Abstract
Pathogen pattern recognition receptors (PRRs) recognize common structural and molecular motifs present on microbial surfaces and contribute to induction of innate immune responses. The mannose receptor (MR), a carbohydrate-binding receptor expressed on subsets of macrophages, is considered one such PRR. In vitro experiments have implicated the MR in phagocytosis of mannose-bearing microbes, including Candida albicans, and enhancement of antifungal response by macrophages. However, the significance of the MR's contribution to immune response during systemic C. albicans infection has never been directly demonstrated. Using MR-deficient mice in an in vivo infection experiment, we examined the role of the MR in immune response during disseminated candidiasis. MR−/− and wild-type control mice were challenged intraperitoneally with C. albicans, and the survival rates, tissue fungal burden, inflammatory cell recruitment, and specific antibody production after infection were evaluated. We found no significant difference in survival between the two mouse strains. MR−/− mice had higher average fungal burdens in some of the organs on days 7 and 21 but exhibited competence in inflammatory cell recruitment and antibody production. We also observed in vitro that MR−/− peritoneal cavity macrophages were equally capable of C. albicans uptake and that phagocytosis could be blocked with β-glucan. We conclude that the MR is not required for the normal host defense during disseminated candidiasis or for the phagocytosis of C. albicans and that a β-glucan receptor may be required for C. albicans phagocytosis.
Candida albicans, an opportunistic fungal pathogen, is a major cause of morbidity and mortality among immunocompromised individuals. The mortality rate among these patients with disseminated candida infection is about 30%, in spite of antifungal drug treatment (34). Predisposing factors such as neutropenia and low CD4-T-cell counts in human immunodeficiency virus patients suggest that both innate and adaptive cell-mediated immune mechanisms are critical in preventing this normal commensal organism from establishing an invasive infection (20). The coordination of the innate and adaptive arms of antifungal defense may also be important; it is believed that Th1, not Th2, response confers protection against candidiasis and that induction of Th1 CD4+ cells depends on activation of phagocytic cells (17).
Phagocytosis of serum-opsonized C. albicans is carried out by neutrophils, macrophages, and eosinophils (7, 13), presumably via complement and Fc receptors. In contrast, uptake of unopsonized C. albicans has been attributed to lectin-like receptors that function as pattern recognition receptors (PRRs). PRRs distinguish self from nonself by recognizing common structural and molecular motifs that are present on microbes but absent from mammalian proteins. It is believed that upon the recognition of foreign motifs PRRs induce innate immune responses (8). The mannose receptor (MR) has been implicated as one such PRR, which can directly bind specific glycans found on C. albicans (28).
The MR is a 180-kDa Ca2+-dependent lectin that functions as an endocytic receptor. It was first isolated from macrophages, but it has been found on a variety of other cells types, including dendritic cells (24), hepatic endothelial cells (29), retinal pigment epithelial cells (27, 30), and kidney mesangial cells (14). The receptor consists of 10 extracellular domains followed by a transmembrane region and short cytoplasmic tail. The extracellular domains are the amino-terminal cysteine-rich domain, a fibronectin type II repeat domain, and eight tandem carbohydrate recognition domains. The carbohydrate recognition domains bind accessible mannose, fucose, and N-acetylglucosamine residues but have a higher affinity for complex ligands with multiple binding sites, such as mannan on yeast surface (31, 32).
Involvement of the MR in phagocytosis of C. albicans and antifungal response has been demonstrated in vitro. Transfection with an MR expression vector enabled COS-1 cells to ingest C. albicans (3), and uptake of unopsonized C. albicans by human monocyte-derived macrophages was inhibited by MR ligands, e.g., mannan and mannosylated bovine serum albumin (BSA) (15). Furthermore, the MR has been implicated in enhancement of C. albicans killing and cytokine production. CSF-1 has been shown to augment fungicidal activity by increasing MR-mediated uptake of C. albicans (11). Likewise, gamma interferon (IFN-γ)-mediated increase in C. albicans phagocytosis and subsequent killing was abolished by addition of MR ligands (16). An alternate receptor-blocking approach, antisense MR oligonucleotide treatment, abolished increases in mRNA levels of interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor in C. albicans-stimulated macrophages (35). Despite extensive in vitro evidence suggesting a role for the MR in immune responses, the function of the MR in host defense has never been determined in vivo.
To examine the physiological importance of the MR in host defense against C. albicans, we studied the course of experimental fungal infection in MR-deficient mice (12).
MATERIALS AND METHODS
Mice.
MR knockout (MR−/−) mice were generated on the 129SvJ × C57BL/6 background and were backcrossed to the C57BL/6 strain for 7 generations (12). The wild-type controls for all experiments were the offspring of backcrossed MR−/− littermates.
Infection and survival curves.
Wild-type and MR−/− mice were sex and age matched, and more than 30 mice per strain were used for each experiment. For survival rates two independent infection experiments were conducted. C. albicans (ATCC 18804) was cultured in 2% Sabouraud's dextrose broth (Difco Laboratories, Becton Dickinson, Sparks, Md.) in a 30°C shaker for 24 h. The fungi were washed in phosphate-buffered saline (PBS) (Gibco BRL, Grand Island, N.Y.) two times before injection. Mice, 6 to 9 weeks old, were challenged intraperitoneally with 8 × 107 C. albicans blastoconidia and were observed for 28 days.
Determination of tissue fungal burden.
Fungal burden in the organs of infected mice was determined by quantitating CFU as described earlier (10). On days 3, 7, 14, and 21 after infection, wild-type and MR−/− mice were sacrificed by CO2 asphyxiation. The organs were excised and weighed before homogenization in 0.1% Triton X-100 (LabChem, Pittsburgh, Pa.). Serial dilutions were plated onto Sabouraud's dextrose agar (Difco Laboratories) in duplicates. After 24 to 36 h of incubation at 37°C, yeast colonies were counted and the number of CFU for each organ was determined. Data from two rounds of infection are shown. The CFU values from four mice per experimental group in each experiment were used to calculate the mean log10 CFU per gram of organ.
Immunohistology.
Fungi in organs were visualized by C. albicans-specific immunostaining of frozen tissue sections. On 3, 7, 14, and 21 days after infection, organs were removed and frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, Calif.). Sections of 6- to 8-μm thickness (10 μm for brain) were cut and dried overnight at 4°C in a desiccated container, followed by fixation in acetone at 4°C for 10 min. For detecting fungi, sections were incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-C. albicans antibody (Cortex Biochem, San Leandro, Calif.) for 60 to 90 min at room temperature. The DAB substrate kit for peroxidase (Vector Laboratories, Burlingame, Calif.) was used for visualizing the antibody. For staining of inflammatory cells, slides were treated with the following antibodies at room temperature for 60 min: biotinylated rat anti-mouse CD45R/B220 (BD Pharmingen, San Diego, Calif.) for B cells; hamster anti-mouse CD3ɛ (BD Pharmingen), and biotinylated anti-hamster cocktail (BD Pharmingen) for T cells and rat anti-mouse CD68 clone FA-11 (Serotec, Raleigh, N.C.); and phosphatase-labeled anti-rat immunoglobulin G (IgG) (Kierkegaard & Perry Laboratories, Gaithersburg, Md.) for macrophages. For visualization of the antibodies, avidin-peroxidase complex and peroxidase substrate from the Vectastain ABC-P Vector Blue kit or phosphatase substrate from the Vectastain ABC-AP Vector Blue kit (Vector Laboratories) were used. Permount medium (Fisher Scientific, Pittsburgh, Pa.) was used for mounting coverslips.
Candida-specific antibody titer assay.
The enzyme-linked immunosorbent assay (ELISA) was used to measure C. albicans-specific antibody titers from infected mice. Ninety-six-well plates were coated with 107 CFU of heat-killed C. albicans, and the plate contents were incubated at 4°C overnight. The plates were then washed and blocked with 1% BSA in PBS for 1 h at 37°C. Serial dilutions of serum samples were added to wells and incubated for 1 h at 37°C. After being washed, wells were incubated with HRP-conjugated anti-mouse IgM and anti-mouse IgG antibodies (Jackson ImmunoResearch, West Grove, Pa.) for 1 h at 37°C and immunoreactivity was revealed with HRP substrate (Bio-Rad, Hercules, Calif.). The colorimetric changes were read at 405 nm on the Versamax Microplate reader (Molecular Devices, Sunnyvale, Calif.). The antibody titers were measured and are expressed as the reciprocal of the dilution giving an absorbance of 0.1 above that of the control (pooled uninfected serum). The geometric mean titers were also calculated for comparison.
Candida phagocytosis assay.
Peritoneal cells were isolated by peritoneal lavage with cold serum-free RPMI 1640 (Gibco BRL). Thioglycolate-elicited cells were obtained from mice (5 days after intraperitoneal injection of 1.5 ml of 4% BBL Brewer modified thioglycolate medium) (Becton Dickinson) and pooled for each experiment. Cells were plated at 2 × 105/well and were allowed to adhere in 24-well plates (lined with 12-mm glass coverslips) for 24 h at 37°C in RPMI 1640 containing 10% fetal bovine serum, 10 mM HEPES, and penicillin-streptomycin. Cells were washed twice in 1× BWD (12.5 mM NaCl, 0.5 mM KCl, 0.5 mM dextrose, 1 mM NaHCO3, 2 mM HEPES, 1 mM CaCl2, 1 mM MgCl, and 0.1 mM KH2PO4) (4). Each well was incubated with 0.75 U at an optical density at 600 nm of C. albicans blastoconidia in the presence or absence of glucan (100 μg/ml) or mannan (500 μg/ml) from baker's yeast (Sigma). After indicated times wells were washed three times with 1× BWD, and glass coverslips were placed on ice. Coverslips were incubated with fluorescein isothiocyanate (FITC)-anti-C. albicans antibody (Cortex Biochem) for 30 min on ice. Subsequently, cells were fixed in 3.7% formaldehyde in PBS for 10 min and were permeabilized with Tris-buffered saline with 0.5% Triton X-100 for 5 min. Permeabilized cells were incubated with rabbit anti-C. albicans antibody and then with Cy3-anti-rabbit IgG antibody (Jackson ImmunoResearch). Each experimental condition was done in triplicates, and each phagocytic experiment was repeated more than once. In quantitation of internalized C. albicans, two or more coverslips from the same experiment were examined. Internalized C. albicans organisms were counted in groups of 10 macrophages from different parts of the same coverslip and from different coverslips; the combined number was averaged. The results were fairly reproducible from experiment to experiment.
Immunofluorescence microscopy was performed on Olympus Microscope AX70 (Olympus America, Melville, N.Y.). Processing of images was done using MetaMorph Imaging System version 4.1.3 (Universal Imaging Corporation, Downington, Pa.).
Statistics.
Survival and fungal burden analyses were performed using SAS 8.0 (SAS Institute Inc., Cary, N.C.). For the survival analysis, Kaplan-Meier methodology was used to generate the survival curves, and the log rank test was used to test for differences in survival between the wild-type and knockout mice. For the CFU data the method of analysis of variance was applied, using mouse strain (wild type or knockout) as the dependent variable and experiment (no. 1 or 2), organ, and an interaction term between experiment and organ as the independent variables. All tests in which P was < 0.05 were considered significant.
RESULTS
Survival rates in C. albicans-infected mice.
To assess the role of MRs in host defense against C. albicans, MR−/− and wild-type control mice were infected intraperitoneally with 8 × 107 blastoconida of C. albicans and their survival was observed for 28 days (Fig. 1). We chose the intraperitoneal route, because we have observed by immunoblotting that peritoneal macrophages express the MR and that this expression is enriched after stimulation with intraperitoneal thioglycolate injection (data not shown). We wished to examine the role of these MR+ macrophages in the initial phase of infection by injecting C. albicans into the peritoneal cavity. The survival experiment was performed twice.
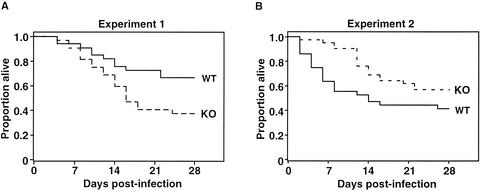
FIG. 1.
Survival of C. albicans-infected wild-type and MR−/− mice. Mice were infected intraperitoneally with 8 × 107 blastoconidia and observed for 28 days. The graphs (A and B) show data from two independent infection experiments; each experiment used 30 or more mice for each genotype. WT, wild-type mice; KO, MR−/− mice.
In the 28-day postinfection period, most incidences of morbidity and mortality in both wild-type and MR−/− strains occurred in the first 2 weeks after infection. After this period few deaths were observed, and most surviving animals exhibited normal appearance and activity.
In terms of survival, at the end of the observation period 67% of the wild-type and 38% of the MR−/− mice in experiment 1 (Fig. 1A) and 42% of the wild-type and 57% of the MR−/− mice in experiment 2 (Fig. 1B) remained alive. In experiment 1 the median survival was not reached for the wild-type mice; for the MR−/− mice the median survival was 16 days (P = 0.02). In experiment 2 the survival trend was reversed. The median survival for the wild-type mice was 14 days, whereas for the MR−/− mice the median survival was not reached (P = 0.05). In summary, differences in survival between the two strains were noted in the two experiments, but they were in opposite directions. When the data from the two experiments were combined, there was no significant difference (P = 0.96) in survival between the wild-type and MR−/− mice. We conclude that there is no significant effect on overall host resistance to systemic candidiasis in MR-deficient mice.
Tissue fungal burden in C. albicans-infected mice.
To determine whether the MR deficiency was associated with higher fungal burdens, organs from C. albicans-infected wild-type and MR−/− mice were examined 3, 7, 14, and 21 days after infection. Brain, lungs, liver, spleen, and kidneys were excised and homogenized, and serial dilutions of organ homogenates were plated to measure the number of CFU. In comparing the fungal burdens of the two mouse strains, the method of analysis of variance was used, allowing for a composite analysis of data from two independent experiments.
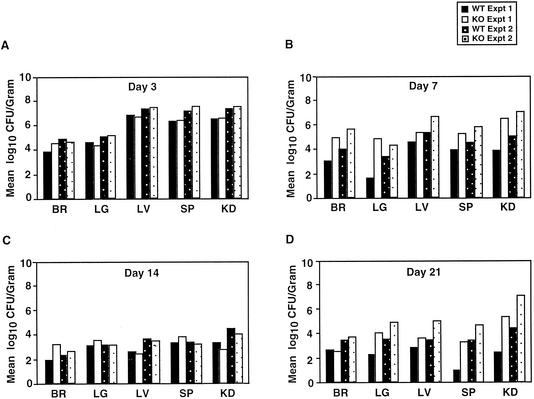
On day 3 no significant difference in the mean number of CFU in the organ was detected between the wild-type and MR−/− groups (Fig. 2A). On day 7 a higher mean number of CFU was observed in all the examined organs of MR−/− mice than in the wild-type mice (Fig. 2B). Only the differences in the brain (P = 0.04) and lungs (P = 0.04) were marginally significant. On day 14 the mean CFU values had decreased in all organs, except in the kidneys, for both the wild-type and MR−/− strains (Fig. 2C). The mean numbers of CFU were higher in wild-type mice for liver and kidneys and in MR−/− mice for brain and lung, but none of these differences was statistically significant. On day 21 the fungal burden continued to remain high in the kidneys for both groups (Fig. 2D). The mean CFU value was higher in the wild type in brain, but in all the other organs the mean values were higher in MR−/− mice; only the differences in the lungs (P = 0.03) and spleen (P = 0.004) were statistically significant.
FIG. 2.
Tissue fungal burden in C. albicans-infected mice. Fungal burden in brain (BR), lungs (LG), liver (LV), spleen (SP), and kidneys (KD) was assessed at 3, 7, 14, and 21 days postinfection. Data from two independent infection experiments (experiment 1 and experiment 2) are shown with separate bars in each graph; four mice for each genotype per experiment were assessed on a given day. The results are expressed as mean log10 CFU per gram of organ. WT, wild type; KO, knockout.
We observed a range of morbidity, evidenced by weight loss, huddled posture, and ruffling of fur, in both strains. We also noted that pronounced morbidity was associated with high fungal burdens in the organs. We observed no marked distinction between the two strains in the range and severity of morbidity observed.
Recruitment of inflammatory cells.
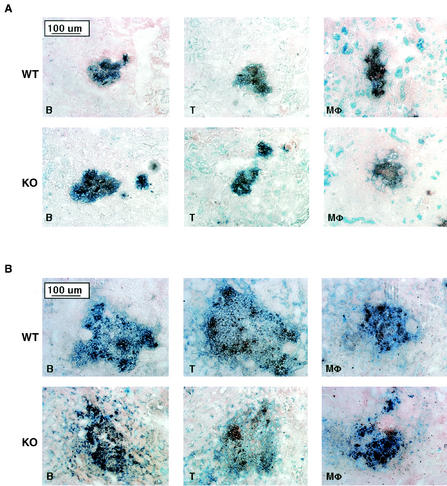
To determine whether MR−/− mice are impaired in recruitment of inflammatory cells to sites of Candida infection, we examined organs from infected wild-type and MR−/− mice immunohistochemically. On day 3 kidneys exhibited multifocal abscesses surrounded by FA-11+ macrophages, B220+ B cells, and CD3+ T cells (Fig. 3A). On day 14 the inflammatory cells were still present around the diffusely expanded fungal abscesses (Fig. 3B)
FIG. 3.
Immunohistochemical analysis of inflammatory lesions in kidneys. Kidneys were harvested from C. albicans-infected mice on day 3 (A) and day 14 (B) postinfection. C. albicans (in brown) and inflammatory cells (B220+ B cells [B], CD3+ T cells [T], and FA-11+ macrophages [Mφ], in blue) were detected by immunohistochemical staining. WT, wild type; KO, knockout.
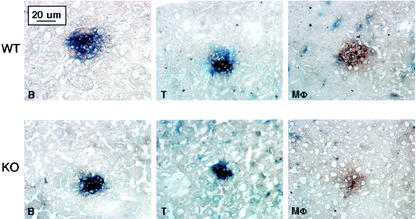
In liver B220+ B and CD3+ T cells but not FA-11+ macrophages were observed around fungal foci on day 3 (Fig. 4). By day 14 few fungi were detected (data not shown). No difference was observed between the wild-type and MR−/− strains. Similar patterns were observed in the lungs of infected mice (data not shown). We conclude that MR−/− mice are not impaired in recruiting inflammatory cells to sites of C. albicans infection.
FIG. 4.
Histopathology of C. albicans-infected liver. On day 3 postinfection liver was harvested for visualization of C. albicans (in brown) and inflammatory cells (B220+ B cells [B], CD3+ T cells [T], and FA-11+ macrophages [Mφ], in blue). WT, wild type; KO, knockout.
Humoral response to C. albicans antigens.
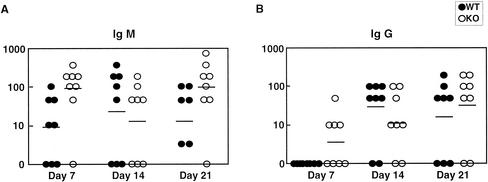
C. albicans-specific antibody titers in infected mice were measured to assess the ability to mount a humoral response against the fungal pathogen. Sera were collected from infected mice on days 3, 7, 14, and 21 days after infection, and C. albicans-specific IgM and IgG levels were measured by ELISA (Fig. 5A and B). On day 3 both the wild-type and MR−/− sera were negative for anti-Candida IgM or IgG (data not shown). On day 7, a higher mean IgM titer was found in MR−/− mice. On day 14 both IgM and IgG titers were higher in the wild-type group, and on day 21 the mean antibody titers were higher in the MR−/− group. As in the case of organ titers, a wide range in antibody titers was observed for each group, and no marked differences in the mean titers, except for the higher IgM titers in MR−/− mice on day 7, were found. The results indicate that MR−/− mice are capable of mounting a specific antibody response to C. albicans.
FIG. 5.
C. albicans-specific antibody titers in sera of infected wild-type (WT) and MR−/− (KO) mice. C. albicans-specific IgM (A) and IgG (B) levels were measured by ELISA. Serial dilutions of serum samples from eight mice per group were individually tested, and the reciprocal of the dilution giving an absorbance of 0.1 above the controls (pooled uninfected serum) was determined. The dots represent the reciprocal values of individual antibody titers, and bars indicate the geometric mean of the reciprocal values.
C. albicans phagocytosis by macrophages.
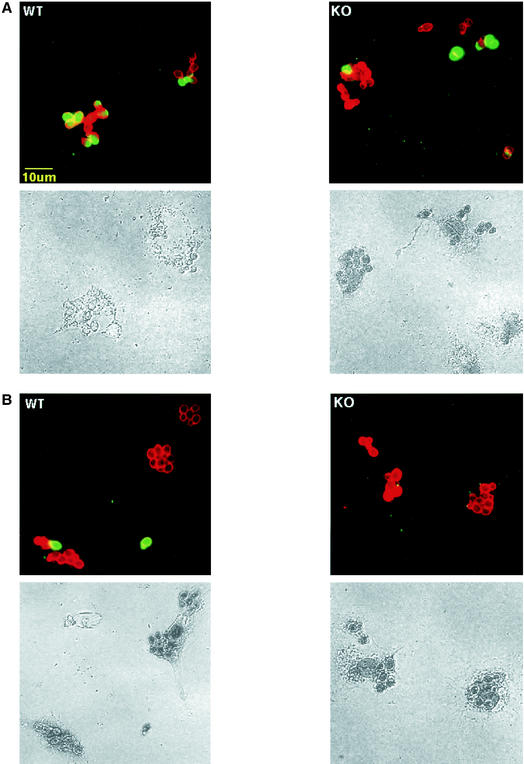
The MR expressed on macrophages has been implicated in the phagocytosis of C. albicans. To examine whether MR−/− macrophages were impaired in their ability to engulf C. albicans, resident peritoneal cavity macrophages were tested for phagocytosis. Macrophages were incubated with unopsonized Candida in serum-free conditions, and the extent of fungal uptake was compared after 15 and 30 min of incubation. To visualize extracellular fungi cells were stained with FITC-conjugated anti-C. albicans antibody. Intracellular fungi were detected by fixation and permeabilization, followed by staining with anti-C. albicans antibody and Cy3-conjugated secondary antibody. The results showed that both wild-type and MR−/− cells were capable of phagocytosing C. albicans with equal efficiency (Table 1). After 15 min some fungi were detected intracellularly and a few were extracellularly bound (Fig. 6A); after 30 min most of the fungi were seen intracellularly (Fig. 6B).
TABLE 1.
Phagocytic index numbersa for the phagocytosis assay
| Mφ sourceb | Incubation conditions (time and concn) | PI for:
|
|
|---|---|---|---|
| Wild type | MR−/− | ||
| RPc | 15 min | 40-9 | 40-5 |
| RP | 30 min | 56-7 | 59-6 |
| TPd | 30 min | 77-13 | 83-15 |
| TP | 30 min, 100 μg of glucan/ml | 0.4-0.05 | 0.3-0.05 |
| TP | 30 min, 500 μg of mannan/ml | 79-10 | 77-6 |
Phagocytic index (PI) numbers indicate the average numbers of CFU of intracellular C. albicans per 10 peritoneal macrophages. To calculate the phagocytic index, 10 groups of 10 macrophages were chosen from either different parts of the same coverslip or from a different coverslip.
Mφ, macrophage.
RP, resident peritoneal Mφ.
TP, thioglycolate-elicited Mφ.
FIG. 6.
In vitro C. albicans phagocytosis by peritoneal resident wild-type (WT) and MR−/− (KO) macrophages. Resident peritoneal cavity macrophages were incubated with C. albicans at 37°C for 15 (A) and 30 (B) min. Immunofluorescence staining of extracellular (FITC-green) and, after fixation and permeabilization, extra- and intracellular (Cy3-red) C. albicans was performed. The upper panels show overlay images; extra- and intracellular C. albicans is seen as yellow-green and red, respectively. The lower panels show the same images acquired with light microscopy.
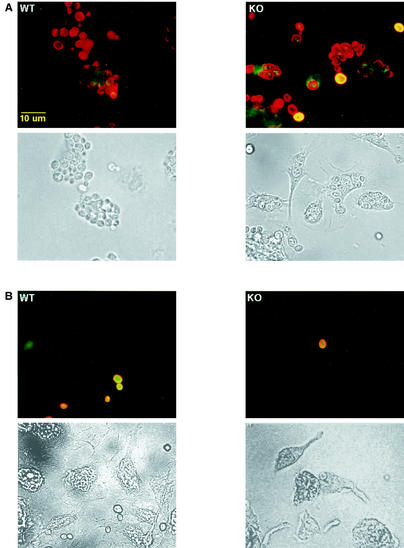
To compare phagocytosis by activated macrophages, wild-type and MR−/− macrophages from thioglycolate-treated mice were assessed. No difference was observed in these two cell populations in their abilities to phagocytose C. albicans (Fig. 7A, Table 1).
FIG. 7.
In vitro C. albicans phagocytosis by thioglycolate-elicited wild-type (WT) and MR−/− (KO) macrophages. After 30 min of incubation in the absence (A) or in the presence (B) of 100 μg of β-glucan/ml at 37°C, immunofluorescence staining was performed as for Fig. 5.
To determine which receptor was being employed for phagocytosing unopsonized yeast, mannan and glucan, which are carbohydrate polymers found on the surface of C. albicans, were tested for potential inhibition of the uptake. For both wild-type and MR−/− cells, only glucan (100 μg/ml) was effective in blocking C. albicans phagocytosis (Fig. 7B; Table 1); no change in uptake was observed with mannan (500 μg/ml) (Table 1).
Glucan inhibition of uptake was C. albicans specific. Phagocytosis of zymosan was similar in wild-type and MR−/− macrophages, and this uptake was blocked by neither glucan nor mannan (data not shown). The results indicate that peritoneal macrophages do not require the MR for the phagocytosis of unopsonized C. albicans and suggest that a receptor for glucan is responsible for this uptake.
DISCUSSION
Our mouse infection model for disseminated candidiasis demonstrated that MR deficiency does not increase the overall susceptibility of a mouse host to systemic C. albicans infection. The survival rates and tissue fungal burdens for wild-type and MR−/− mice failed to reveal a consistent pattern of significant differences, indicating an unaltered level of host resistance against this pathogen in the absence of MR expression. MR−/− mice also demonstrated competence in recruiting inflammatory cells to sites of fungal infection and mounting a fungal-antigen-specific humoral response.
The idea that the MR functions as a PRR was supported by studies in which in vitro phagocytosis of unopsonized C. albicans was blocked by various MR ligands. Our results show that MR−/− peritoneal cavity macrophages, both resident and thioglycolate elicited, can efficiently ingest C. albicans. Interestingly, C. albicans phagocytosis by MR−/−, as well as wild-type, macrophages was completely abolished by addition of β-glucan. This suggests that MR−/− macrophages do not merely compensate for the loss of MR function with another redundant receptor, since wild-type macrophages also appear to require the function of a receptor for β-glucan for phagocytosis of C. albicans. This finding is in agreement with a previous study that showed that β-glucan but not α-mannan blocked C. albicans phagocytosis by human monocyte-derived macrophages (9). However, we cannot exclude the possibility that uptake of the particulate form of glucan used in the experiment may consequently cause internalization of an adjacent receptor that is actually responsible for interacting with C. albicans.
The complement receptor 3 has been implicated in the macrophage adsorption of β-glucan (33). But, recently, a lectin-like receptor called dectin-1 was also identified as a β-glucan receptor on macrophages, and the receptor has been shown to bind and promote phagocytosis of C. albicans and zymosan (1). Further characterization of these molecules using genetic approaches should be helpful in determining which receptors are required for yeast phagocytosis by macrophages.
The function of PRRs goes beyond a mere recognition of a harmful foreign substance; upon encountering a pathogen, PRRs initiate a cascade of signals to induce immune responses. It was previously reported that blocking the MR with mannosylated BSA abolished IFN-γ-enhanced C. albicans uptake and killing in macrophages, suggesting that the MR may be involved in the cytokine-induced activation of phagocytic cells. However, the contradictory finding of decreased MR surface expression and endocytic activity upon IFN-γ treatment was also noted in the same study (16).
The recent finding that the MR is an important clearance receptor for endogenous glycoproteins released during inflammation (12) compounds the doubts on the receptor's role as a PRR. Uptake of endogenous glycoproteins should not result in arming the immune system for pathogen attack. Furthermore, MR expression is downregulated by inflammatory stimuli (2, 6, 25) and upregulated by immunosupressors (26). This pattern of expression is more compatible with the role of the MR as a cleanup receptor for acute-phase reactants in the postinflammatory setting than with a role as a PRR.
Host resistance to C. albicans infection is generally thought to be established by Th1, rather than Th2, response. Mouse infection experiments, in which either Th1 or Th2 cytokines were inactivated with monoclonal antibodies, first demonstrated that Th1-driven cell-mediated immunity conferred resistance and Th2-driven humoral response susceptibility (21-23). It was later shown that mice deficient in IFN-γ, a Th1 cytokine, but not those deficient in IL-4, a Th2 cytokine, showed increased susceptibility to disseminated C. albicans infection (10). In our infection experiment we observed that morbidity was associated with high IgM and IgG antibody titers, concurring with the previous results that humoral response is not critical to host defense against C. albicans during primary systemic infection.
Nevertheless, some results have shown that antibodies can increase resistance to C. albicans infection. Administration of antibodies against certain C. albicans antigens confers protection in naïve mice against primary infection (5, 18, 19). We believe that these seemingly contradictory sets of observations are not necessarily at odds with each other. In host defense against C. albicans, development of a Th1 response may be critical. However, the presence of C. albicans-specific antibodies may enhance this mechanism of defense by opsonizing the pathogen and thereby facilitating phagocytosis, an important part of the Th1 response. Th2-driven humoral response may be counterproductive only because it normally comes at the expense of initial Th1 induction, not because it leads to the production of antibodies that are useless for host resistance. When passively transferring antibodies, it is possible for the immune system to reap the benefits of these antibodies without compromising the development of cell-mediated immunity.
In summary, we conclude that the MR is not essential for host defense against C. albicans and that a receptor for β-glucan may be required for protection against C. albicans.
Acknowledgments
We are indebted to K. Mahnke and S. Greenberg for technical advice on phagocytosis experiments, C. Nathan for many helpful discussions and gifts of thioglycolate broth, T. Eisenreich for technical assistance with infection, and D. Verbel for statistical analysis.
This work was supported by National Institutes of Health Medical Scientist Training Program grant GM07739 (S.J.L.) and the National Institutes of Health and the Howard Hughes Institute (M.C.N.).
Editor: T. R. Kozel
REFERENCES
- 1.Brown, G. D., and S. Gordon. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36-37. [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz, R., M. Hill, and S. Gordon. 1986. Interferon alpha/beta selectively antagonises down-regulation of mannosyl-fucosyl receptors on activated macrophages by interferon gamma. Biochem. Biophys. Res. Commun. 136:737-744. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz, R. A., K. Sastry, P. Bailly, and A. Warner. 1990. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 172:1785-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg, S., F. Di Virgilio, T. H. Steinberg, and S. C. Silverstein. 1988. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J. Biol. Chem. 263:10337-10343. [PubMed] [Google Scholar]
- 5.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris, N., M. Super, M. Rits, G. Chang, and R. A. Ezekowitz. 1992. Characterization of the murine macrophage mannose receptor: demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood 80:2363-2373. [PubMed] [Google Scholar]
- 7.Ishikawa, T., A. C. Dalton, and C. E. Arbesman. 1972. Phagocytosis of Candida albicans by eosinophilic leukocytes. J. Allergy Clin. Immunol. 49:311-315. [DOI] [PubMed] [Google Scholar]
- 8.Janeway, C. A., Jr. 1992. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13:11-16. [DOI] [PubMed] [Google Scholar]
- 9.Janusz, M. J., K. F. Austen, and J. K. Czop. 1988. Phagocytosis of heat-killed blastospores of Candida albicans by human monocyte beta-glucan receptors. Immunology 65:181-185. [PMC free article] [PubMed] [Google Scholar]
- 10.Kaposzta, R., P. Tree, L. Marodi, and S. Gordon. 1998. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 66:1708-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karbassi, A., J. M. Becker, J. S. Foster, and R. N. Moore. 1987. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J. Immunol. 139:417-421. [PubMed] [Google Scholar]
- 12.Lee, S. J., S. Evers, D. Roeder, A. F. Parlow, J. Risteli, L. Risteli, Y. C. Lee, T. Feizi, H. Langen, and M. C. Nussenzweig. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295:1898-1901. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer, R. I., and M. J. Cline. 1969. Interaction of Candida albicans with human leukocytes and serum. J. Bacteriol. 98:996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linehan, S. A., L. Martinez-Pomares, P. D. Stahl, and S. Gordon. 1999. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 189:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marodi, L., H. M. Korchak, and R. B. Johnston, Jr. 1991. Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J. Immunol. 146:2783-2789. [PubMed] [Google Scholar]
- 16.Marodi, L., S. Schreiber, D. C. Anderson, R. P. MacDermott, H. M. Korchak, and R. B. Johnston, Jr. 1993. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Investig. 91:2596-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mencacci, A., E. Cenci, F. Bistoni, A. Bacci, G. Del Sero, C. Montagnoli, C. Fe d'Ostiani, and L. Romani. 1998. Specific and non-specific immunity to Candida albicans: a lesson from genetically modified animals. Res. Immunol. 149:352-361, 517-519. [DOI] [PubMed] [Google Scholar]
- 18.Mourad, S., and L. Friedman. 1968. Passive immunization of mice against Candida albicans. Sabouraudia 6:103-105. [PubMed] [Google Scholar]
- 19.Pearsall, N. N., B. L. Adams, and R. Bunni. 1978. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J. Immunol. 120:1176-1180. [PubMed] [Google Scholar]
- 20.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 21.Romani, L., E. Cenci, A. Mencacci, R. Spaccapelo, U. Grohmann, P. Puccetti, and F. Bistoni. 1992. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect. Immun. 60:4950-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romani, L., A. Mencacci, U. Grohmann, S. Mocci, P. Mosci, P. Puccetti, and F. Bistoni. 1992. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J. Exp. Med. 176:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani, L., P. Puccetti, A. Mencacci, E. Cenci, R. Spaccapelo, L. Tonnetti, U. Grohmann, and F. Bistoni. 1994. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J. Immunol. 152:3514-3521. [PubMed] [Google Scholar]
- 24.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd, V. L., R. Abdolrasulnia, M. Garrett, and H. B. Cowan. 1990. Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J. Immunol. 145:1530-1536. [PubMed] [Google Scholar]
- 26.Shepherd, V. L., M. G. Konish, and P. Stahl. 1985. Dexamethasone increases expression of mannose receptors and decreases extracellular lysosomal enzyme accumulation in macrophages. J. Biol. Chem. 260:160-164. [PubMed] [Google Scholar]
- 27.Shepherd, V. L., B. I. Tarnowski, and B. J. McLaughlin. 1991. Isolation and characterization of a mannose receptor from human pigment epithelium. Investig. Ophthalmol. Vis. Sci. 32:1779-1784. [PubMed] [Google Scholar]
- 28.Stahl, P. D., and R. A. Ezekowitz. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50-55. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, K., M. J. Donovan, R. A. Rogers, and R. A. Ezekowitz. 1998. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 292:311-323. [DOI] [PubMed] [Google Scholar]
- 30.Tarnowski, B. I., V. L. Shepherd, and B. J. McLaughlin. 1988. Expression of mannose receptors for pinocytosis and phagocytosis on rat retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 29:742-748. [PubMed] [Google Scholar]
- 31.Taylor, M. E., K. Bezouska, and K. Drickamer. 1992. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J. Biol. Chem. 267:1719-1726. [PubMed] [Google Scholar]
- 32.Taylor, M. E., and K. Drickamer. 1993. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J. Biol. Chem. 268:399-404. [PubMed] [Google Scholar]
- 33.Thornton, B. P., V. Vetvicka, M. Pitman, R. C. Goldman, and G. D. Ross. 1996. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156:1235-1246. [PubMed] [Google Scholar]
- 34.Wenzel, R. P., and M. A. Pfaller. 1991. Candida species: emerging hospital bloodstream pathogens. Infect. Control Hosp. Epidemiol. 12:523-524. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]