Abstract
Lactobacilli derived from the endogenous flora of normal donors are being increasingly used as probiotics in functional foods and as vaccine carriers. However, a variety of studies done with distinct strains of lactobacilli has suggested heterogeneous and strain-specific effects. To dissect this heterogeneity at the immunological level, we selected two strains of lactobacilli that displayed similar properties in vitro and studied their impact on mucosal and systemic B-cell responses in monoxenic mice. Germfree mice were colonized with Lactobacillus johnsonii (NCC 533) or Lactobacillus paracasei (NCC 2461). Bacterial loads were monitored for 30 days in intestinal tissues, and mucosal and systemic B-cell responses were measured. Although both Lactobacillus strains displayed similar growth, survival, and adherence properties in vitro, they colonized the intestinal lumen and translocated into mucosal lymphoid organs at different densities. L. johnsonii colonized the intestine very efficiently at high levels, whereas the number of L. paracasei decreased rapidly and it colonized at low levels. We determined whether this difference in colonization correlated with an induction of different types of immune responses. We observed that colonization with either strain induced similar germinal center formation and immunoglobulin A-bearing lymphocytes in the mucosa, suggesting that both strains were able to activate mucosal B-cell responses. However, clear differences in patterns of immunoglobulins were observed between the two strains in the mucosa and in the periphery. Therefore, despite similar in vitro probiotic properties, distinct Lactobacillus strains may colonize the gut differently and generate divergent immune responses.
The gut-associated lymphoid tissues are highly compartmentalized. The Peyer's patches are organized lymphoid tissues in the wall of the small intestine that contain B-lymphoid follicles and interfollicular populations of T cells. The lamina propria consists of large numbers of lymphoid and myeloid cells, particularly immunoglobulin A-positive (IgA+) plasmablasts, scattered under the gut epithelium. Intraepithelial lymphocytes composed mostly of CD8+ T-cell subsets are interspersed within the enterocyte monolayer. It is becoming clear that the quantity and quality of the cells that populate these compartments depend on continuous stimulation provided by the endogenous intestinal microflora (9).
Early studies by Schaedler et al. (36, 37) contributed to the identification of members of the indigenous gut flora of mice and described the colonization of germfree mice with monocultures of these bacteria, mainly lactobacilli, enterococci, coliforms, and clostridia. Colonization of germfree mice with different mixtures of such commensal bacteria led to the rapid appearance of IgA+ plasma cells in the lamina propria (13, 28). Since then, germfree mice have become the model of choice to study the impact of the microflora on gut-associated lymphoid tissue development (7, 9).
It was reported by several groups (10, 24, 44, 45) that segmented filamentous bacteria, a major population of commensal gram-positive bacteria found in many animals, could induce a profound mucosal IgA response in the Peyer's patches and lamina propria of monoassociated mice. Elegant studies with germfree SCID mice showed that the level and timing of colonization of segmented filamentous bacteria in monoassociated pups are regulated by both maternal and newborn IgA (21). Similar studies with a gram-negative commensal bacterium, Morganella morganii, showed a transient self-limiting humoral mucosal response elicited by this bacterium while permanently colonizing the gut (40). Thus, early colonization of the neonatal gut by commensal bacteria engenders a transient and controlled immune response at weaning, characterized by IgA secretion, which could be implicated in controlling bacterial adherence to the mucosal epithelium or cause clumping of bacteria near the mucosa to enhance “washout” by peristalsis.
On the other hand, whether secretory IgA is also important for preventing translocation of resident bacteria into mucosal tissues remains less clear. In vitro experiments with an Ussing chamber showed that secretory IgA prevented transepithelial passage of Escherichia coli across the epithelial layer (1). In contrast, earlier work by Berg and colleagues demonstrated that oral immunization of germfree mice with heat-killed E. coli vaccines before colonization with these strains did not reduce the incidence of translocation despite markedly increased levels of intestinal IgA (5). Instead, this group showed that T cells and macrophages play an important role in controlling translocation of endogenous bacteria, including lactobacilli (17, 30). Furthermore, there was a direct relationship between the numbers of a particular bacterial strain in the cecum and the numbers of viable bacteria of this strain present in the mesenteric lymph nodes of germfree mice associated with a whole cecal flora (42), and there were differences in translocation rate among the various types of indigenous bacteria (43).
The description in recent years of a number of strains of gram-positive lactic acid bacteria eliciting probiotic properties (3) and immunomodulatory functions (20, 27) has heightened the interest in the effects of nonpathogenic commensal enteric bacteria on local and systemic immunity (12, 14, 31, 32, 34, 35). Several Lactobacillus strains have been reported to display stimulatory properties on cells of the innate immune system in vitro (18, 19, 22, 23). This stimulation was mirrored in vivo in animal models (31, 32, 33, 34) and in humans given fermented milk products containing probiotics (25, 38, 39). It appears from most of these studies that these effects were strain specific. However, due to a lack of consistency among the models used and the methods of preparation and administration of the bacteria, it has been difficult to establish a physiological basis for this functional heterogeneity.
The current selection criteria for probiotic strains are mostly based on in vitro experiments that addressed inherent properties such as growth rate, survival in simulated gastric juices, and adherence to intestinal epithelial cell lines (15, 29). We used gnotobiotic mice monoassociated with lactobacilli to demonstrate that very different patterns of colonization and of immune response were elicited from two distinct strains that displayed similar properties in vitro, suggesting different applications for each strain. It is therefore also important to select proper probiotic strains for specific applications depending on their activity in vivo.
MATERIALS AND METHODS
Mice.
Six-week-old C3H/n germfree female mice were purchased from the Centre National de la Recherche Scientifique, Orleans, France, and maintained in flexible film isolators in our animal facilities at the Nestlé Research Center, Lausanne, Switzerland, in accordance with the ethical regulations of the Veterinary Service of the Canton de Vaud, Switzerland. All mice were screened on a weekly basis for germfree or monoassociated status by sampling feces sterilely and culturing on MRS-agar plates under aerobic and anaerobic conditions. At time zero, germfree mice received a single gavage of 109 CFU of bacteria (monoassociated mice), as estimated by plating dilutions, and colonization was readily detectable after 24 h in the feces. Ex-germfree conventional mice were transferred at time zero in a conventional animal facility, where they were reared in the presence of conventional mice. After 2 days, the colonization of conventional mice was confirmed in feces. C3H/n conventional mice were purchased from Iffa Credo (Paris, France). Mice were sacrificed by cervical dislocation under 3% Isoflurane (Abbot SA) anesthesia.
Bacteria.
L. johnsonii (strain NCC 533 from the Nestlé Culture Collection) and L. paracasei (NCC 2461) were originally isolated from human feces. L. johnsonii (NCC 533) belongs to the L. acidophilus group, is catalase negative, and produces dl-lactic acid. L. paracasei (NCC 2461) belongs to the L. casei group, is catalase negative, and produces l-lactic acid. Both strains were previously shown to grow equally well in vitro in MRS broth without acetate at 37°C under anaerobic conditions and in the presence of bile salts at a concentration of up to 0.4%. Both strains resisted simulated gastric juice conditions similarly (pepsin at 3 g/liter in sterile saline [0.5% wt/vol], pH 2.0, adjusted with concentrated HCl) (8) (Rochat et al., unpublished data).
For the preparation of gavages, the lactobacilli were grown in MRS broth without acetate at 37°C under anaerobic conditions (GasPak system from AnaeroGen, Hampshire, United Kingdom) for 8 h (exponential growth phase). Bacteria were harvested by centrifugation (4,000 × g, 10 min). Pelleted bacteria were then washed two times in sterile 0.9% NaCl and resuspended at 109 CFU in 0.2 ml, as estimated by plating dilutions. For the gavage, bacteria were inoculated intragastrically with a sterile 22-gauge stainless steel feeding needle. Bacterial crude lysate were prepared by resuspending the pellet in distilled water and by sequential sonication five times for 2 min each at maximum power and an amplitude of 18 μm (Soniprep 150 MSE). Protein concentrations were estimated by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
Adhesion of lactobacilli to Caco-2 cells.
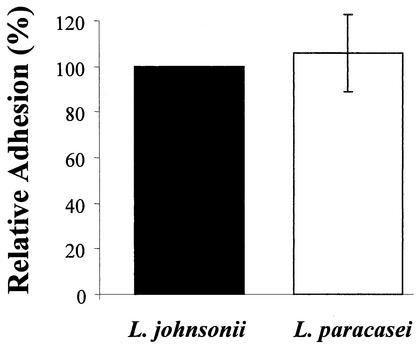
The adherence assays were performed as previously described (19). Briefly, bacteria were metabolically labeled for 24 h with [3H]adenine (24 Ci/mmol; Amersham Pharmacia Biotech, Dübendorf, Switzerland). Caco-2 cells were incubated for 1 h with bacteria (the cell-bacteria ratio was 1:10), washed three times with phosphate-buffered saline (PBS), and dissolved in 0.2 N NaOH. Adhesion was evaluated by liquid scintillation counting (TopCount; Packard Biosciences B.V., Groningen, The Netherlands). Cell adhesion was calculated as the means of triplicate cultures, and L. johnsonii adhesion was arbitrarily set at 100% in each experiment.
Colonization and translocation of bacteria.
At different times following gavage with L. johnsonii or L. paracasei, translocation of the bacteria to Peyer's patches and mesenteric lymph nodes, and the density of colonization in the small intestine and colon was measured. Four mice per group were used, and these experiments were repeated three times. All Peyer's patches and mesenteric lymph nodes were removed aseptically, washed five times in an excess volume of sterile PBS, and homogenized in 2 ml of sterile PBS-15% glycerol (Merck, Darmstadt, Germany). One hundred microliters of each of the homogenates was plated on MRS agar plates. The final dilution on the plate was therefore 1:20, and the detection limit was 20 CFU/organ. At each time point, Peyer's patches were also sampled from control conventional mice to ensure that the Peyer's patch sampling was done sterilely (Peyer's patches were always found to be sterile in conventional mice).
Intestinal and colon contents were flushed with an excess volume of sterile PBS and mixed. After 5 min of sedimentation to remove large remains, bacteria were collected from the supernatant, washed three times with PBS, and resuspended in 10 ml of PBS-15% glycerol. One hundred microliters of bacterial suspension was plated on MRS agar plates. Therefore, the initial dilution on the plate was 10−2 and the detection limit was 102 CFU/g. In order to express bacterial levels in CFU per gram of luminal suspension, the luminal contents were weighed (weight of flushed intestine or colon subtracted from weight of intact intestine or colon). Feces were collected sterilely from the rectum and resuspended in 5 ml of saline (10−2 dilution). Serial dilutions of all samples were plated on MRS agar and incubated anaerobically at 37°C for 48 h, after which colony counts were recorded.
Collection of intestinal contents for secretory IgA.
Small intestines (including jejunum and ileum) were cut into small pieces and homogenized mildly with a Polytron (Kinematica, Lausanne, Switzerland) in 3 ml of PBS solution containing 0.01% soybean trypsin inhibitor, 36 mM EDTA (Sigma, St. Louis, Mo.), and 1% bovine serum albumin (Sigma). Supernatants were collected after centrifugation at 2,000 × g for 10 min at 4°C. After adding 0.1 M phenylmethylsulfonyl fluoride (Sigma), intestinal contents were collected from the supernatants following ultracentrifugation at 10,000 × g for 20 min at 4°C and frozen at −20°C.
Peyer's patch organ cultures.
The method for Peyer's patch organ cultures was described previously (40). Briefly, Peyer's patches were dissected from the small intestine of mice and replaced in cold RPMI-10% fetal calf serum (FCS). The Peyer's patches were washed by three successive transfers through fresh medium. Each Peyer's patch was halved with a sharp sterile razor blade and placed in a sterile flat-bottomed well of a 24-well plate (Costar, Cambridge, Mass.) in 2.0 ml of RPMI-10% FCS and cultured for 10 days under 5% CO2 at 37°C.
Isolation of lamina propria lymphocytes and flow cytometric analysis.
Small intestines (jejunum and ileum) from pooled from two mice were opened lengthwise, cut into small pieces, and washed four times with Hanks' balanced salt solution containing 15 mM HEPES (Life Technologies). Intestinal pieces were mixed with warm Hanks' balanced salt solution containing 10% FCS (Amimed, Allschwill, Switzerland) and 50 mM EDTA (Sigma) at least four times. Then they were digested three times for 30 min each at 37°C with RPMI 1640 (Life Technologies) containing 10% FCS and 100 U of collagenase (Sigma) per ml. Lamina propria lymphocytes were collected in the supernatant, washed twice in RPMI-10% FCS, and resuspended in culture medium. The surface expression of IgA on lamina propria lymphocytes was analyzed by staining with biotinylated goat anti-mouse IgA (Southern Biotechnology Associates, Inc.), followed by phycoerythrin-conjugated streptavidin (Pharmingen). The samples were analyzed on a FACScalibur (Becton Dickinson) by measuring the mean fluorescence intensity of surface IgA expression on lamina propria lymphocytes.
ELISA.
Lactobacillus-specific immunoglobulins were detected by coating bacterial crude lysate at 100 μg/ml in borate-buffered saline 3 h at 37°C on Maxisorp enzyme-linked immunosorbent assay (ELISA) plates (Life Technologies AG, Basel, Switzerland). After four washes in PBS containing 0.05% Tween 20, wells were blocked overnight at 4°C with PBS containing 20% FCS and 0.05% Tween 20. Samples were incubated for 4 h at 4°C, and after further washing, secondary biotinylated monoclonal antibodies (biotinylated goat anti-mouse IgA or biotinylated goat anti-mouse IgG1 or IgG2a [Southern Biotechnology Associates, Inc., Birmingham, Ala.]) were added at a concentration of 0.5 μg/ml for 1 h at room temperature. Wells were then washed, and 1 μg of horseradish peroxidase-labeled streptavidin (KPL, Gaithersburg, Md.) per ml was incubated for 30 min at room temperature. The plates were washed one last time and revealed with TMB microwell substrate (KPL). Reactions were stopped with 1 M phosphoric acid. Optical density was read at 450 nm.
Lactobacillus-specific IgG isotypes were expressed as arbitrary units calculated from an immune serum obtained from mice injected intraperitoneally with 100 μg of crude bacterial lysate. Lactobacillus-specific IgA levels were expressed in optical density units because no positive standard was available. For serum levels of total IgE, the same method was used with anti-mouse IgE (R35-72; Pharmingen, San Diego, Calif.) and biotinylated rat anti-mouse IgE (Southern Biotechnology Associates, Inc., Birmingham, Ala.) as the coating and detecting antibodies, respectively. IgE levels were compared to an internal standard (mouse IgE; Pharmingen).
Histological analysis.
Small intestine tissues were fixed in 10% buffered formaldehyde and paraffin embedded. Then 5-μm-thick sections were cut and deparaffinized in xylol with two changes, hydrated gradually through graded alcohols, and rinsed in distilled water. Sections were stained either with hematoxylin and eosin (Fluka Chemie GMBH, Buchs, Switzerland) or with biotinylated anti-mouse IgA followed by horseradish peroxidase-labeled steptavidin (KPL).
To quench endogenous peroxidase activity, sections were incubated for 15 min in 1% hydrogen peroxide (Aldrich-Chemie, Steinheim, Germany) and then washed twice in PBS. For the immunoperoxidase staining, nonspecific binding was blocked with 10% goat serum in PBS and tissues were stained for 1 h at room temperature with a biotinylated goat anti-mouse IgA (Southern Biotechnology Associates, Inc.) in PBS-1% bovine serum albumin. Then sections were incubated in horseradish peroxidase-labeled streptavidin (KPL, Gaithersburg, Md.) and washed extensively in PBS. Staining was revealed with diaminobenzidine tetrahydrochloride (NEN Life Scientific Products, Inc., Boston, Mass.), and the reaction was stopped with distilled water.
Statistical analysis.
Student's t test was used if two groups were compared, while analysis of variance was used for multiple comparisons over time or among several treatment groups. Differences were considered significant when the P value was equal to or less than 0.05.
RESULTS
L. johnsonii colonized more efficiently than L. paracasei in monoxenic mice.
The two Lactobacillus strains used in this study were chosen for several similarities, including similar growth curves in MRS medium without acetate at 37°C under anaerobic conditions and similar resistance to simulated gastric juice conditions (pepsin at 3 g/liter in sterile saline [0.5% wt/vol] at pH 2.0 adjusted with concentrated HCl) (see Materials and Methods). As shown in Fig. 1, the two strains also displayed a similar capacity to adhere to Caco-2 cells in vitro.
FIG. 1.
Adhesion of L. johnsonii and L. paracasei to differentiated Caco-2 cells in vitro. Results represent the mean ± standard deviation from six individual experiments. The adhesion value for L. johnsonii in each experiment was set at 100%. Each experiment was performed in triplicate, and the percentages were calculated from the means of these triplicate cultures.
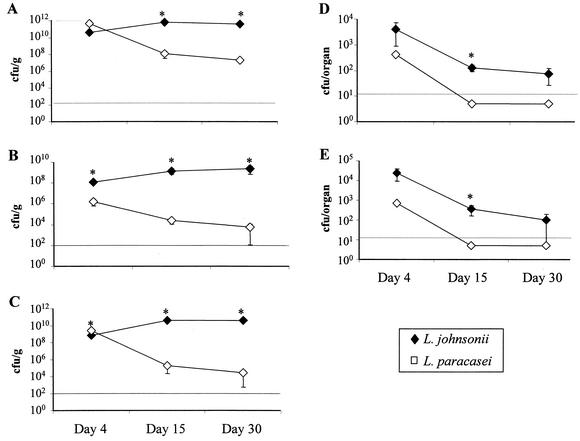
Considering that these two Lactobacillus strains had similar adhesion properties in vitro, we wanted to compare their patterns of colonization and translocation in vivo. For this purpose, germfree mice were colonized by administration of a single gavage of 109 CFU in 0.2 ml of saline, and bacterial loads were determined in the feces, small intestine, colon, Peyer's patches, and mesenteric lymph nodes at 4, 15, and 30 days after the gavage. As shown in Fig. 2, both strains were found in the feces, small intestine, colon, Peyer's patches, and mesenteric lymph nodes just 4 days after gavage. Counts of L. johnsonii were clearly higher in the small intestine, Peyer's patches, and mesenteric lymph nodes than those of L. paracasei. In the feces and colon, however, the densities of the two bacteria were roughly the same and were much greater than in the small intestine.
FIG. 2.
Kinetics of bacterial translocation in monoassociated mice. Germfree C3H/n mice received a single gavage with 109 CFU of L. johnsonii or L. paracasei at weaning. Bacterial loads were counted in fecal pellets extracted from the rectum (A), the luminal contents of the small intestine (B), and the colon (C). Bacteria were also counted in Peyer's patches (D) and mesenteric lymph nodes (E). Counts were made by gavage at days 4, 15, and 30 after colonization. This is one representative experiment out of three performed individually. Results are expressed as mean (± standard error of the mean) CFU per gram for feces, small intestine, and colon or as CFU per organ for Peyer's patches and mesenteric lymph nodes for four mice per group. The horizontal dashed line indicates the limit of detection due to the initial dilution of the samples (see Materials and Methods), and data points below that line represent negative cultures. *, P < 0.05 between the two groups.
At day 15, L. paracasei decreased in feces and in the lumen of the small intestine and colon and was no longer present in Peyer's patches and mesenteric lymph nodes (under the limit of detection, 20 CFU/organ). In contrast, L. johnsonii counts were maintained or increased in the feces, small intestine, and colon, and it was still found, albeit at lower levels than at day 4, in Peyer's patches and mesenteric lymph nodes. At day 30 of colonization, L. johnsonii was still found in Peyer's patches and mesenteric lymph nodes, although at very low levels, and was still more abundant than L. paracasei in the feces, small intestine, and colon. These data indicate that, under monoxenic conditions, L. johnsonii persisted at high levels in the lumen of the small intestine and colon and translocated into mucosal lymphoid organs more efficiently than L. paracasei. Furthermore, although both strains permanently colonized the lumen (albeit at different concentrations), their levels were eventually decreased in the mucosal lymphoid organs.
Colonization with both L. johnsonii and L. paracasei activated mucosal B-cell responses.
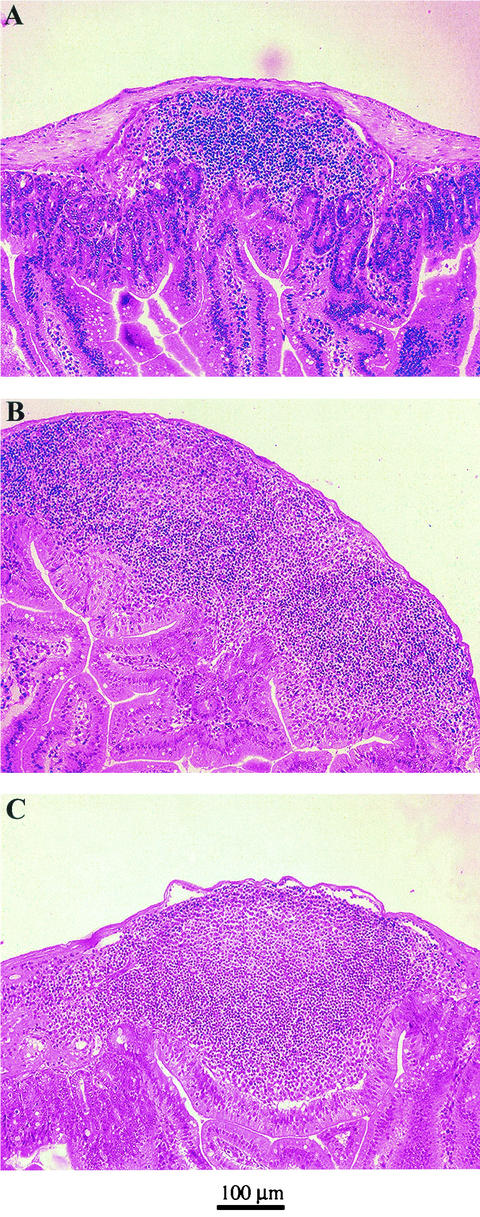
Because the two Lactobacillus strains colonized the gut of monoxenic mice at different densities, we wanted to determine how they induced B-cell reactivity in the mucosa. To do this, we monitored structural changes in the Peyer's patches by analyzing the formation of lymphoid aggregates at day 30 after gavage. Tissue sections from the Peyer's patches of L. johnsonii-colonized mice, L. paracasei-colonized mice, ex-germfree mice reconstituted with a normal mouse flora (conventionalized), and germfree mice were stained with hematoxylin and eosin. As described earlier, we observed that the number and size of Peyer's patches in the small intestine of germfree mice were reduced compared to those of ex-germfree mice colonized with a normal mouse flora (11). The lymphoid follicles in the Peyer's patches of L. johnsonii-colonized mice (Fig. 3B) and L. paracasei-colonized mice (Fig. 3C) were in general larger than in germfree mice (Fig. 3A), but not as large as in conventionalized mice (data not shown).
FIG. 3.
Hematoxylin- and eosin-stained histological section of Peyer's patches of monoassociated mice. After 30 days of colonization, the jejunum was taken from germfree and monoassociated mice, and 5-μm-thick paraffin-embedded tissue sections were made from Peyer's patches of germfree controls (A), L. johnsonii-colonized mice (B), and L. paracasei-colonized mice (C).
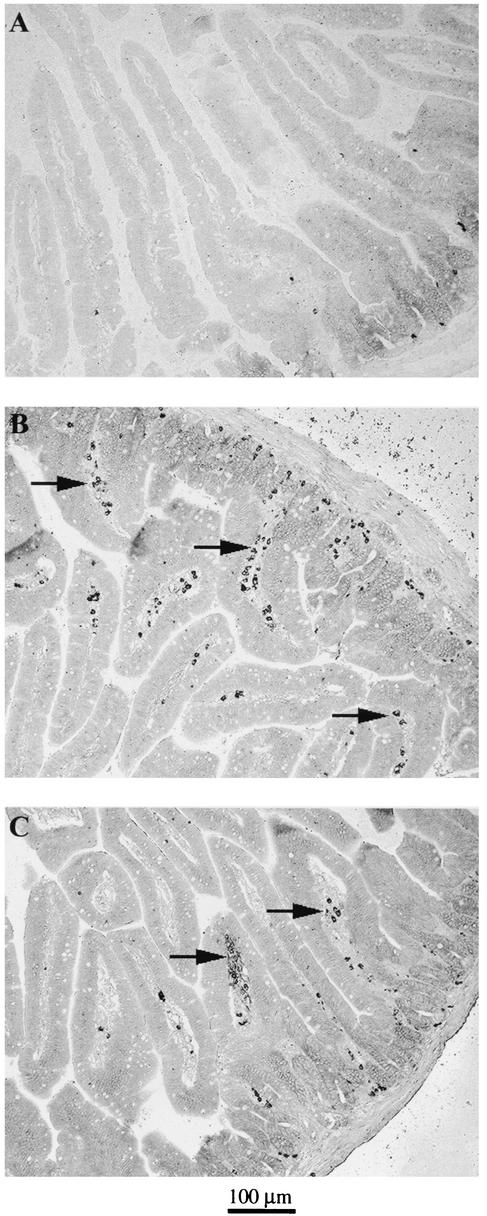
We also analyzed the prevalence of IgA+ B cells in the mucosa 30 days after monoassociation. In the lamina propria, mice monoassociated with either Lactobacillus strain contained increased numbers of IgA+ plasma blasts (Fig. 4B and 4C) compared with germfree mice, which had few or no IgA+ cells (Fig. 4A). These data suggested that monoassociation with L. johnsonii or L. paracasei stimulated lymphoid aggregate formation in the Peyer's patches and homing of IgA+ blasts into the lamina propria.
FIG. 4.
Increased number of IgA+ cells in the lamina propria of monoassociated mice. After 30 days of colonization, the jejunum was taken from germfree and monoassociated mice, and 5-μm-thick paraffin-embedded tissue sections from intestinal tissue of germfree controls (A), L. johnsonii-colonized mice (B), and L. paracasei-colonized mice (C) were stained with biotinylated goat anti-mouse IgA followed by horseradish peroxidase-labeled streptavidin.
Mice monoassociated with L. johnsonii but not L. paracasei secrete increased amounts of Lactobacillus-specific IgA.
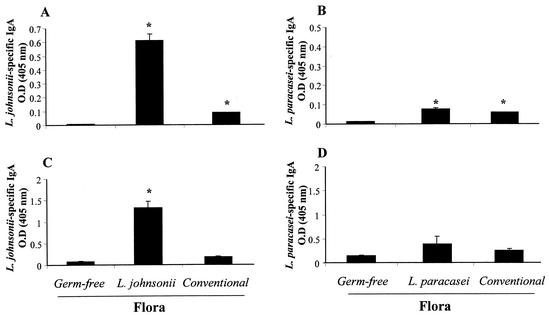
We then wanted to measure the functional properties of mucosal B cells, and therefore, intestinal levels of Lactobacillus-specific IgA were monitored. In all experiments, sampling was performed at 30 days after colonization. We first measured the production of IgA in whole-organ cultures of Peyer's patches. The supernatants from these cultures were analyzed for Lactobacillus-specific IgA (Fig. 5A and 5B).
FIG. 5.
Levels of Lactobacillus-specific IgA in Peyer's patch whole-organ culture supernatants and intestinal contents of monoxenic mice. After 30 days of colonization, Peyer's patches were collected, washed, and incubated for 10 days in culture. The whole small intestine was taken to extract intestinal contents. Culture supernatants from Peyer's patch cultures (A and B) and intestinal contents (C and D) were assessed for L. johnsonii-specific (A and C) and L. paracasei-specific (B and D) IgA by ELISA. Results are expressed as optical density at 405 nm of specific IgA antibodies and represent means of duplicates (A and B) and of eight mice per group (C and D) (± standard error of the mean). The data show results from one representative experiment out of three. *, significantly increased above values for germfree controls (P < 0.05).
There was a significant increase in L. johnsonii-specific IgA in Peyer's patch cultures from both L. johnsonii-colonized and conventional mice compared to germfree mice, albeit at much higher levels in L. johnsonii-associated mice (Fig. 5A). The production of L. paracasei-specific IgA was also significantly increased in Peyer's patch cultures from both L. paracasei-monoassociated and conventional mice compared to germfree mice (Fig. 5B). In this case, however, culture supernatants from Peyer's patches of mice monoassociated with L. paracasei contained amounts of specific IgA that were similar to those found in conventional mice and were clearly lower than in L. johnsonii-colonized mice (Fig. 5A). These data showed that B cells from the Peyer's patches of mice monoassociated with L. johnsonii produced higher levels of specific IgA than those colonized with L. paracasei. In addition, they suggest that the IgA response observed in L. johnsonii-colonized mice was more antigen specific than that in mice monoassociated with L. paracasei.
Next, we measured secretory IgA production in intestinal contents (Fig. 5C and 5D). The data were very similar to those found with the Peyer's patch organ cultures. There was increased production of L. johnsonii-specific IgA in the lumen of L. johnsonii-colonized mice compared to germfree mice (Fig. 5C). In contrast, only a minor (nonsignificant) increase in L. paracasei-specific IgA was seen in the intestinal contents of L. paracasei-colonized mice (Fig. 5D). Again, there was a minor but detectable presence of L. johnsonii- or L. paracasei-specific IgA in the intestinal contents of conventional mice (Fig. 5C and D). Thus, as seen in the supernatants of Peyer's patch cultures, these data suggested that L. johnsonii-colonized mice produced more lactobacillus-specific IgA than L. paracasei-colonized mice.
We also directly measured the intensity of IgA surface expression on B cells in the lamina propria by flow cytometry. As shown in Table 1, the mean fluorescence intensity of surface IgA expression in lamina propria B cells was much higher in conventional mice than in germfree mice. The mean fluorescence intensity of surface IgA was also substantially increased in L. johnsonii-colonized mice, albeit not as much as in conventional mice. In contrast, the mean fluorescence intensity of surface IgA was only weakly increased over that of the germfree controls in L. paracasei-colonized mice. Altogether, these data suggested that colonization with L. johnsonii and L. paracasei induced a secretory IgA response but that this IgA response was stronger and highly specific for L. johnsonii antigens in L. johnsonii-colonized mice, whereas it was weaker and poorly specific for L. paracasei antigens in L. paracasei-colonized mice.
TABLE 1.
Surface IgA expression on B lymphocytes in the lamina propria of germfree and conventional mice or in mice monoassociated with L. johnsonii or L. paracasei
| Mice | Mean fluorescence intensity of IgAa
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Germfree | 221 | 476 |
| L. johnsonii colonized | 533 | 1,461 |
| L. paracasei colonized | 274 | 884 |
| Conventional | 1,039 | 2,545 |
Means for lymphocytes from pooled intestines of two mice.
L. johnsonii and L. paracasei induced different systemic immunoglobulin isotype profiles.
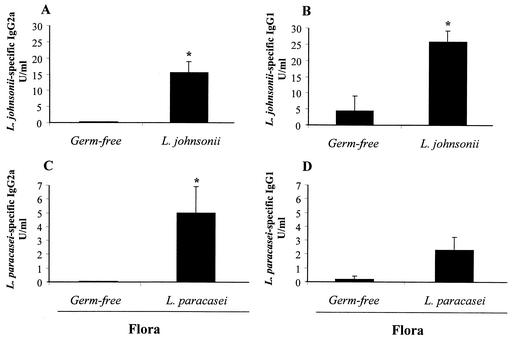
We also observed a difference in circulating immunoglobulin isotypes between the two groups of monoassociated mice. Figure 6 shows the levels of circulating L. johnsonii- and L. paracasei-specific IgG1 and IgG2a isotypes measured in the serum. L. johnsonii-colonized mice produced higher levels of specific IgG2a and IgG1 (Fig. 6A and B) compared to germfree mice, with the predominant isotype being IgG1 (IgG2a/IgG1 ratio = 0.6). In line with this, there was also a clear but not significant (P > 0.05) increase in serum IgE (73 ± 30 ng/ml) in L. johnsonii-colonized mice that almost reached the values observed in conventional mice (80.6 ± 6.5 ng/ml). In contrast, colonization with L. paracasei induced predominantly L. paracasei-specific IgG2a (Fig. 6C) (IgG2a/IgG1 ratio = 2.2) and did not increase the levels of total IgE above that in the germfree controls (27 ± 5 ng/ml versus 17 ± 5 ng/ml). These data suggested that the two Lactobacillus strains induced distinct immunoglobulin isotype switching programs in the periphery.
FIG. 6.
Lactobacillus-specific IgG2a and IgG1 isotypes in the serum of monoassociated mice. After 30 days of colonization, serum was collected from L. johnsonii-associated mice and assayed for L. johnsonii-specific IgG2a (A) and IgG1 (B). Serum from L. paracasei-colonized mice was assayed for L. paracasei-specific IgG2a (C) and IgG1 (D). Results were extrapolated from a positive serum standard from mice immunized systemically with L. johnsonii or L. paracasei crude antigens and represent means for eight mice per group (± standard error of the mean). The data show results from one representative experiment out of three. *, significantly increased above values for germfree controls (P < 0.05).
DISCUSSION
In the present study, we investigated the impact of two different Lactobacillus strains on the development of mucosal and systemic antibody responses. We have shown that the two strains followed distinct patterns of colonization and translocation and promoted different immune responses at the mucosal and systemic levels despite the fact that they exhibited similar growth and enterocyte adherence capacity in vitro. Viable-cell counts of L. johnsonii were much higher than those of L. paracasei in the small intestine and colon of monoxenic mice. This demonstrates that the capacity of a given Lactobacillus strain to adhere in vitro is not the only parameter that will allow inferences about its ability to colonize the gut. It should nevertheless be kept in mind that this conclusion derives from cross-species data, as the Lactobacillus strains were derived from human feces and the in vitro adhesion assays used the human Caco-2 cell line, while the in vivo data were derived from mice. Therefore, it cannot be completely excluded that the data presented reflect a differential colonization potential and immunogenicity in the mouse.
As already described earlier for gnotobiotic mice (4, 42), we found an important rate of translocation by both Lactobacillus strains into the Peyer's patches and mesenteric lymph nodes. This translocation does not occur in specific-pathogen-free mice, suggesting that the mechanisms that normally prevent translocation in conventional mice are impaired in germfree mice (4). It was shown that administration of a whole cecal flora restored these protective mechanisms in germfree mice (6). In our study, the viable-cell counts of L. johnsonii found in the Peyer's patches and mesenteric lymph nodes were much more important than those of L. paracasei, suggesting that L. johnsonii translocated at a higher rate than L. paracasei. Since bacterial loads of L. johnsonii in the lumen were far greater than L. paracasei, this was in line with the previous observation made in gnotobiotic mice that the number of viable bacteria recovered from the mesenteric lymph nodes is directly related to the numbers of bacteria in the intestinal lumen (42, 43).
However, we observed that despite the maintenance of very high levels of L. johnsonii in the lumen of the small intestine and colon throughout the experiment, the quantity of bacteria recovered from the Peyer's patches and mesenteric lymph nodes decreased dramatically with time. This indicated that, despite maintenance of high bacterial counts in the lumen, an active mechanism prevented further translocation of the bacteria into the Peyer's patches and mesenteric lymph nodes. This was in marked contrast to previous studies showing that the numbers of translocating E. coli cultured from mesenteric lymph nodes of E. coli-monoassociated mice were the same after either 2 days or 100 days of monoassociation (6). Conceivably, this difference could be explained by the fact that the types of immune responses generated in the mucosa by E. coli may be very different from those induced by lactobacilli.
Many factors may influence bacterial survival and translocation in the gut mucosa, the most prominent one probably being the immune response (17, 21, 40). We therefore undertook to study the specific humoral responses elicited by the two strains in the mucosa. We have observed that both lactobacilli induced B-cell stimulation, as characterized by the formation of lymphoid aggregates in Peyer's patches and the presence of IgA+ B cells in the lamina propria. These data suggested that, despite substantial quantitative differences in colonization and translocation, both lactobacilli induced a mucosal B-cell response. However, when we analyzed the nature of the B-cell responses, we observed clear differences between the two strains. Colonization with L. johnsonii promoted substantial production of specific secretory IgA, whereas L. paracasei induced only marginal secretory IgA at levels that were in the same range as the cross-reactive IgA found in conventional mice.
Assuming that secretory IgA plays a key role in the maintenance of bacterial homeostasis in the lumen (21, 40), the latter observation was difficult to reconcile with the finding that bacterial loads of L. johnsonii were by far higher than those of L. paracasei in the lumen. This emphasized that other factors of the innate immune response, distinct from secretory IgA, probably also play an important role in controlling bacterial levels in the lumen and adherence to the mucosa. These could include, for example, the differential activation of α-defensin secretion by Paneth's cells, as shown previously by others (2).
There may be several explanations for why the IgA responses were so different between the two strains. First, due to less efficient colonization and translocation, L. paracasei-specific IgA was transiently produced and was no longer detectable 30 days after colonization. Interestingly, it has previously been shown that colonization of germfree mice with the gram-negative bacterium Morganella morganii also induced little specific secretory IgA (40) due to transient production with a peak at 14 days. Second, IgA in L. paracasei-monoassociated mice may have a low affinity for L. paracasei epitopes. As reported earlier in another system (44), it is not excluded that L. paracasei-specific IgA epitopes were hidden in our ELISA, although this was not the case for L. paracasei-specific IgG. It should also be underlined that secretory IgA from conventional mice cross-reacted to some extent with the Lactobacillus strains, although neither L. johnsonii nor L. paracasei has been reported as being a normal member of the mouse intestinal microbiota. This may not be surprising, because control of the endogenous flora probably requires a broad spectrum of reduced-affinity secretory IgA. Indeed, changes in the intestinal flora result in induction of secretory IgA responses through a pathway independent of T-cell help that may not be highly specific (26).
Further evidence that the two strains of lactobacilli induced different immune responses was given by measuring systemic IgG isotypes. L. johnsonii-colonized mice produced higher levels of specific IgG1 isotypes, in contrast to L. paracasei-colonized mice, which clearly produced preferentially specific IgG2a antibodies. Since it has been described that gamma interferon causes murine B cells to switch towards the IgG2a isotype (16, 41) and interleukin-4 promotes IgG1 (43), this suggested preferential Th1 and Th2 patterns induced by L. paracasei and L. johnsonii. Dissection of the induction of distinct patterns of cytokines in mucosal CD4+ T cells from monoassociated mice should give a clear indication of whether the two strains induce different T-helper responses. This is currently under investigation.
In conclusion, we have shown that Lactobacillus strains with apparently similar properties in vitro may have distinct patterns of colonization and may induce heterogeneous immune responses in vivo. These data underline the heterogeneity that prevails among probiotic Lactobacillus strains. It is only after thorough understanding of their mechanisms of action in vivo that certain strains of probiotics may be used for specific health applications.
Acknowledgments
We gratefully acknowledge the technical assistance of Kim-Yen Saudan, Thérèse Sauthier, Senda-Nton Mafuala-Muana, Catherine Schwartz, Robert Beumer, and Angele Boenzli-Bruand. We thank Jalil Benyacoub and Irène Corthésy for helpful discussion and critical reading.
Editor: B. B. Finlay
REFERENCES
- 1.Albanese, C. T., S. D. Smith, S. Watkins, A. Kurkchubasche, R. L. Simmons, and M. I. Rowe. 1994. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J. Am. Coll. Surg. 179:679-688. [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 3.Bengmark, S. 1998. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 42:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, R. D., and A. W. Garlington. 1979. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 23:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg, R. D., and A. W. Garlington. 1980. Translocation of Escherichia coli from the gastrointestinal tract to the mesenteric lymph nodes in gnotobiotic mice receiving E. coli vaccines before colonization. Infect. Immun. 30:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg, R. D., and W. E. Owens. 1979. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect. Immun. 25:820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg, R. D., and D. C. Savage. 1975. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect. Immun. 11:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1994. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 10.Cebra, J. J., N. A. Bos, E. R. Cebra, D. R. Kramer, F. G. Kroese, and C. E. Schrader. 1995. Cellular and molecular biologic approaches for analyzing the in vivo development and maintenance of gut mucosal IgA responses. Adv. Exp. Med. Biol. 371A:429-434. [DOI] [PubMed] [Google Scholar]
- 11.Cebra, J. J., S. B. Periwal, G. Lee, F. Lee, and K. E. Shroff. 1998. Development and maintenance of the gut-associated lymphoid tissue (gut-associated lymphoid tissues): the roles of enteric bacteria and viruses. Dev. Immunol. 6:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen, H. R., H. Frokiaer, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171-178. [DOI] [PubMed] [Google Scholar]
- 13.Crabbe, P. A., H. Bazin, H. Eyssen, and J. F. Heremans. 1968. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int. Arch. Allergy Appl. Immunol. 34:362-375. [DOI] [PubMed] [Google Scholar]
- 14.Delneste, Y., A. Donnet-Hughes, and E. J. Schiffrin. 1998. Functional foods: mechanisms of action on immunocompetent cells. Nutr. Rev. 56:S93-S98. [DOI] [PubMed] [Google Scholar]
- 15.Dunne, C., L. O'Mahony, L. Murphy, G. Thornton, D. Morrissey, S. O'Halloran, M. Feeney, S. Flynn, G. Fitzgerald, C. Daly, B. Kiely, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S-392S. [DOI] [PubMed] [Google Scholar]
- 16.Finkelman, F. D., I. M. Katona, T. R. Mosmann, and R. L. Coffman. 1988. interferon-γ regulates the isotypes of Ig secreted for in vivo humoral immune responses. J. Immunol. 140:1022-1027. [PubMed] [Google Scholar]
- 17.Gautreaux, M. D., E. A. Deitch, and R. D. Berg. 1994. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect. Immun. 62:2874-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller, D., S. Blum, C. Bode, W. P. Hammes, and E. J. Schiffrin. 2000. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect. Immun 68:752-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller, D., C. Bode, W. P. Hammes, A. M. Pfeifer, E. J. Schiffrin, and S. Blum. 2000. Nonpathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 47:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isolauri, E., Y. Sutas, P. Kankaanpaa, H. Arvilommi, and S. Salminen. 2001. Probiotics: effects on immunity. Am. J. Clin. Nutr. 73:444S-450S. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, H. Q., N. A. Bos, and J. J. Cebra. 2001. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect. Immun. 69:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato, I., T. Yokokura, and M. Mutai. 1983. Macrophage activation by Lactobacillus casei in mice. Microbiol. Immunol. 27:611-618. [DOI] [PubMed] [Google Scholar]
- 23.Kato, I., T. Yokokura, and M. Mutai. 1984. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol. Immunol. 28:209-217. [DOI] [PubMed] [Google Scholar]
- 24.Klaasen, H. L., P. J. Van der Heijden, W. Stok, F. G. Poelma, J. P. Koopman, M. E. Van den Brink, M. H. Bakker, W. M. Eling, and A. C. Beynen. 1993. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect. Immun 61:303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link-Amster, H., F. Rochat, K. Y. Saudan, O. Mignot, and J. M. Aeschlimann. 1994. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 10:55-63. [DOI] [PubMed] [Google Scholar]
- 26.Macpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T-cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 27.Meydani, S. N., and W. K. Ha. 2000. Immunologic effects of yogurt. Am. J. Clin. Nutr. 71:861-872. [DOI] [PubMed] [Google Scholar]
- 28.Moreau, M. C., R. Ducluzeau, D. Guy-Grand, and M. C. Muller. 1978. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect. Immun. 21:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morelli, L. 2000. In vitro selection of probiotic lactobacilli: a critical appraisal. Curr. Issues Intest. Microbiol. 1:59-67. [PubMed] [Google Scholar]
- 30.Owens, W. E., and R. D. Berg. 1980. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect. Immun. 27:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdigon, G., S. Alvarez, and H. A. Pesce de Ruiz. 1991. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J. Dairy Res. 58:485-496. [DOI] [PubMed] [Google Scholar]
- 32.Perdigon, G., S. Alvarez, M. Rachid, G. Agüero, and N. Gobbato. 1995. Immune system stimulation by probiotics. J. Dairy. Sci. 78:1597-1606. [DOI] [PubMed] [Google Scholar]
- 33.Perdigon, G., M. E. de Macias, S. Alvarez, G. Oliver, and H. de Ruiz. 1986. Effect of perorally administered lactobacilli on macrophage activation in mice. Infect. Immun. 53:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perdigon, G., M. E. de Macias, S. Alvarez, G. Oliver, and H. de Ruiz. 1988. Systemic augmentation of the immune response in mice by feeding fermented milks with Lactobacillus casei and Lactobacillus acidophilus. Immunology 63:17-23. [PMC free article] [PubMed] [Google Scholar]
- 35.Perdigon, G., D. M. M. Nader, S. Alvarez, G. Oliver, and A. A. Pesce de Ruiz Holgado. 1987. Enhancement of immune response in mice fed with Streptococcus thermophilus and Lactobacillus acidophilus. J. Dairy Sci. 70:919-926. [DOI] [PubMed] [Google Scholar]
- 36.Schaedler, R. W., R. Dubos, and R. Costello. 1965. Association of germfree mice with bacteria isolated from normal mice. J. Exp. Med. 122:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaedler, R. W., R. Dubos, and R. Costello. 1965. The development of the bacterial flora in the gastrointestinal tract of mice. J. Exp. Med. 122:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiffrin, E. J., D. Brassart, A. L. Servin, F. Rochat, and A. Donnet-Hughes. 1997. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am. J. Clin. Nutr. 66:515S-520S. [DOI] [PubMed] [Google Scholar]
- 39.Schiffrin, E. J., F. Rochat, H. Link-Amster, J. M. Aeschlimann, and A. Donnet-Hughes. 1995. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 78:491-497. [DOI] [PubMed] [Google Scholar]
- 40.Shroff, K. E., K. Meslin, and J. J. Cebra. 1995. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun 63:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snapper, C. M., C. Peschel, and W. E. Paul. 1988. interferon-γ stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 140:2121-2127. [PubMed] [Google Scholar]
- 42.Steffen, E. K., and R. D. Berg. 1983. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect. Immun. 39:1252-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steffen, E. K., R. D. Berg, and E. A. Deitch. 1988. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J. Infect. Dis. 157:1032-1038. [DOI] [PubMed] [Google Scholar]
- 44.Talham, G. L., H. Q. Jiang, N. A. Bos, and J. J. Cebra. 1999. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 67:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein, P. D., and J. J. Cebra. 1991. The preference for switching to IgA expression by Peyer's patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J. Immunol. 147:4126-4135. [PubMed] [Google Scholar]