Short abstract
Aquaporins are intrinsic membrane proteins found in all organisms, from archaea to mammals. They selectively allow water or other small uncharged molecules to pass along the osmotic gradient.
Abstract
Water is the major component of all living cells, and efficient regulation of water homeostasis is essential for many biological processes. The mechanism by which water passes through biological membranes was a matter of debate until the discovery of the aquaporin water channels. Aquaporins are intrinsic membrane proteins characterized by six transmembrane helices that selectively allow water or other small uncharged molecules to pass along the osmotic gradient. In addition, recent observations show that some aquaporins also facilitate the transport of volatile substances, such as carbon dioxide (CO2) and ammonia (NH3), across membranes. Aquaporins usually form tetramers, with each monomer defining a single pore. Aquaporin-related proteins are found in all organisms, from archaea to mammals. In both uni- and multicellular organisms, numerous isoforms have been identified that are differentially expressed and modified by post-translational processes, thus allowing fine-tuned tissue-specific osmoregulation. In mammals, aquaporins are involved in multiple physiological processes, including kidney and salivary gland function. They are associated with several clinical disorders, such as kidney dysfunction, loss of vision and brain edema.
Gene organization and evolutionary history
The aquaporins are a family of small (24-30 kDa) pore-forming integral membrane proteins. This ancient protein family was first named after its archetype, the major intrinsic protein (MIP) of mammalian lens fibers [1,2], which is now designated AQP0 (see Table 1). When, later on, MIP homologs were shown to function as water channels, the term 'aquaporin' was suggested for the family. The aquaporin family has representatives in all kingdoms, including archaea, eubacteria, fungi, plants and animals. Following a functional classification, MIP homologs with exclusive water permeability are referred to as aquaporins (sometimes called AQPs and in this article referred to as 'classical' aquaporins), whereas water- and glycerol-permeable homologs are referred to as aquaglyceroporins (or glycerol facilitation-like proteins, GLP, although some proteins of this subfamily have 'AQP' in their names); the term MIP is widely used if the function is uncertain. It is worth noting that there is an ongoing debate about the MIP nomenclature because some scientists believe that a secondary function, not the exclusive water permeability observed initially, is of physiological importance. For example, it turned out for some members of the protein family that an increase in gas or small solutes transport could be more relevant than a change in the water permeability.
Table 1.
Classification of aquaporin sequences from phylogenetic analyses
| Name | Organism | Glycerol permeability |
| AQP subfamily (classical aquaporins) | ||
| AQP0 | Mammals | No |
| AQP1 | Mammals | No |
| AQP2 | Mammals | No |
| AQP4 | Mammals | No |
| AQP5 | Mammals | No |
| AQP6 | Mammals | No |
| AQP8 | Mammals | No |
| AQPZ | E. coli | No |
| AQY1 | Yeast | No |
| AQY2 | Yeast | No |
| Plasma-membrane intrinsic proteins (PIPs) | Plants | * |
| Tonoplast intrinsic proteins (TIPs) | Plants | * |
| Nodulin-like intrinsic proteins (NIPs or NLMs) | Plants | * |
| Small basic intrinsic proteins (SIPs) | Plants | NK |
| GLP subfamily | ||
| AQP3 | Mammals | Yes |
| AQP7 | Mammals | Yes |
| AQP9 | Mammals | Yes |
| AQP10 | Mammals | Yes |
| GlpF | E. coli | Yes |
| GLPY1 | Yeast | Yes |
| GLPY2 | Yeast | Yes |
*Glycerol permeability has been demonstrated for individual members of the subgroup. NK, not known.
The three-dimensional structures of the human water-channel protein AQP1 [3] and the bacterial aquaglyceroporin GlpF [4] are highly similar, although the sequence identity between them is less than 30% at the amino-acid level. This indicates that the overall structure of aquaporins (classical aquaporins and aquaglyceroporins) is conserved over 2 to 3 billion years of evolution.
Many eubacteria have a single AQP and a single GLP. In archaea, aquaporin-like sequences have been identified that are permeable to both water and glycerol. The genome of the yeast Saccharomyces cerevisiae contains two highly similar classical aquaporin genes, AQY1 and AQY2, and at least two aquaglyceroporins [2]. The diversification into classical aquaporins and aquaglyceroporins is also found in other fungi, such as Dictyostelium, Candida and Ustilago. Recently, aquaporins from protozoans such as Trypanosoma and Plasmodium have been characterized (see [5] and references therein). Many multicellular organisms express a range of aquaporin isoforms that differ in their tissue specificity and subcellular localization [1]. An overview on the aquaporin family, including representatives from eubacteria, yeast, plants and mammals, is given in Table 1.
So far, 11 different aquaporins have been found in vertebrates, corresponding to the human proteins AQP0-AQP10 [6]. Of these, four (AQP3, AQP7, AQP9 and AQP10) promote glycerol transport and have thus been assigned to the GLP subfamily. Human AQP8 and its orthologs from other metazoan species are more divergent from other mammalian classical aquaporins than the latter are from each other (see Figure 1), indicating that the diversification and specialization of the other metazoan members of the subfamily occurred after the split of AQP8 from the others [5]. Human aquaporin genes have four to eight introns, and gene size varies between 3.6 kilobases (kb) and 47 kb. They have been mapped to chromosomes 1, 7, 9, 10, 12, 15 and 16, with the genes encoding AQP0, AQP2, AQP5 and AQP6 clustering on chromosome 12. Splice variants have been found for the genes encoding human AQP1, AQP4 and AQP6.
Figure 1.
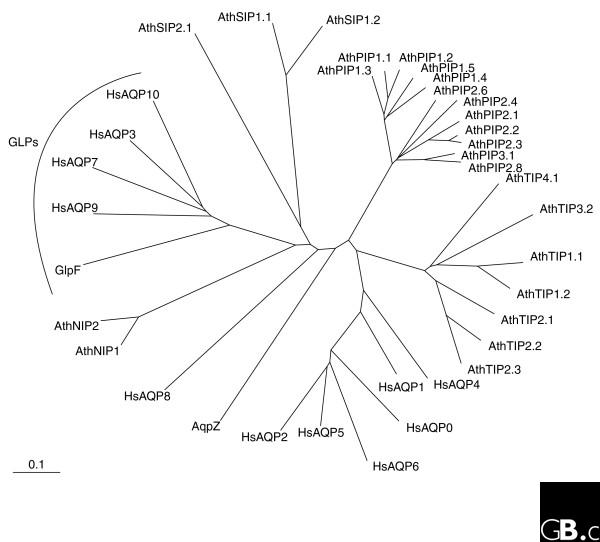
The evolutionary relationships of aquaporins. A phylogenetic tree was generated from human (Hs), Arabidopsis (Ath) and E. coli (AqpZ and GlpF) aquaporin sequences using ClustalX. Members of the aquaglyceroporin (GLP) subfamily are indicated; all other proteins shown belong to the classical aquaporin subfamily.
MIP genes are particularly abundant in plants. They show greater diversity than the metazoan homologs, a fact that has been attributed to the higher degree of compartmentalization of plant cells and their greater necessity for fine-tuned water control [7]. Sequences of more than 35 different genes encoding aquaporin-like proteins were found in the genome of the model plant Arabidopsis thaliana [7,8]. The plant aquaporins comprise four major groups: plasma-membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), NOD26-like intrinsic proteins (NLMs or NIPs), and small basic intrinsic proteins (SIPs). The PIP subfamily can be further subdivided into two groups, PIP1 and PIP2; these differ in the lengths of their amino and carboxyl termini, the amino termini being longer in the PIP1 proteins. SIPs are the most divergent aquaporins in plants, and they show a high level of diversity even within the subfamily.
The first aquaporin-like sequences identified from plants were representatives of the NIP subfamily, including the NLM protein found in the peribacteroid membrane of soybean symbiotic root nodules [9], although members of this subfamily are also found in non-legume plants. The NIPs have glycerol transport activity [10] and thus can be regarded as plant glycerol transporters. NIPs are more similar to the bacterial aquaporin AqpZ than to glycerol facilitators in the GLP subfamily, however [1]. This suggests that the common ancestor of plant aquaporins lacked glycerol transport activity and that this activity was acquired later during evolution to compensate for the lack of GLPs in plants [3].
In Arabidopsis, the 35 aquaporin genes are spread over all five chromosomes. Their structural organization has been extensively analyzed [7]: introns are preferentially located in regions encoding loops connecting the transmembrane helices, and both the position and the number of introns are remarkably well conserved within each subfamily.
Characteristic structural features
The first member of the aquaporin family to be extensively described was the channel-like integral membrane protein CHIP28, the 28 kDa protein of the human erythrocyte membrane [11]. On the basis of functional analyses, it was later renamed aquaporin-1 (AQP1) [12]. Hydropathy plot analyses of the primary sequence predicted six transmembrane helices (I-VI) connected by five loops (loops A-E; Figure 2). Loops A, C and E are extracellular and loops B and D are intracellular. The protein comprises two internal tandem repeats, covering roughly the amino- and carboxy-terminal halves of the protein. Each repeat consists of three transmembrane helices and a highly conserved loop following the second transmembrane helix (loops B and E, respectively). This loop includes a conserved signature motif, asparagine-proline-alanine (NPA). Loops B and E form short α helices that fold back into the membrane, with loop B entering the membrane from the cytoplasmic side and loop E from the extracellular side. A seventh transmembrane domain in which the two NPA boxes are orientated 180 degrees to each other is thus formed (Figure 3), creating an aqueous pathway through the proteinaceous pore [13].
Figure 2.
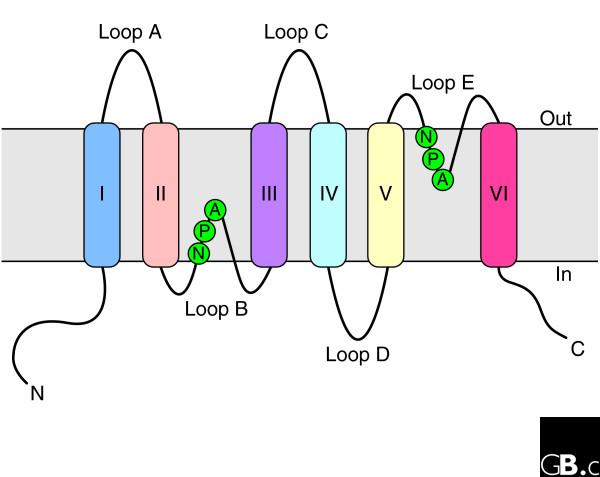
Topology of an aquaporin protein within the membrane. The protein consists of six transmembrane helices (I-VI) connected by five loops (A-E) and includes two internal tandem repeats (I-III and IV-VI, respectively). Loops B and E, containing the conserved NPA motifs (in the single-letter amino-acid code), form short α helices that fold back into the membrane from opposite sides. C, carboxyl terminus; N, amino terminus.
Figure 3.
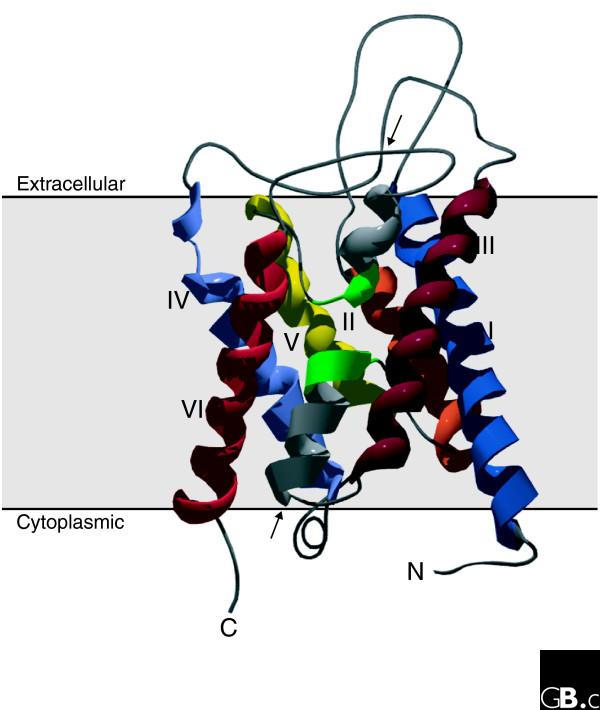
Three-dimensional structure of an aquaporin subunit monomer (a ribbon model of NtAQP1, a PIP1 protein from tobacco). The structure shows six tilted membrane-spanning helices (I-VI) and two pore-forming domains made up of two short α helices entering the membrane from the extracellular and intracellular surfaces (arrows). The two NPA boxes are indicated in green. Amino- and carboxy-terminal domains are oriented to the cytoplasmic side of the membrane. The figure was generated using MODELLER7v7 and Swiss-Pdb Viewer.
This 'hourglass model' has been confirmed by three-dimensional maps of AQP1 using cryoelectron microscopy [14]. These maps also showed that aquaporins have a tetrameric organization: the four subunits are arranged in parallel, forming a fifth pore in the center of the tetramer. It is generally accepted that all aquaporin-like proteins assemble into tetramers. Each monomer alone can facilitate water flow, however. Recent experiments have indicated conductance of ions (K+, Cs+, Na+ and tetramethylammonium) through the central pore of the AQP1 tetramer [15,16].
Localization and function
Since the discovery of the Escherichia coli water channel AqpZ, the pathway of rapid water fluxes through membranes by which microorganisms adapt to abrupt changes in osmolarity has begun to be understood [17]. This channel is selectively permeable to water, has a role in both the short-term and the long-term osmoregulatory response, and is required by rapidly growing cells. AqpZ-like proteins seem to be necessary for the virulence of some pathogenic bacteria. Microbial aquaporins are also likely to be involved in spore formation and/or germination.
The diversity of aquaporins in multicellular organisms highlights the diverse requirements for osmoregulation and transmembrane water movement in different tissues, organs and developmental stages. In mammals, aquaporins are localized in epithelia that need a high rate of water flux, such as the collecting duct of the kidney, the capillaries of the lung and the secretory cells of the salivary glands. Mammalian aquaporins differ in their transcriptional regulation, post-transcriptional regulation and subcellular distribution.
Members of the aquaporin family are implicated in numerous physiological processes (reviewed in [6]). In the kidney, for example, AQP1 is extremely abundant in both the apical and the basolateral membranes of the renal proximal tubules and in the capillary endothelium. It contributes to the counter-current mechanism for urine concentration. In the salivary gland, AQP3 is found in basolateral membranes, where water is taken up from the interstitium, and AQP5 is in the apical membrane, where water is released. A wide range of clinical disorders have been attributed to the loss or dysfunction of aquaporins, including abnormalities of kidney function, loss of vision, onset of brain edema and starvation [6,18]. AQP1 was recently shown to be involved in angiogenesis, wound healing, organ regeneration and carcinogenesis [19].
Our knowledge of the molecular functions of plant aquaporins with regard to their specificity for water and small neutral solutes has increased substantially in recent years [20,21]. In plant cells, the cytoplasm is in fact enclosed between two membranes: the plasma membrane, which forms the outer boundary of the cell, and the tonoplast, which surrounds the vacuolar compartment. Aquaporins located in the plasma membrane (PIPs) or tonoplasts (TIPs) contribute to intracellular water balance and transcellular water flow. NIPs, which were initially found in the peribacteroid membrane of legume symbiotic root nodules [9], are presumed to be involved in exchange of metabolites between the host and the symbiont; the subcellular localization and physiological function of NIPs in non-leguminous plants is not known. SIPs have recently been localized to endoplasmic reticulum membranes; their physiological functions remain to be elucidated [22].
Much of our information on the physiological relevance of aquaporins in plants comes from analyses of transgenic plants with modified expression of various aquaporins, or from analysis of aquaporin mutants. The first evidence for a function in cellular water uptake and whole-plant water transport came from plants expressing antisense RNA for PIP proteins, which developed a larger root system than the controls [23]. In tobacco, the plasma-membrane aquaporin NtAQP1 was shown to be important for hydraulic conductivity and water stress resistance in roots [24]. Studies on plants with impaired expression of two different aquaporins (PIP1 and PIP2) indicated that these proteins are important in the recovery from water deficiency [25]. Overexpression of an Arabidopsis plasma-membrane aquaporin in tobacco resulted in increased growth rates under optimal irrigation [26], which was interpreted as the sum of effects on water uptake and photosynthesis. Besides their function in water management, plant aquaporins have a role during leaf movement, a process involving high rates of cellular water transport [27,28].
In addition to their role in water transport and osmoregulation, some aquaporins facilitate the passage of gases such as CO2 and NH3 across membranes (reviewed in [29]). The physiological significance of AQP1-facilitated CO2 transport is still a matter of debate. AQP1 knockout mice did not show differences in CO2 exchange rates in lung and kidney [30], but plants with impaired expression of a PIP1 aquaporin showed several differences, not only in water transport [24] but also in CO2-limited processes such as photosynthesis and stomatal conductance [31]. Studies with inhibitors of aquaporin function in plants suggest that NIPs are involved in NH3 permeability [32] and perhaps in nutrient exchange between the host plant and endosymbiotic bacteria.
Mechanism
Given that all aquaporins are structurally related and have highly similar consensus regions, particularly in the pore-forming domains, a similar transport mechanism can be assumed. The hydrophobic domain created by the loops B and E (Figure 2) has been suggested to be involved in substrate specificity and/or size restriction. The pathway through the aquaporin monomer is lined with conserved hydrophobic residues that permit rapid transport of water in the form of a single-file hydrogen-bonded chain of water molecules [4]. The pore contains two constriction sites: an aromatic region comprising a conserved arginine residue (Arg195) forms the narrowest part of the pore [33], and the highly conserved NPA motifs form a second filter, where single water molecules interact with the two asparagine side chains [4]. Because of a direct interaction between water molecules and the NPA motifs, the dipolar water molecule rotates 180 degrees during passage through the pore. Both filter regions build up electrostatic barriers, which prevent the permeation of protons [34]. In human AQP1, a hydrophobic phenylalanine side chain (Phe24) intrudes into the pore and enhances the interaction of single permeating water molecules with the NPA loops. In the bacterial glycerol facilitator GlpF, this residue is replaced by the smaller amino acid leucine (Leu21). Phe24 acts as a size-exclusion filter, preventing the passage of larger molecules such as glycerol through AQP1 [34].
The water permeability and selectivity of aquaporins varies considerably, however. The water permeabilities for human aquaporins have been estimated to be between 0.25 × 10-14 cm3/sec for AQP0 and 24 × 10-14 cm3/sec for AQP4 [35]. Plant plasma-membrane aquaporins also have differing levels of aquaporin activity [36]. Coexpression and heteromerization of PIP1 and PIP2 isoforms from maize induced an increase in permeability above that obtained for expression of single isoforms [37]. Heteromerization seems to be important not only in heterologous expression systems, but also in the plant, as was demonstrated by analysis of PIP1 and PIP2 antisense Arabidopsis plants [25].
The mechanism by which aquaglyceroporins promote glycerol transport has been investigated for the E. coli glycerol facilitator GlpF [5,33]. This protein also contains the conserved NPA motifs at comparable positions to those in the water-selective aquaporins, but the preference for glycerol is achieved by aromatic amino acids at the periplasmic side. Trp48, Phe200 and Arg206 form a constriction, and the arginine residue forms hydrogen bonds with two hydroxyl groups of the glycerol molecule. As a result, the carbon backbone of the glycerol molecule faces into the cavity assembled by the two aromatic amino acids (Phe200 and Trp48). Glycerol is separated from other linear polyols and passes the pore in a single file. The GlpF pore is completely amphipathic, with polar residues opposite a hydrophobic wall.
Frontiers
Since the description of the first aquaporin [11,12] by Peter Agre and his colleagues, which was rewarded with the Nobel Prize for Chemistry in 2003, much information on the physiological significance of these channel proteins has accumulated. Additional functions in osmoregulation and metabolite transport have been attributed to this large and multifunctional protein family, and new physiological functions will probably be found in the future. As more biological roles of aquaporins are discovered, their potential in medicine, pharmacology and agrobiotechnology is also becoming clear.
Our knowledge of the structural determinants of the pore's selectivity will enable the development of channel-modulating agents for therapy. Detailed studies of aquaporin gene expression and regulation will lead to a more refined understanding of the involvement of aquaporins in pathophysiological processes.
Integration of data from studies in vitro and in intact plants will provide a more complete picture of the interaction and regulation of aquaporins in plants. Insight into the mechanisms of regulation with regard to subcellular distribution, heterotetramerization or other means of regulation will improve our understanding of water control and solute homeostasis in plants. This will help to develop plants with improved salt or drought resistance, more efficient water use and/or greater biomass production, through manipulation of the expression of individual aquaporins.
References
- Heymann JB, Engel A. Aquaporins: phylogeny, structure, and physiology of water channels. News Physiol Sci. 1999;14:187–193. doi: 10.1152/physiologyonline.1999.14.5.187. [DOI] [PubMed] [Google Scholar]
- Hohmann S, Bill RM, Kayingo G, Prior BA. Microbial MIP channels. Trends Microbiol. 2000;8:33–38. doi: 10.1016/S0966-842X(99)01645-5. [DOI] [PubMed] [Google Scholar]
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- Fu DX, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- Zardoya R. Phylogeny and evolution of the major intrinsic protein family. Biol Cell. 2005;97:397–414. doi: 10.1042/BC20040134. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley F, Rosenberg J, Shachar-Hill Y, Bohnert H. From genome to function: the Arabidopsis aquaporins. Genome Biol. 2001;3:research0001.1–0001.17. doi: 10.1186/gb-2001-3-1-research0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal NN, Marcker KA. Soybean nodulin 26 is homologous to the major intrinsic protein of the bovine lens fiber membrane. Nucleic Acids Res. 1988;16:9347–9348. doi: 10.1093/nar/16.19.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM. Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochemistry. 1999;38:347–353. doi: 10.1021/bi982110c. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The three-dimensional structure of aquaporin-1. Nature. 1997;387:624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. Water and ion permeation of aquaporin-1 in planar lipid bilayers: major differences in structural determinants and stoichiometry. J Biol Chem. 2001;276:31515–31520. doi: 10.1074/jbc.M104267200. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Weinstein AM. New roles for old holes: ion channel function in aquaporin-1. News Physiol Sci. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- Calamita G. The Escherichia coli aquaporin-Z water channel. Mol Microbiol. 2000;37:254–262. doi: 10.1046/j.1365-2958.2000.02016.x. [DOI] [PubMed] [Google Scholar]
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/S0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Exp Bot. 1999;50:1055–1071. doi: 10.1093/jexbot/50.suppl_1.1055. [DOI] [Google Scholar]
- Luu DT, Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 2005;28:85–96. doi: 10.1111/j.1365-3040.2004.01295.x. [DOI] [Google Scholar]
- Ishikawa F, Suga S, Uemura T, Sato MH, Maeshima M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 2005;579:5814–5820. doi: 10.1016/j.febslet.2005.09.076. [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U. Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J. 1998;14:121–128. doi: 10.1046/j.1365-313X.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell. 2002;14:869–876. doi: 10.1105/tpc.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 2002;130:2101–2110. doi: 10.1104/pp.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell. 2003;15:439–447. doi: 10.1105/tpc.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R. Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell. 2002;14:727–739. doi: 10.1105/tpc.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R. The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J. 2004;37:147–155. doi: 10.1046/j.1365-313x.2003.01947.x. [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Zhou YH, Bouyer P, Grichtchenko II, Boron WF. Transport of volatile solutes through AQP1. J Physiol. 2002;542:17–29. doi: 10.1113/jphysiol.2002.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XH, Yang BX, Matthay MA, Verkman AS. Evidence against aquaporin-1-dependent CO2 permeability in lung and kidney. J Physiol. 2002;542:63–69. doi: 10.1113/jphysiol.2001.013813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425:734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000;465:110–114. doi: 10.1016/S0014-5793(99)01729-9. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Grubmuller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1062459. [DOI] [PubMed] [Google Scholar]
- de Groot BL, Frigato T, Helms V, Grubmuller H. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol. 2003;333:279–293. doi: 10.1016/j.jmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of Aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000;122:1025–1034. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell. 2004;16:215–228. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]


