klok CJ, Chown SL. Temperature-dependence of metabolic rate in Glossina morsitans morsitans (Diptera, Glossinidae) does not vary with gender, age, feeding, pregnancy or acclimation. Journal of Insect Physiology. 2005a;51:861–870. doi: 10.1016/j.jinsphys.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Terblanche JS, Sinclair BJ, Klok CJ, McFarlane ML, Chown SL. The effects of acclimation on thermal tolerance, desiccation resistance and metabolic rate in Chirodica chalcoptera (Coleoptera: Chrysomelidae) Journal of Insect Physiology. 2005b;51:1013–1023. doi: 10.1016/j.jinsphys.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Thomson LJ, Robinson M, Hoffmann AA. Field and laboratory evidence for acclimation without costs in an egg parasitoid. Functional Ecology. 2001;15:217–221. [Google Scholar]
- Tracy RL, Walsberg GE. Developmental and acclimatory contributions to water loss in a desert rodent: investigating the time-course of adaptive change. Journal of Comparative Physiology B. 2001;171:669–679. doi: 10.1007/s003600100218. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Franklin CE. Testing the beneficial acclimation hypothesis. Trends in Ecology & Evolution. 2002;17:66–70. [Google Scholar]
- Winston PW, Bates DH. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]
- Woods HA. Patterns and mechanisms of growth of fifth-instar Manduca sexta caterpillars following exposure to low- or high-protein food during early instars. Physiological and Biochemical Zoology. 1999;72:445–454. doi: 10.1086/316678. [DOI] [PubMed] [Google Scholar]
- Woods HA, Harrison JF. The beneficial acclimation hypothesis versus acclimation of specific traits: physiological change in water-stressed Manduca sexta caterpillars. Physiological and Biochemical Zoology. 2001;74:32–44. doi: 10.1086/319302. [DOI] [PubMed] [Google Scholar]
- Zamudio KR, Huey RB, Crill WD. Bigger isn't always better: body size, temperature and male territorial success in Drosophila melanogaster. Animal Behaviour. 1995;49:671–677. [Google Scholar]
- Zeilstra I, Fischer K. Cold tolerance in relation to developmental and adult temperature in a butterfly. Physiological Entomology. 2005;30:92–95. [Google Scholar]


