Synthetic oligonucleotides are employed to downregulate intracellular RNA expression through mechanisms involving RNA interference,1-3 ribozyme, and antisense, as well as, to inhibit protein function through aptamer approach.4-6 Based on these technologies, two drugs, Vitravene™ (antisense) and Macugen™ (aptamer) have been approved for clinical use. Oligonucleotides also find applications in target validation, functional genomics, diagnostics, and as an experimental tool in molecular biology. 4-6
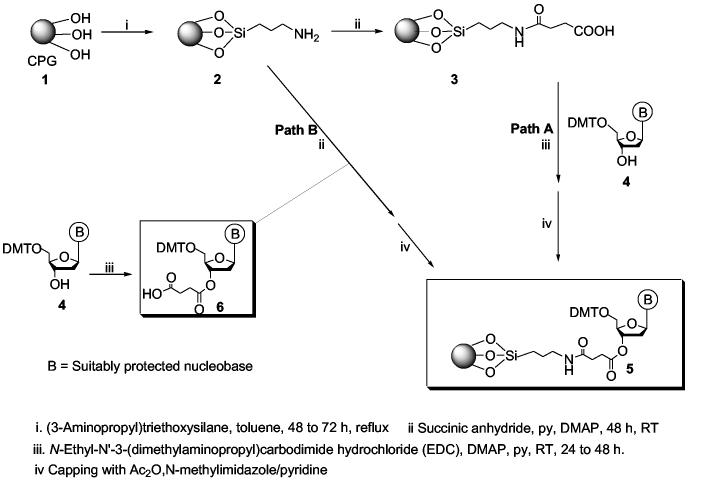
The synthesis and manufacture of the oligonucleotides are carried out using automated synthesizers in conjunction with solid-phase phosphoramidite chemistry.7 The assembly of these oligonucleotides involves the sequential attachment of monomeric units to a leader nucleoside bound to a solid support.8 Consequently, the availability of appropriate nucleoside-loaded supports is critical for the manufacture of oligonucleotides. Although several improvements in oligonucleotide synthesis have been reported over the past two decades,8,9 only limited studies have been reported10-14 on the functionalization of solid supports and preparation of support-bound nucleosides (Scheme 1). The functionalization and loading of supports are presently carried out according to Scheme 110-14 wherein native controlled-pore-glass (CPG) 1 functionalized as the aminopropylsilyl derivative 2, is succinylated to produce the carboxy-tethered CPG derivative 3. In a final step, a 5′-DMT-protected nucleoside 4 is coupled to the carboxy terminus of 3 by esterification to give 5 (Path A, Scheme 1). Alternatively, Path B is followed where 2 is contacted with the pre-synthesized nucleoside hemisuccinate derivative 6 to produce 5. Although, the reported protocols for producing 5 from 1 have helped advance oligonucleotide synthesis technology, the protocols are time-consuming, labor-intensive, and show high batch to batch variability in loading.
Scheme 1.
Functionalization and loading of nucleoside on solid support
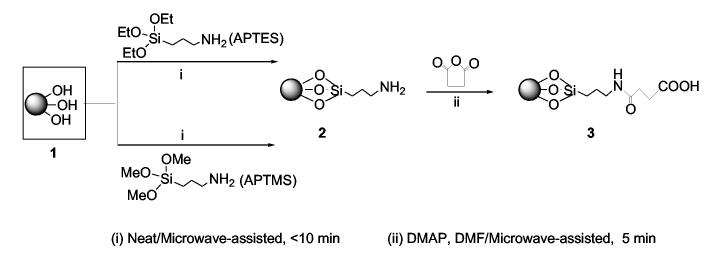
In connection with our program on antiviral drug discovery and development,15,16 we needed access to large quantities of supports with high nucleoside loadings for solid-phase synthesis. Recently, we disclosed our results on microwave-assisted functionalization of solid supports that provides rapid access to amino-terminated (CPG) 2, and carboxy-terminated CPG 3 starting from native CPG 1 (Scheme 2).17,18 Reported herein is highly efficient loading of nucleosides on succinylated-CPG 3 using a specially fabricated reactor in conjunction with N,N-dimethylformamide (DMF) as a solvent, with simultaneous recovery of excess nucleoside thereby resulting in high economy of operations.
Scheme 2.
Microwave-assisted functionalization of solid support
For the loading studies, adequate quantities of functionalized supports 2 and 3 were desired. For this purpose, 100 g batches of native CPG (500 [unk]) 1 were functionalized using microwave-assisted amination to give amino-CPG 2 with loadings of 100 to 110 micromol/g (Scheme 2).17 The amino-CPG 2 was converted to carboxy-terminated CPG 3 using microwave-assisted succinylation as reported elsewhere.17 We then attempted the loading of nucleosides on carboxy-terminated solid support 3 using reported methods.14 Thus, a large excess of nucleoside 4, condensing reagents, along with catalysts were contacted with the functionalized solid support 3 in the presence of pyridine, and mixed in an orbital shaker over a long period until sufficient loading was obtained (Scheme 1). Though, nucleoside-loaded CPG could be obtained using these procedures, there was wide variability in the loadings that ranged from 30 to 80 micromol/g starting from amino-CPG 2 of 100 to 110 micromol/g amino loading. Additionally, during large-scale work, there was a potential risk of continued exposure to pyridine and air-borne particles of the solid supports. Therefore, we explored alternate approaches for the loading of nucleoside on succinylated support 3.19
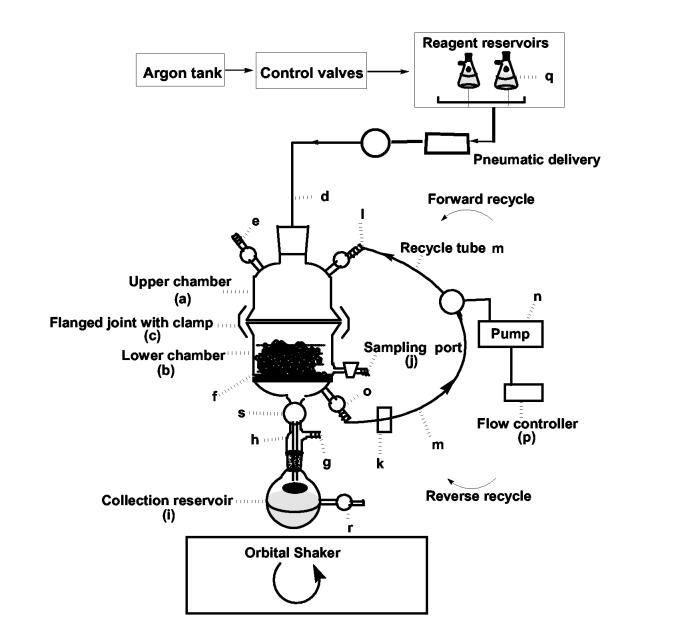
We rationalized that effective mixing and contact of the functionalized solid support with the nucleoside and various reagents are necessary to achieve efficient and high loading of supports in these multi-phase reactions. Additionally, performing all operations in a closed system could minimize exposure to toxic solvents and air-borne support particles. Accordingly, we designed a novel reactor equipped with a work station called “LOTUS” (Figure 1)20 that incorporates three design elements to facilitate solid-liquid mixing: (a) orbital shaking of the solid and liquid phases, (b) fluidization of phases induced by inert gas , and (c) active recycling of liquid phase. We have designated the mixing process in LOTUS as “orbital shaking coupled with active recycling” (OSCAR).
Fig. 1.
Prototype LOTUS reactor and workstation. The LOTUS reaction vessel comprises of an upper chamber a and a lower chamber b held together by the clamp c. The upper chamber has a gas/solid/liquid inlet d. The inlet d is used to introduce fresh chemical reactants into the reaction vessel under argon. A pressure release valve e is provided on the upper chamber a. The lower chamber, equipped with frit f (medium porosity), is connected via valve s to an adapter h to the collection reservoir i. The adapter h further comprises a gas port g, suitable for connecting to a vacuum line to draw excess reagents into the collection reservoir as desired. The contents of the collection reservoir can be drained through the valve r. The entire reaction vessel including the collection reservoir I is mounted on an orbital shaker to ensure thorough and efficient mixing of the solid, liquid and gas phases during the reaction.The reaction chamber and accessories are maintained under slightly positive inert atmosphere so that air and moisture sensitive reactions can be performed. The lower chamber b comprises a sampling port j that allows a sample of solid matrix to be drawn for analysis during reactions, and process operations. Liquid reactants can be independently sampled from auxiliary port k in the recycle path. The online monitoring of both solid and liquid phases allows better process control during nucleoside loading. In order to ensure continuous and close contact between liquid and solid phases, the concept of active recycling is incorporated in the reactor design. Thus, the upper chamber a is fitted with a recycled reagent inlet port l adapted for connection to flexible, chemically resistant tubing m that fits into the pump head of a peristaltic pump n. The lower chamber b comprises of a recycled reagent outlet port o that is adapted for connection to flexible tubing. The recycled reagent outlet port o of the lower chamber b is connected to the recycled reagent inlet port l of the upper chamber a via tubing through the pump.The pump n serves several functions: (a) It can be optionally used to draw the reactants from a reservoir into the reaction vessel, (b) It helps in recycling the liquid contents of the reaction vessel through recycled reagent ports o and l, (c) It helps modulate the delivery of reagent or the rate of its recycling throughout the reaction via the flow controller p. The recycling can be done in both forward, as well as, reverse directions, at different speeds. Forward recycling generates turbulent forces that help in mixing of solid and liquid phases, and reverse recycling assists fluidization of solid and liquid phases thereby aiding mixing. The combined fluid forces generated by forward and reverse recycling facilitates thorough mixing of the solid and liquid reaction phases thereby resulting in rapid reaction kinetics and efficient and reproducible loading without causing mechanical abrasion of solid support.LOTUS has been designed to incorporate pneumatic systems for better control of fluid flow while maintaining inert atmosphere. LOTUS I has two pneumatically controlled solenoid valves (McMaster Carr) for delivery of reagents from solvent reservoirs q under argon. Pressure release valves placed in the argon line provides outlet for release of excess pressure. The activation of the solenoid valves, forces pressurized gas into the solvent and reactant chambers thus allowing liquids to enter into the reactor.
LOTUS consists of a reaction chamber equipped with accessories and a workstation to control operations by which both recycling and orbital shaking can be optionally and independently controlled. As shown in Fig. 1, since all operations are performed in a closed system, human exposure to reagents, solvents, and supports are minimized while maintaining anhydrous conditions and inert atmosphere.
As representative examples, we carried out the loading of 5′-O-4,4-dimethoxytrityl-N-benzoyl-deoxyadenosine (DMT-NBzdA) and 5′-O-4,4-dimethoxytrityl thymidine (DMT-T) on succinylated CPG 3, using LOTUS in conjunction with anhydrous DMF as solvent. Using 100 g supports in each batch, we were able to achieve high nucleoside loading of 70 to 80 μmol/g with only three equivalents of nucleoside within 12 to 14 hours. Following the reaction, both filtration and drying operations were performed in LOTUS. The nucleoside-loaded CPG was then treated with CAP A and CAP B in LOTUS. Following OSCAR, the reaction mixture was filtered, washed and dried. Thus, nucleoside loading, as well as, the attendant process operations could be conveniently performed in LOTUS. Additionally, the use of DMF instead of pyridine prevented any unpleasant odor and also aided the facile recovery of excess nucleoside, which is especially desirable while using expensive nucleosides. Indeed, the recovery of excess nucleoside was carried out by simple aqueous work-up of the DMF filtrate. Subsequently, the recovered nucleoside (DMT-NBzdA) was successfully loaded onto succinylated CPG. We believe that efficient intermixing of phases, induced by OSCAR, along with on-line monitoring, is the key to faster reactions and dramatically improved nucleoside loading on solid support using LOTUS. The nucleoside-loaded support thus obtained was successfully used in the large-scale synthesis of dinucleotide analogs15 by solid-phase synthesis.
It is pertinent to mention that nucleoside loading on succinylated CPG could also be carried out by simple orbital shaking using DMF as a solvent. Thus, we carried out loading on succinylated CPG (100 g batches) using five equivalents of DMT-NBzdA and DMT-T. Using this procedure, nucleoside-loaded CPG, with loadings ranging from 60 to 70 micromol/g, was obtained. Using similar procedures, we have also carried out nucleoside loadings on carboxy-terminated wide-pore silica, Tentagel, and aminomethyl polystyrene. Nevertheless, the disadvantages of this protocol have been mentioned above.
In conclusion, significantly improved procedures for nucleoside loading on functionalized supports have been achieved using DMF as a solvent in conjunction with LOTUS workstation. Since functionalized supports can be rapidly prepared using microwave-assisted procedures,17 nucleoside-loaded supports can be readily prepared. Furthermore, we have carried out a number of other solid-phase reactions efficiently using LOTUS. The results of such studies will be reported in due course.
EXPERIMENTAL
Materials
DMT-NBzdA and DMT-T were obtained from Reliable Biopharmaceuticals, (St. Louis, MO) and used as such. Anhydrous pyridine, triethylamine, and dimethylformamide, obtained from reputed vendors, were freshly distilled from CaH2 prior to use. CPG was obtained from Prime Synthesis, Inc (Aston, PA). Cap A and Cap B were obtained from American International Chemicals (Natick, MA). Other reagents such as EDC, N,N-Dimethylaminopyridine (DMAP), and triethylamine were obtained from reputed vendors and used as received. The amino, and nucleoside loading on CPG was determined as per protocols described.14
Preparation of succinylated CPG 3 from native CPG
The succinylated CPG 3 was prepared in two steps from native CPG 1 using previously reported microwave-assisted procedures.17,18 All microwave reactions were conducted in a domestic microwave oven using a specially fabricated heavy-walled glass chamber. The chamber was fitted with Teflon screw cap with a chemically resistant o-ring. These microwave reactions should not be attempted in common laboratory glassware. All microwave reactions should be carried out behind safety shields. Brief procedures are given below:
Native CPG (50 to 100 g) was subjected to microwave-assisted amination (MAA) using (3-aminopropyl)triethoxysilane (3.5mL/g to form a slurry) to give the amino product 2 within a few minutes. Following filtration and washings, amino CPG 2, with amino-loadings of 90 to 113 micromol/g, was obtained. To obtain 3, microwave-assisted succinylation (MAS) of 2 (50 to 100 g scale) was carried out in the presence of catalytic amount of DMAP in DMF as solvent, in less than 5 min. Typically, 0.4 g of succinic anhydride and 50 mg of DMAP (each per g of amino CPG) was employed for MAS. Since this reaction was exothermic, each microwave exposure was carried out in 30 sec cycles with intermittent cooling. Completion of the reaction was ascertained by testing for the absence of amino group on a sample of the CPG. After the reaction, the colored slurry was filtered, washed with dichloromethane, methanol, and hexanes, and dried. The use of DMF instead of pyridine, under microwave conditions, makes MAS procedure highly attractive for the preparation of succinylated CPG 3.
Loading of nucleoside on succinylated CPG using LOTUS: Loading of DMT-NBzdA
Succinylated CPG (100g, 103 μmol/g amino loading), DMT-NBzdA (19.7g, 3 eq, 30 mmol) and DMAP (3.66 g, 3 eq, 30 mmol) were added to the LOTUS reactor20 under a blanket of argon. Anhyd. DMF (400ml) was introduced under argon atmosphere and the whole contents were mixed well using the orbital shaker. After the addition of anhydrous triethylamine (4.2 ml, 30 mmol), OSCAR was initiated for 5-10 min. Finally EDC (5.76 g, 3 eq, 30 mmol) was added and the reaction mixture was mixed under orbital shaking and recycling. OSCAR was carried out both in the forward and reverse directions for 8 h and orbital shaking alone for about 8 h. Periodically, aliquots of recycling liquid were withdrawn and trityl analysis carried out to determine the amount of DMT-NBzdA consumed. If needed, additional amount of EDC (2.95 g), DMAP (3.7g), and triethylamine (5 ml) were added and OSCAR continued. The contents of the reactor were filtered under vacuum and the filtrate was collected to recover the excess unreacted nucleoside. The loaded support was washed twice with methanol, dichloromethane and hexanes (∼400 ml each) under OSCAR, and at the end of washing, the support was dried thoroughly under vacuum in LOTUS. A sample of solid support isolated showed a typical nucleoside loading of 75 - 80 μmol/g by trityl analysis.
The capping of the support was also performed in LOTUS. For capping of the support, the nucleoside-loaded CPG was mixed with 325 ml each of CAP A and CAP B mixture for 3 hours using OSCAR and the capped support was filtered, washed twice with methanol, DCM, and finally with hexanes (300 ml each). The loading of dried support was typically around 70 to 80 micromol/g. The nucleoside-loaded support was stored at 4 °C.
Loading of DMT-NBzdA on succinylated solid support 3. General procedure using orbital shaking
We carried out the loading of nucleosides (DMT-NBzdA, and DMT-T) in anhydrous pyridine and anhydrous DMF using an excess of nucleoside in the presence of EDC, DMAP, and triethylamine (rt, 24 to 72 h) using an orbital shaker. Following loading, the support was washed, filtered, and dried. The support was then taken up in acetic anhydride/N-methylimidazole/pyridine and placed in an orbital shaker to cap unreacted hydroxyl, and amino groups. Experimental details are provided below:
(a) Using pyridine. To succinylated CPG 3 (30g, 110 μmol/g amino loading) in an r.b. flask, was added anhydrous pyridine (100 ml), followed by the addition of DMT-NBzdA (6.5 g, 3 eq), DMAP (1.2 g, 3 eq), triethylamine (1.4 ml), and finally EDC (1.9 g, 3 eq). The reaction flask was sealed with rubber septa and mixed under orbital shaking (∼150 rpm) overnight. If the loading of a representative sample from the reaction was acceptable (≥ 40 μmol/g), the reaction was processed further. Otherwise, additional equivalents of nucleoside, EDC, DMAP, and triethylamine were added. At the end of the reaction, the reaction mixture was washed twice with methanol, dichloromethane (DCM), DCM:MeOH (1:1), and hexanes (100 ml each) and dried in air overnight. Using this procedure, nucleoside loading of the support was found to be variable and best results obtained were in the 50-60 μmol/g range.
(b) Using DMF. To succinylated CPG (65g, 87μmol/g amino loading) in an r.b. flask, was added anhydrous DMF (260 ml) followed by DMT-NBzdA (18.7 g, 5 eq), DMAP (3.5 g, 5 eq), triethylamine (4 ml), and finally EDC (5.47 g, 5 eq). The reaction flask was sealed with rubber septa and mixed under orbital shaking (∼150 rpm) overnight. A sample of the support was subjected to trityl analysis. If the determined loading was acceptable (≥ 60 μmol/g), the reaction was processed further. Otherwise, additional equivalents of nucleoside, EDC, DMAP, and triethylamine were added. The reaction mixture was filtered, washed with methanol (2X 200 mL), DCM, and hexanes (200 ml each) and dried in air overnight. The nucleoside-loaded CPG was then mixed under orbital shaking with 325 ml each of CAP A and CAP B mixtures for 3 hours and the capped support was filtered, washed twice with methanol, DCM and hexanes (300 ml each). In a number of experiments, the loading of dried support was typically found to be around 60 to 65 micromol/g. The nucleoside-bound support was stored at 4 °C.
Acknowledgements:
Support of this research from the National Institutes of Health, under a Research Project Cooperative Agreement Grant Award 5 UO1 AI058270-02, is gratefully acknowledged.
REFERENCES
- 1.Sharp PA. Genes Dev. 2001;15:485. doi: 10.1101/gad.880001. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ. Nature. 2002;418:244. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 3.Iyer RP, Pandey R, Kuchimanchi S. Drugs of the Future. 2003;28:51. [Google Scholar]
- 4.Verma S, Eckstein F. Annu. Rev. Biochem. 1998;67:99. doi: 10.1146/annurev.biochem.67.1.99. [DOI] [PubMed] [Google Scholar]
- 5.Sproat BS. J. Biotechnol. 1995;41:221. doi: 10.1016/0168-1656(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 6.Vlassov VV, Vlassova IE, Pautova LV. Prog. Nucl. Acids Res. Mol. Biol. 1997;57:95. doi: 10.1016/s0079-6603(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 7.Beaucage SL, Caruthers MH. Tetrahedron Lett. 1981;22:1859. [Google Scholar]
- 8.Beaucage SL, Iyer RP. Tetrahedron. 1992;48:2223. [Google Scholar]
- 9.Iyer RP, Beaucage SL. “Oligonucleotide Synthesis”. In: Kool ET, editor. Comprehensive Natural Products Chemistry. Vol 7. Elsevier Science; London: 1999. pp. 105–152. [Google Scholar]
- 10.Majors R, Hopper M. J. Chrom. Sci. 1974;12:767. [Google Scholar]
- 11.Tundo P, Venturello P. J. Am. Chem. Soc. 1979;101:660. [Google Scholar]
- 12.Matteucci MD, Caruthers MH. Tetrahedron Lett. 1980;21:719. [Google Scholar]
- 13.Damha MJ, Giannaris PA, Zabarylo SV. Nucl. Acids Res. 1990;18:3813. doi: 10.1093/nar/18.13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pon RT. Current Protocols in Nucleic Acids Chemistry. John Wiley; New York: 1999. “Attachments of Nucleosides to Solid-phase Supports”; pp. 287–298. For an excellent review see: S. L. Beaucage, D. E. Bergstrom, G. D. Glick and R. A. Jones. [DOI] [PubMed] [Google Scholar]
- 15.Iyer RP, Jin Y, Roland A, Morrey JD, Mounir S, Korba B. Antimicrob. Agents Chemother. 2004;48:2199. doi: 10.1128/AAC.48.6.2199-2205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer RP, Roland A, Jin Y, Mounir S, Korba B, Julander JG, Morrey JD. Antimicrob Agents Chemother. 2004;48:2318. doi: 10.1128/AAC.48.6.2318-2320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padmanabhan S, Coughlin JE, Iyer RP. Tetrahedron Lett. 2005;46:343. [Google Scholar]
- 18.Padmanabhan S, Iyer RP. Patent pending.
- 19.Preliminary results of nucleoside loading on functionalized supports 2 and 3 (Scheme 1, Path A and Path B) using microwave conditions have been encouraging
- 20.Iyer RP, Coughlin JE, Padmanabhan S. Patent pending.