Abstract
Catch is characterized by maintenance of force with very low energy utilization in some invertebrate muscles. Catch is regulated by phosphorylation of the mini-titin, twitchin, and a catch component of force exists at all [Ca2+] except those resulting in maximum force. The mechanism responsible for catch force was characterized by determining how the effects of agents that inhibit the low to high force transition of the myosin cross-bridge (inorganic phosphate, butanedione monoxime, trifluoperazine, and blebbistatin) are modified by twitchin phosphorylation and [Ca2+]. In permeabilized anterior byssus retractor muscles from Mytilus edulis, catch force was identified as being sensitive to twitchin phosphorylation, whereas noncatch force was insensitive. In all cases, inhibition of the low to high force transition caused an increase in catch force. The same relationship exists between catch force and noncatch force whether force is varied by changes in [Ca2+] and/or agents that inhibit cross-bridge force production. This suggests that myosin in the high force state detaches catch force maintaining structures, whereas myosin in the low force state promotes their formation. It is unlikely that the catch structure is the myosin cross-bridge; rather, it appears that myosin interacts with the structure, most likely twitchin, and regulates its attachment and detachment.
INTRODUCTION
Catch is a mechanical state in muscle characterized by maintenance of force and resistance to stretch with very low energy utilization. Catch is observed in some invertebrate muscles and has historically been thought of as a very slowly decreasing force output after cessation of contractile activation. Under such conditions, there is an absence of force redevelopment after unloading of the muscle (1), and intracellular [Ca2+] has returned to near-basal concentrations (2) even though force is maintained. Catch force is relaxed by activation of serotonergic nerves (3), which results in an increase in [cAMP] (4) and activation of cAMP-dependent protein kinase (5). Twitchin is the target of phosphorylation, and as such, is the regulator of the catch state (6,7). Twitchin from the anterior byssus retractor muscle (ABRM) of Mytilus edulis is a mini-titin (∼530 kDa) consisting of 24 Ig, 15 Fn, and a single kinase domain (8). It is associated with thick filaments in catch muscles (9), and is dephosphorylated during activation of the muscle (6), most likely through the action of the calcium-dependent protein phosphatase 2B (10,11). As long as twitchin is dephosphorylated, there is maintenance of catch force when [Ca2+] is decreased below that necessary for activation of actin-activated myosin ATPase activity (12).
The identification of the central role that phosphorylation of twitchin plays in regulation of the catch state has allowed detection of a catch component of force output at calcium concentrations that support myosin cross-bridge cycling. It was found that phosphorylation of twitchin leads to a decrease in steady-state isometric force output at all [Ca2+] except those that result in maximum force (7). The lower the degree of activation of the muscle, the larger is the relative effect of twitchin phosphorylation on force. The decrease in force caused by phosphorylation of twitchin was not associated with a change in ATPase activity, suggesting that it did not result directly from cycling myosin cross-bridges (12). Rather, the force appeared to result from a structure that maintained force with little or no energy input, as is the case with catch force maintenance after cessation of activation.
For many years, there has been debate about the mechanism responsible for force maintenance in the catch state. The “linkage” hypothesis (for review, see Lowy and Millman (13)) is based on the idea that the same structures responsible for development of active force (myosin cross-bridges) are also responsible for catch force maintenance, presumably through regulation of the detachment rate of the cross-bridge from actin. The “independent” catch hypothesis ((14), and for a review, see Ruegg (15)) suggests that a structure other than myosin maintains catch force. That is, myosin cross-bridge cycling is responsible for force development and active force maintenance, but an independent catch structure maintains force when myosin cross-bridges detach during relaxation. The recent findings of a lack of effect of vanadate, phosphate, and 2,3-butanedione monoxime on catch force at pCa > 8 (16) and mechanical studies suggesting a lack of effect of twitchin phosphorylation on myosin head detachment (17) support such a model.
The fact that there is a catch component of the steady-state force output at suprabasal, but subsaturating [Ca2+], puts limitations on the characteristics of a possible myosin-independent catch force maintaining structure. The structure must participate in force development, and as such cannot be totally independent of the cycling myosin cross-bridge, as suggested by some models (18). Also, it has been shown that at intermediate [Ca2+], catch force can redevelop after a quick release (12). Thus, the structures responsible for catch force seem to be able to detach and reattach (cycle) during muscle shortening. Given evidence such as this, we have favored the view that myosin cross-bridges are the catch force maintaining structures (12,19).
To further characterize the mechanism responsible for catch force, we have determined how the effects of agents that inhibit the low to high force transition of the myosin cross-bridge are modified by twitchin phosphorylation and [Ca2+]. Force output was identified as catch force if it was sensitive to twitchin phosphorylation and noncatch force (i.e., from cycling cross-bridges) if it remained after twitchin was phosphorylated. We find that inhibition of the low to high force transition causes an increase in catch force. The same relationship exists between catch force and noncatch force no matter whether force is varied by changes in [Ca2+] and/or agents that inhibit cross-bridge force production. Thus, myosin in the high force state leads to detachment of catch structures, whereas myosin in the low force state promotes their formation. This makes it unlikely that the catch force maintaining structure is the myosin cross-bridge. Rather, myosin interacts with the structure, most likely twitchin, and regulates its attachment and detachment. The catch force structure seems to be a force-bearing link between thick and thin filaments that effectively extends the duty cycle of the cross-bridge by allowing a portion of the force developed by the cross-bridge to persist after cross-bridge detachment.
MATERIALS AND METHODS
Muscle preparation
M. edulis were obtained from Anastasi's Fish Market (Philadelphia, PA). Mussels were housed in an aquarium containing aerated filtered seawater (Instant Ocean, Carolina Biological Supply, Burlington, NC) at 5°C. On the day of the experiment, the shell was opened, the anterior byssus retractor muscle was exposed, and the pedal ganglia removed. Muscle bundles (0.2–0.3 mm in diameter and up to 1 cm in length) were mounted in holders and incubated in an aerated artificial seawater solution at 20°C until use. The artificial seawater contained KCl, 10 mM; MgCl2, 50 mM; CaCl2, 10 mM; NaCl, 428 mM; and N-[2-hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid], 10 mM at pH 7.4. The muscles were permeabilized by incubation in 1% Triton X-100 in rigor solution for 30 min and then rinsed in rigor solution before further experimental manipulation. All experiments were done at 20°C.
Solutions
Relaxing and activating solutions for permeabilized muscles
Relaxing solutions consisted of the following: 3 mM Mg ATP; 5 mM phosphocreatine; 20 mM EGTA; 3 mM free Mg2+; 0.5 mM leupeptin; 1 mM dithiothreitol; 30 mM piperazine-N,N'-bis[2-ethanesulfonic acid]; and 1 mg/ml, creatine phosphokinase. Ionic strength was maintained at 202 mM with 1,6-diaminohexane-N,N,N',N'-tetraacetic acid, and the pH was 6.8. A computer program provided by Dr. R. J. Barsotti (Thomas Jefferson University) was used to solve the multiple binding equilibria. The [Ca2+] of the relaxing solutions with no added calcium was considered to be pCa > 8. The activating solutions were similar to the relaxing solutions, with the exception that [Ca2+] was varied by adjusting the amount of CaEGTA, whereas the total EGTA was maintained at 20 mM. In experiments testing the effect of inorganic phosphate, [Pi] was varied from 0 to 25 mM. Solutions that did not contain inorganic phosphate included sucrose (10 mM) and sucrose phosphorylase (0.15 units/ml) as a Pi sink.
Other solutions
Rigor solution was similar to relaxing solution, except that it contained no ATP and phosphocreatine, and the EGTA concentration was 2 mM. cAMP was used at 100 μM. 2,3-butanedione monoxime (BDM) and trifluoperazine (TFP) were obtained from Sigma-Aldrich (St. Louis, MO) and ICN Biomedicals (Aurora, OH), respectively. A 20 mM stock solution of TFP was freshly prepared in water (pH adjusted to ∼6 with KOH) and diluted (1:100) in the muscle solution. (±)-Blebbistatin (Calbiochem, San Diego, CA) was dissolved in 90% DMSO, 10% water for stock solutions of 0.046 to 4.6 mM and diluted into muscle solutions. The highest DMSO content in the final muscle solution was 2.7%. When testing the effect of blebbistatin, an identical concentration of DMSO was used in solutions for control muscles.
Mechanical measurements
Muscle bundles of ∼5 mm in length were mounted on a myograph similar to that described previously (6,20). Isometric force output was measured with a DSC-6 transducer (Kistler Morse, Spartanburg, SC) and was recorded on both a strip chart recorder and a digital storage oscilloscope (Nicolet, Madison, WI).
ATPase activity
ATPase was measured in permeabilized muscles as the rate of 3H-ADP formation from 3H-ATP in the solution over a 10 min period. All solutions contained 1 mM MgATP (2 μCi/ml 3H-ATP) and 0.2 mM P1,P5-di(adenosine-5′) pentaphosphate in addition to the standard constituents. No phosphocreatine or creatine phosphokinase was present. For each muscle, aliquots of the bathing media were collected after a 10 min incubation at pCa > 8 and a subsequent 10 min incubation in pCa 5. This allowed determination of suprabasal ATPase. At the end of the procedure, the volume of the muscle was determined from the tritium content of the blotted muscle compared to a known volume of the incubating solution. Blebbistatin (25 μM) was included in both the pCa > 8 and pCa 5 solutions for the experimental muscles, but not for control muscles. 3H-ADP in the solution was separated from 3H-ATP by high-performance liquid chromatography and quantitated by liquid scintillation counting (21). Data are reported as micromoles of ADP formed per liter of muscle volume per second.
Statistics
Data are expressed as mean ± SE. Statistical comparisons were performed using the t-test or one-way ANOVA, and P < 0.05 was considered to be significant.
RESULTS
At calcium concentrations that result in maximum force, phosphorylation of twitchin has little effect on isometric force, whereas at lower calcium concentrations, the phosphorylation causes a significant decrease in force with no detectable change in ATPase activity (7,12). The twitchin phosphorylation-sensitive force that is not associated with an energy input is considered to be catch force, and the effects of agents that inhibit force production were tested at different [Ca2+] where there are different initial amounts of catch force.
Effect of inorganic phosphate
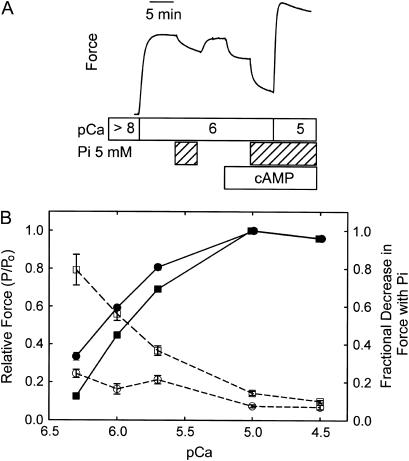
Fig. 1 A shows a typical force trace from a muscle subjected to a design in which the effect of twitchin phosphorylation on the decrease in force resulting from addition of inorganic phosphate (5 mM) was determined at pCa 6 where there is a significant decrease in force associated with twitchin phosphorylation. The addition of Pi caused a 15 ± 3% Po (referred to pCa 5) decrease in force when twitchin was unphosphorylated, and a significantly larger decrease (29 ± 1% Po) when twitchin was phosphorylated after addition of cAMP (n = 5). The fractional decrease in force caused by Pi (i.e., the change in force normalized to the force immediately before the addition of Pi) shows even a larger effect of twitchin phosphorylation (0.6 phosphorylated vs. 0.2 unphosphorylated). This is due to the fact that twitchin phosphorylation causes both a smaller developed force to begin with and a larger absolute change in force when Pi is added. The decrease in force resulting from twitchin phosphorylation was also larger when Pi was present (30 ± 2% Po vs. 16 ± 1% Po in the presence and absence of Pi, respectively). The results from similar designs at various calcium concentrations are summarized in Fig. 1 B. Also shown is the effect of twitchin phosphorylation on the relationship between force and calcium concentration in the absence of inorganic phosphate. When twitchin is unphosphorylated, inorganic phosphate caused a <10% fractional decrease in force at high [Ca2+], and the effect increased to ∼25% at pCa 6.3. There was a relatively small effect of twitchin phosphorylation on the fractional decrease in force with Pi addition at pCa 4.5 and 5.0, but, as [Ca2+] decreased, a much larger fraction of force was sensitive to Pi. At pCa 6.3, the phosphorylation of twitchin increased the fractional decrease in force with the addition of Pi from ∼0.25 to ∼0.80. Clearly, the phosphorylation state of twitchin plays an important role in modulating the response of force to inorganic phosphate at subsaturating calcium concentrations. Conversely, inorganic phosphate also increases the force susceptible to relaxation by twitchin phosphorylation.
FIGURE 1.
Effect of twitchin phosphorylation on the inorganic phosphate (Pi)-mediated decrease in force. (A) A typical force trace showing the effect of 5 mM inorganic phosphate on force at pCa 6 before and after the addition of cAMP and associated phosphorylation of twitchin. (B) Relative force and the fractional decrease in force with addition of inorganic phosphate as a function of [Ca2+] and twitchin phosphorylation. Relative force (minus inorganic phosphate), −cAMP (•), +cAMP (▪). Fractional decrease in force caused by addition of 5 mM inorganic phosphate, −cAMP (○), +cAMP (□). Mean ± SE, N = 5–11.
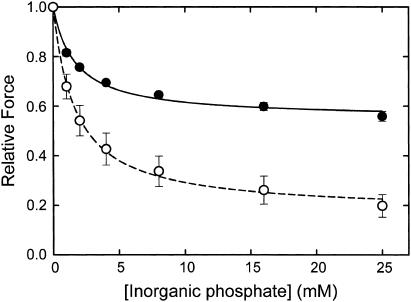
Fig. 2 shows the effect of twitchin phosphorylation on the relationship between relative force and inorganic phosphate concentration at pCa 6. At every concentration studied, the addition of phosphate caused a much larger decrease in force when twitchin was phosphorylated. In contrast, there is no significant effect of twitchin phosphorylation on the concentration of phosphate that causes the half-maximal decrease in force (1.7 ± 0.2 mM, twitchin unphosphorylated; 1.7 ± 0.4 mM, twitchin phosphorylated). These data suggest that the characteristics of phosphate binding are not changed by the state of phosphorylation of twitchin; rather, a much larger fraction of the relative force is susceptible to inhibition by phosphate when twitchin is phosphorylated.
FIGURE 2.
Effect of twitchin phosphorylation on the relationship between relative force and inorganic phosphate concentration at pCa 6. Force (relative to that in the absence of Pi) is shown as a function of [Pi] in the presence (○, dashed line) and absence (•, solid line) of cAMP. The lines show the best fits of the data to simple concentration-response relationships. EC50 and maximum decrease in force are 1.7 ± 0.4 mM and 0.83 ± .04, respectively, in the presence of cAMP and 1.7 ± 0.2 mM and 0.45 ± .01 in its absence. Data are mean ± SE, N = 4.
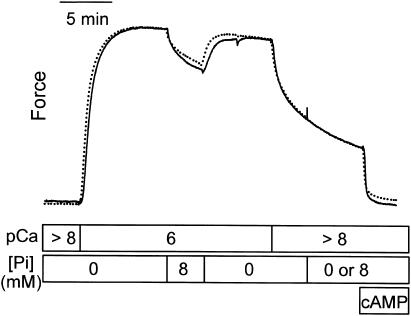
The design shown in Fig. 3 was used to test the effect of phosphate on catch force at pCa >8. When twitchin is unphosphorylated, addition of phosphate (8 mM) at pCa 6 causes the expected decrease in force, which is readily reversible upon removal of phosphate. When, in another muscle, 8 mM phosphate was also added during catch (pCa >8), there was no effect on force output (dotted line). These data show that catch force maintaining structures are not reversed to the low force state by phosphate binding.
FIGURE 3.
Lack of an effect of phosphate on catch force. Typical force traces showing the effect of 8 mM phosphate on force in pCa 6 and the absence of an effect of 8 mM phosphate on force output when the muscle is in catch at pCa > 8. The dotted line shows a muscle to which phosphate was added during catch, whereas the solid line shows the response of a muscle to which no phosphate was added during catch.
Effect of butanedione monoxime
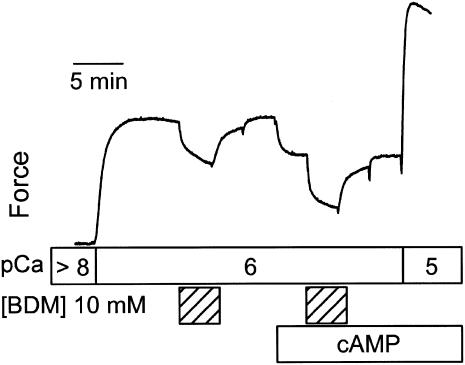
A typical force response to treatment with BDM at pCa 6 is shown in Fig. 4. BDM (10 mM) causes a significant decrease in force that is readily reversible, and which is greatly enhanced when the muscle is treated with cAMP and twitchin is phosphorylated. At pCa 6, the fractional decrease in force with BDM when twitchin is unphosphorylated is 0.22 ± 0.03, and when twitchin is phosphorylated is 0.49 ± 0.05 (N = 5). As is the case with inorganic phosphate, the same concentration of BDM has a much smaller effect on force at pCa 5, but there was still a significant effect of twitchin phosphorylation on the fractional decrease in force (0.044 ± 0.005, twitchin unphosphorylated; 0.069 ± 0.007, twitchin unphosphorylated; N = 5). It was also found that BDM had no effect on catch force at pCa > 8 (data not shown) as has been previously reported (16).
FIGURE 4.
Effect of butanedione monoxime and twitchin phosphorylation on force at pCa 6. A typical force trace shows the effect of 10 mM BDM on force before and after addition of cAMP and associated phosphorylation of twitchin.
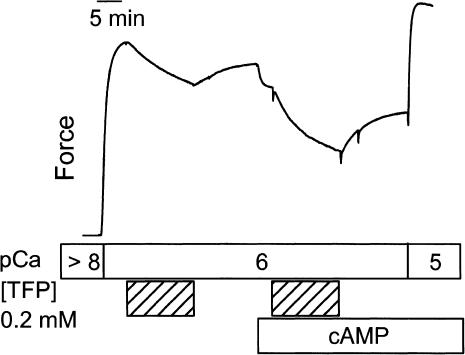
Effect of trifluoperazine
The phosphorylation state of twitchin also affects the extent to which TFP inhibits force production. Fig. 5 shows the effect of TFP at pCa 6. In such a design, TFP (0.2 mM) caused a decrease in force of 0.20 ± 0.02 and 0.26 ± 0.03 Po when twitchin was unphosphorylated and phosphorylated, respectively. When responses are compared in the same muscle, this is a 26 ± 5% (N = 6) larger change in force with TFP when twitchin was phosphorylated. When the change in force caused by TFP is normalized to the force immediately before addition of TFP, then the fractional decrease in force is 0.27 ± 0.03 when twitchin is unphosphorylated and 0.45 ± 0.04 (N = 6) when it is phosphorylated. As shown in Fig. 5, the effect of TFP was not fully reversible, and this could result in an underestimation of the modulation of the TFP effect by twitchin phosphorylation. Another experiment was performed to directly test the extent to which TFP modified the sensitivity of force to twitchin phosphorylation. When TFP was present for 15 min (5 min in pCa > 8, 10 min in pCa 6), the subsequent addition of cAMP and resulting phosphorylation of twitchin caused a fractional decrease in force of 0.42 ± 0.03 (N = 4) compared to 0.13 ± 0.01 (N = 4) in the absence of TFP. This large increase in the fraction of force that is sensitive to twitchin phosphorylation suggests that TFP increases the amount of catch force output at pCa 6.
FIGURE 5.
Effect of trifluoperizine and twitchin phosphorylation on force at pCa 6. A typical force trace shows the effect of 0.2 mM TFP on force before and after addition of cAMP and associated phosphorylation of twitchin.
Effect of blebbistatin
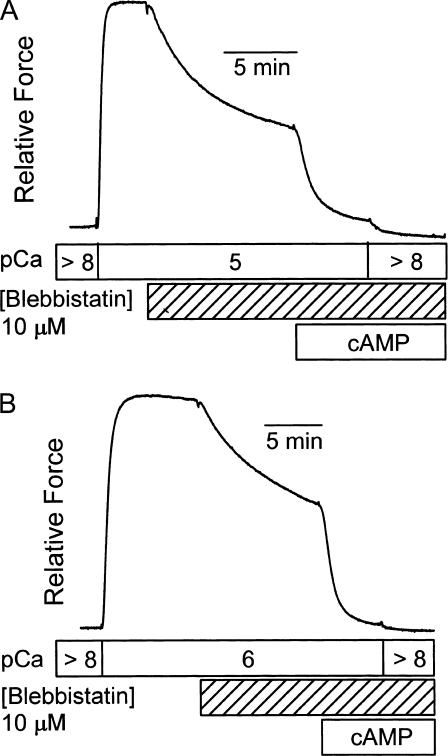
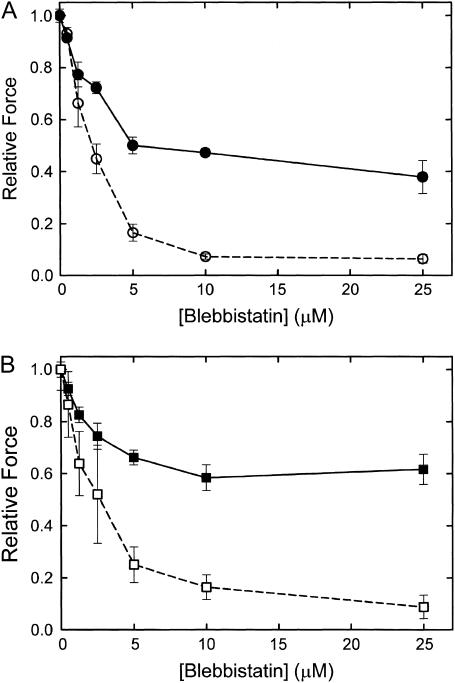
The ATPase activity resulting from an increase in [Ca2+] from pCa > 8 to pCa 5 is 26 ± 6 μM/s (N = 4), and it is totally inhibited (0 ± 1 μM/s, N = 4) in the presence of 25 μM blebbistatin. This confirms that blebbistatin inhibits actin-activated myosin ATPase in this catch muscle. The effect of blebbistatin on force output and on the sensitivity of force to twitchin phosphorylation is illustrated in Fig. 6. At pCa 5 (Fig. 6 A), blebbistatin (10 μM) causes a 50–60% decrease in force and almost all of the remaining force is removed with phosphorylation of twitchin. A subsequent decrease in [Ca2+] causes only a slight further decrease in force. The appearance of a large effect of twitchin phosphorylation on force at pCa 5 is quite surprising and unique to blebbistatin treatment. The effect of blebbistatin at pCa 6 (Fig. 6 B) is similar except that the extent of inhibition of force is somewhat less. Treatment with cAMP and the associated phosphorylation of twitchin also results in complete loss of force at this lower [Ca2+]. Similar types of experiments were performed to determine how inhibition of force depends on blebbistatin concentration and the state of phosphorylation of twitchin. The results are shown in Fig. 7. At both pCa 5 and pCa 6, blebbistatin almost totally inhibits force when twitchin is phosphorylated. The concentration of blebbistatin that causes 50% inhibition of force is ∼2.5 μM. When twitchin is unphosphorylated, there is only partial inhibition of force at high blebbistatin concentrations. Even at 100 μM blebbistatin, there is still significant force output that is relaxed when twitchin is phosphorylated (data not shown). There is no effect of Pi (25 mM) on the force remaining in the presence of blebbistatin (25 μM) at both pCa 6 and pCa 5 (data not shown). The fact that force output at pCa 5 in the presence of blebbistatin occurs with little or no myosin ATPase activity, is relaxed with twitchin phosphorylation, and is insensitive to Pi strongly suggests that it is catch force.
FIGURE 6.
Effect of blebbistatin on force output and on the sensitivity of force to twitchin phosphorylation. Panel A shows the effect of addition of blebbistatin (10 μM) at pCa 5 as well as the subsequent phosphorylation of twitchin by addition of cAMP. Note that the addition of cAMP decreased force in pCa 5 almost to that present in pCa > 8 plus cAMP. Panel B shows a force trace from a similar experiment at pCa 6.
FIGURE 7.
Relationship between force and blebbistatin concentration in the presence and absence of twitchin phosphorylation. The experimental design was similar to that shown in Fig. 6. (A) pCa 5; (B) pCa 6. (Solid symbols, solid lines) −cAMP; (open symbols, dashed lines) +cAMP. Mean ± SE, N = 3–7.
Relationship between force and the effect of twitchin phosphorylation
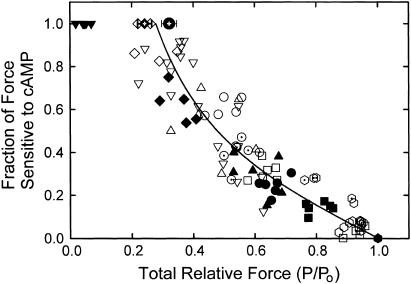
Each of the inhibitors of force output described above causes an increase in the fraction of force that is relaxed when twitchin is phosphorylated. This finding suggests that a decrease in force output may be inherently associated with an increase in the fraction of force that is sensitive to removal with twitchin phosphorylation. If this were the case, then it may be that force output determines sensitivity to twitchin phosphorylation and the amount of catch force present rather than other factors such as [Ca2+], etc. To investigate this possibility, the fraction of force sensitive to removal by twitchin phosphorylation was plotted as a function of the total force (relative to maximum force obtained at pCa 5). The results are shown in Fig. 8, and include data from all of the inhibitors described above at various [Ca2+], as well as controls. Each data point represents one muscle except for averages from experiments in which the catch force remaining was determined 10 and 20 min after transfer of a muscle from pCa 6 to pCa > 8. The results show that when force output is near maximum, there is little, if any, decrease in force resulting from phosphorylation of twitchin. At lower forces, the fractional change with twitchin phosphorylation increases. At a given force, the sensitivity to phosphorylation is similar no matter the calcium concentration or the presence or absence of an inhibitor. For example, the amount of force at pCa 5 with 10–25 μM blebbistatin is similar to that at pCa 6.3 in the absence of phosphorylation, and the decrease in force resulting from phosphorylation of twitchin is also similar. The catch force that can be maintained after 10–20 min at pCa > 8 is ∼0.25–0.35 Po, and all of this force is removed as a result of twitchin phosphorylation. These data from muscles in catch at pCa > 8 are consistent with the relationship between cAMP-sensitive force and total force under the other conditions shown, and demonstrate that almost all force is sensitive to twitchin phosphorylation when total force is ∼0.3 Po or lower.
FIGURE 8.
Relationship between the fractional decrease in force caused by phosphorylation of twitchin and the total force output (P/Po) before twitchin phosphorylation. Data for individual muscles are shown as the following: pCa 4.5, + Pi (⊡); pCa 5, control ( ), Pi 5 mM (
), Pi 5 mM ( ), Pi 25 mM (
), Pi 25 mM ( ), blebbistatin 1.25–25 μM (∇); pCa 5.7, control (▪), Pi 5 mM (□); pCa 6, control (•, ▴), Pi 5 mM (○), BDM 10 mM (▵), TFP 200 μM (⊙); pCa 6.3 (♦), Pi 5 mM (⋄); pCa 7, (▾). Mean ± SE (N = 16) for catch force remaining 10 min (
), blebbistatin 1.25–25 μM (∇); pCa 5.7, control (▪), Pi 5 mM (□); pCa 6, control (•, ▴), Pi 5 mM (○), BDM 10 mM (▵), TFP 200 μM (⊙); pCa 6.3 (♦), Pi 5 mM (⋄); pCa 7, (▾). Mean ± SE (N = 16) for catch force remaining 10 min ( ) and 20 min (
) and 20 min ( ) after switch from pCa 6 to pCa > 8. The line is a least-squares quadratic fit to the data.
) after switch from pCa 6 to pCa > 8. The line is a least-squares quadratic fit to the data.
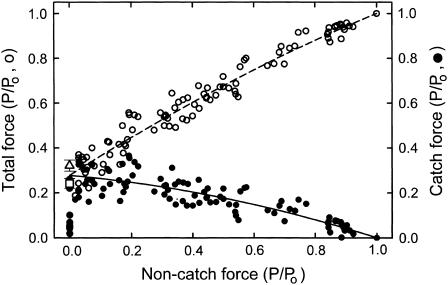
Total force under conditions when twitchin is unphosphorylated is the sum of catch force (the decrease in force resulting from cAMP treatment) and noncatch force (that which remains after cAMP treatment). Noncatch force most likely results from calcium activated myosin cross-bridge cycling. Inasmuch as it has been shown that there is no detectable change in ATPase activity associated with the phosphorylation of twitchin (12), it is likely that cross-bridge cycling and associated noncatch force output are independent of the phosphorylation state of twitchin. Fig. 9 shows how catch force and total force in the absence of twitchin phosphorylation depend on the noncatch or cycling cross-bridge-mediated force. As noncatch force decreases, catch force increases almost linearly to a maximum of ∼0.3 Po. Total force decreases as noncatch force decreases and, by definition, shows the same intercept as catch force when noncatch force is zero. These results suggest that a decrease in force output from cycling cross-bridges, whether by a decrease in calcium concentration or by a decrease in force output by an inhibitor of the low to high force cross-bridge transition, is associated with an increase in catch force.
FIGURE 9.
Dependence of total force and catch force on noncatch force under various conditions. The data are derived from the same experiments shown in Fig. 8. Noncatch force is that remaining after addition of cAMP, whereas catch force (•, solid line) is the change in force resulting from addition of cAMP. Total force (○, dashed line) is the force before cAMP addition. Also shown are the mean ± SE for catch force remaining 10 min (▵) and 20 min (□) after switch from pCa 6 to pCa > 8. The lines are least-squares quadratic fits to the data.
DISCUSSION
The results show that inorganic phosphate causes a decrease in Ca2+-activated isometric force output as described in previous studies on catch muscle (16,22) as well as on a variety of other muscle types (23–27). The lack of an effect of Pi on catch force at very low [Ca2+] (16) was also confirmed. It was, however, surprising that the degree to which Pi inhibited force depended on the state of phosphorylation of twitchin.
Several studies (for a review, see Takagi et al. (28)) suggest that the mechanism of force generation by myosin is as shown in Scheme 1:
Figure .
It involves an isomerization (reaction 1) of the AM.ADP.Pi low force state to an AM′.ADP.Pi high force state followed by the release of inorganic phosphate (reaction 2). An increase in [Pi] results in reversal of reaction 2, an increase in AM′.ADP.Pi, and a subsequent reversal of reaction 1 leading to a decrease in force with an increase in the population of low force AM.ADP.Pi. The fact that inorganic phosphate does not affect catch force at pCa >8 suggests that it acts only on the calcium-bound cycling cross-bridges and not on the structures responsible for catch force maintenance. Inasmuch as the phosphorylation state of twitchin does not appear to control cycling cross-bridges (12), one might expect the change in force caused by inorganic phosphate at intermediate [Ca2+] to be independent of twitchin phosphorylation. This, however, is not the case. At pCa 6, there is almost a two-fold larger decrease in force in response to 5 mM Pi when twitchin is phosphorylated. Even though the magnitude of the response depends on the state of phosphorylation of twitchin, the [Pi] that causes the half-maximal decrease in force does not.
How do the structures responsible for catch force maintenance play a role in determining the change in force resulting from an increase in [Pi]? Simply stated, when twitchin is unphosphorylated, inorganic phosphate causes a decrease in total force, but also causes an increase in catch force. As a result, there is a smaller effect of Pi when twitchin is unphosphorylated compared to when twitchin is phosphorylated and catch force cannot increase.
This scenario is illustrated by the results obtained for 5 mM Pi at pCa 6. When twitchin is phosphorylated, Pi causes a decrease in force of 0.29 Po (0.50 → 0.21 Po). In this case, the decrease in force reflects the effect of Pi on only cycling cross-bridges, because catch force is not present when twitchin is phosphorylated. When twitchin is unphosphorylated, the same addition of Pi causes a change of just 0.15 Po (0.66 → 0.51). Here, the change in total force is the sum of the change in force from cycling cross-bridges plus any change in catch force. If the characteristics of the cycling cross-bridges are independent of the state of phosphorylation of twitchin, it follows that the change in force from cycling cross-bridges caused by Pi is also independent of twitchin phosphorylation. The Pi-induced change in force from cycling cross-bridges would thus be 0.29 Po when twitchin is unphosphorylated (i.e., the same as that when twitchin is phosphorylated), whereas the change in total force is only 0.15 Po. This means that Pi caused an increase in catch force of 0.14 Po, and it is to this extent that there is a larger effect of twitchin phosphorylation on force in the presence compared to the absence of Pi.
The relationship among these parameters is shown in Fig. 9. When twitchin is unphosphorylated, a decrease in noncatch force (from cycling myosin cross-bridges) is not matched by an equal decrease in total force because catch force increases. This suggests that the fraction of cross-bridges in the strong binding, high force state is an important regulator of the structure responsible for catch force maintenance. The higher the number of cycling cross-bridges in the high force state, the lower the catch force.
According to the analysis given above, the large Pi-induced fractional decrease in force when twitchin is phosphorylated (as shown in Fig. 1 B) at low [Ca2+] results from two factors. The first is that the full effect of inorganic phosphate on cycling myosin cross-bridges is seen when twitchin is phosphorylated, and the second is that total force output is decreased when twitchin is phosphorylated by removal of catch force maintaining structures that show no sensitivity to inorganic phosphate. At pCa 6.3, these factors combine to increase the fractional decrease in force with 5 mM Pi from ∼0.25 when twitchin is unphosphorylated to ∼0.80 when twitchin is phosphorylated.
BDM is a noncompetitive inhibitor of myosin ATPase activity and force output in permeabilized muscles (29–31). It is thought to act by reducing the fraction of myosin in the strong binding, high force state and increasing the fraction in the weak binding, low force state. Studies on permeabilized soleus muscle support the idea that the BDM-induced decrease in force results from a lowering of the equilibrium constant of the force producing isomerization shown as reaction 1 in Scheme 1 (32), most likely by decreasing the forward rate constant. The effect of BDM on force output in catch muscle is strikingly similar to inorganic phosphate. There is a much larger effect of BDM at pCa 6 when twitchin is phosphorylated, and conversely there is a larger effect of twitchin phosphorylation in the presence compared to the absence of BDM. As is the case for Pi, it seems that a BDM-induced decrease in the fraction of cycling cross-bridges in the strong binding, high force conformation results in an increase in catch force.
TFP causes an inhibition of actin-activated ATPase activity of myosin from scallop striated adductor muscle (33) and other smooth and striated muscles (34). The inhibition occurs at a TFP concentration (0.2 mM) that is an order of magnitude lower than that causing removal of light chains from myosin. The lack of a dramatic effect of inhibition of a small fraction of myosin molecules on movement of actin filaments in in vitro motility assays suggests that TFP inhibits the transition of cross-bridges from the weak to strong binding states (34). That is, TFP locks myosin in the weak binding, low force state. The inhibition of force output by TFP (0.2 mM) at pCa 6 in catch muscle was significantly greater when twitchin was phosphorylated. In addition, the decrease in force resulting from phosphorylation of twitchin is larger in the presence of TFP. Inhibition of force output by TFP thus seems to result in an increase in catch force if twitchin is unphosphorylated. The similarities of the effects of twitchin phosphorylation on TFP inhibition of force with those of Pi and BDM are striking.
Blebbistatin is an inhibitor of the actin-activated ATPase activities of several vertebrate and invertebrate striated muscle myosins as well as vertebrate nonmuscle myosin IIA and IIB (35,36). The IC50 for inhibition is between 0.5 and 5 μM. Turkey gizzard smooth muscle myosin is much less susceptible to inhibition by blebbistatin (IC50 ∼ 80 μM). Blebbistatin blocks myosin in a state with ADP and phosphate bound and with low actin affinity (37). That is, it is thought to inhibit the isomerization of myosin into force-producing states. Blebbistatin has been shown to bind to myosin at the 50 kDa cleft near the γ-phosphate-binding pocket (38), and this structure is consistent with it stabilizing the low force state that precedes the force generating step. Blebbistatin is an effective inhibitor of myosin ATPase in activated catch muscle. When twitchin is phosphorylated, blebbistatin is also a potent inhibitor of force output at pCa 5 with an IC50 of ∼2.5 μM, which is very similar to the reported IC50 of 2.3 μM for scallop striated muscle actin-activated myosin ATPase activity (36). Interestingly, when twitchin is unphosphorylated, there is only partial inhibition of force, although myosin ATPase is totally inhibited. The force that is resistant to inhibition with blebbistatin is relaxed by phosphorylation of twitchin and is not affected by Pi. These characteristics clearly identify the force output at pCa 5 in presence of high concentrations of blebbistatin as catch force. Because there is normally little or no catch force apparent at pCa 5 (i.e., no effect of twitchin phosphorylation on force), blebbistatin treatment leads to a substantial increase in catch force.
Implications for the mechanism of catch force maintenance and its regulation
Even though the mechanisms of action of the inhibitors of force output used in this study are likely to be very different, all seem to act by inhibiting the transition of myosin into a force generating state. In doing so, all of these agents increase catch force maintenance by the muscle. Indeed, there seems to be an invariant relationship between the force output from cycling cross-bridges (noncatch force) and the amount of catch force. The inverse relationship between catch force and noncatch force suggests that cycling myosin cross-bridges in the high force state lead to detachment of the catch force maintaining structure.
We have proposed a model in which catch force results from myosin cross-bridges that exhibit a very slow rate of ADP release resulting from unbinding of activating Ca2+ from the cross-bridge while it is in the high force state (12,19). Phosphorylation of twitchin was proposed to relax force by allowing ADP release and subsequent detachment of the calcium-free cross-bridges. It has been suggested that the observation that Pi does not affect catch force argues against such an ADP-bound cross-bridge as the catch force maintaining structure (16). But, as noted earlier (22), it is possible that the reversal of reactions 1 and/or 2 in Scheme 1 also could be inhibited when the calcium-free cross-bridge is in the high force state. If this were the case, the catch cross-bridge would essentially be trapped in the high force conformation. Although such a model is consistent with the effects of the inhibitors at pCa >8 when there are no cycling cross-bridges, it does not provide a simple explanation of the increase in catch force that results from a decrease in force output from calcium-bound cycling cross-bridges. At every [Ca2+] that supports cross-bridge cycling, inhibition of the low force to high force transition was found to increase catch force. The limitation of such a model is immediately apparent given the effects of blebbistatin at pCa 5, a nearly saturating [Ca2+] for force output. Under such conditions, a blebbistatin-mediated inhibition of the transition into the high force state should result in a total inhibition of force, because the myosin cross-bridge with calcium bound would have a high rate of ADP release and subsequent detachment from actin. That is, catch cross-bridges should not build up because there are no calcium-free cross-bridges. This limitation of the model can also be extended to include all of the above described inhibitors at all suprabasal [Ca2+]. As long as the fraction of myosin that has calcium bound does not change, the model predicts that there would be less catch force, not more, if there is an increase in myosin in the low force conformation. Although there is some evidence that TFP decreases calcium binding to scallop myosin (33), the effect is small compared to the extent of inhibition of myosin ATPase. Also, it is very unlikely that Pi, BDM, and blebbistatin all inhibit calcium binding given the similarities of the effects of these agents on many different types of myosin, most of which are not regulated by calcium binding directly to myosin.
The results suggest that myosin in the high force state leads to detachment of catch force maintaining structures, whereas myosin in the low force state promotes formation of such structures. Therefore, it is unlikely that the catch force maintaining structure is the myosin cross-bridge. Others have also recently questioned whether myosin is the link responsible for catch (16,17). But we have previously shown that the catch force maintaining structure must readjust upon muscle shortening such that catch force is redeveloped at a shorter muscle length (12). The detachment and reattachment of the structure during shortening may be driven by myosin cross-bridge cycling, which would include the transition of the myosin cross-bridge into and out of the high force state, which, as described above, tends to decrease and increase, respectively, the amount of catch force. So, rather than being the catch force maintaining structure, it is possible that myosin interacts with it and regulates its attachment and detachment. It is also possible that rather than interacting with myosin, the structure may somehow be controlled by structural changes in the thin filament resulting from force production by the myosin cross-bridge.
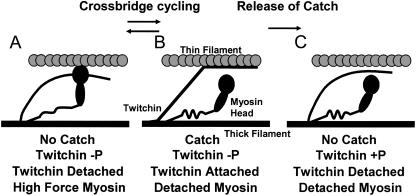
Twitchin is an obvious candidate for the structure responsible for catch force maintenance because its phosphorylation state controls catch force. In addition, it is located on the thick filament (9); has a putative actin-binding motif in the portion of the molecule including one of the regulatory phosphorylation sites (8); interacts with the thin filament in a phosphorylation dependent manner (39); and is sufficient in combination with actin and myosin to cause catch-like behavior in an in vitro system (40). Fig. 10 shows a cartoon of a possible mechanism by which twitchin and myosin may interact to result in catch force. In the catch state (B), the cross-bridge is in the low force or detached state, but force is maintained by dephosphorylated twitchin, which provides a link between the thick and thin filaments. When the muscle is activated, myosin enters the high force state (A) and displaces twitchin as a link between the filaments. Cross-bridge cycling involves the interconversion between states A and B with twitchin attachment and detachment alternating between low and high force myosin cross-bridge states, respectively. Phosphorylation of twitchin causes detachment (B to C) of the twitchin link when myosin is in the low force state. This releases catch force.
FIGURE 10.
Cartoon showing a possible mechanism of how actin, myosin, and twitchin interact to give rise to catch force output. See text for details.
In such a model, inactivation of the muscle either by a decrease in [Ca2+] or by inhibition of the low force to high force transition causes the amount of myosin in state (B) to increase, resulting in an increase in catch force as long as twitchin is dephosphorylated. This mechanism allows continued maintenance of a portion of the force produced by the cross-bridge in the transition to the high force state when the cross-bridge is subsequently detached. From the data shown in Fig. 9, the maximum force that can be maintained by the catch link between thick and thin filaments is ∼0.3 Po. Inasmuch as there is only one twitchin molecule present for every 14 double-headed myosins, (6) it is not likely that every myosin interacts with a twitchin molecule. On the other hand, more than one myosin molecule could cause the detachment of a single twitchin molecule. So, at high levels of activation when there is a significant fraction of cross-bridges in the high force state, all twitchin molecules could be detached. This would result in no effect of twitchin phosphorylation at very high force output as found experimentally.
In summary, inhibition of the low to high force transition of the myosin cross-bridge causes an increase in catch force. The same relationship exists between catch force and noncatch force no matter whether force is varied by changes in [Ca2+] and/or agents that inhibit cross-bridge force production. This suggests that myosin in the high force state detaches catch force maintaining structures, whereas myosin in the low force state promotes their formation. It is unlikely that the catch structure is the myosin cross-bridge; rather, it appears that myosin interacts with the structure, which may be twitchin, and regulates its attachment and detachment.
Acknowledgments
This work was supported by National Institutes of Health grant AR042758.
References
- 1.Jewell, B. R. 1959. The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J. Physiol. 149:154–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishii, N., A. W. M. Simpson, and C. C. Ashley. 1989. Free calcium at rest during “catch” in single smooth muscle cells. Science. 243:1367–1368. [DOI] [PubMed] [Google Scholar]
- 3.Twarog, B. M. 1954. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J. Cell. Physiol. 44:141–163. [DOI] [PubMed] [Google Scholar]
- 4.Cole, R. A., and B. M. Twarog. 1972. Relaxation of catch in a molluscan smooth muscle. I. Effects of drugs which act on the adenyl cyclase system. Comp. Biochem. Physiol. 43:321–330. [DOI] [PubMed] [Google Scholar]
- 5.Castellani, L., and C. Cohen. 1987. Myosin rod phosphorylation and the catch state of molluscan muscles. Science. 235:334–337. [DOI] [PubMed] [Google Scholar]
- 6.Siegman, M. J., S. U. Mooers, C. Li, S. Narayan, L. Trinkle-Mulcahy, S. Watabe, D. J. Hartshorne, and T. M. Butler. 1997. Phosphorylation of a high molecular weight (approximately 600 kDa) protein regulates catch in invertebrate smooth muscle. J. Muscle Res. Cell Motil. 18:655–670. [DOI] [PubMed] [Google Scholar]
- 7.Siegman, M. J., D. Funabara, S. Kinoshita, S. Watabe, D. J. Hartshorne, and T. M. Butler. 1998. Phosphorylation of a twitchin-related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc. Natl. Acad. Sci. USA. 95:5383–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funabara, D., S. Watabe, S. U. Mooers, S. Narayan, C. Dudas, D. J. Hartshorne, M. J. Siegman, and T. M. Butler. 2003. Twitchin from molluscan catch muscle: primary structure and relationship between site-specific phosphorylation and mechanical function. J. Biol. Chem. 278:29308–29316. [DOI] [PubMed] [Google Scholar]
- 9.Vibert, P., S. M. Edelstein, L. Castellani, and B. W. Elliott. 1993. Mini-titins in striated and smooth molluscan muscles: structure, location and immunological crossreactivity. J. Muscle Res. Cell Motil. 14:598–607. [DOI] [PubMed] [Google Scholar]
- 10.Castellani, L., and C. Cohen. 1992. A calcineurin-like phosphatase is required for catch contraction. FEBS Lett. 309:321–326. [DOI] [PubMed] [Google Scholar]
- 11.Yamada, A., M. Yoshio, A. Nakamura, K. Kohama, and K. Oiwa. 2004. Protein phosphatase 2B dephosphorylates twitchin, initiating the catch state of invertebrate smooth muscle. J. Biol. Chem. 279:40762–40768. [DOI] [PubMed] [Google Scholar]
- 12.Butler, T. M., S. U. Mooers, C. Li, S. Narayan, and M. J. Siegman. 1998. Regulation of catch muscle by twitchin phosphorylation: effects on force, ATPase, and shortening. Biophys. J. 75:1904–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowy, J., and B. M. Millman. 1963. The contractile mechanism of the anterior byssus retractor of Mytilus edulis. Proc. R. Soc. Lond. B Biol. Sci. 246:105–148. [Google Scholar]
- 14.Ruegg, J. C. 1961. On the tropomyosin-paramyosin system in relation to the viscous tone of lammellibranch “catch” muscle. Proc. R. Soc. Lond. B Biol. Sci. 154:224–249. [Google Scholar]
- 15.Ruegg, J. C. 1971. Smooth muscle tone. Physiol. Rev. 51:201–248. [DOI] [PubMed] [Google Scholar]
- 16.Galler, S., M. C. Hopflinger, O. Andruchov, O. Andruchova, and H. Grassberger. 2005. Effects of vanadate, phosphate and 2,3-butanedione monoxime (BDM) on skinned molluscan catch muscle. Pflugers Arch. 449:372–383. [DOI] [PubMed] [Google Scholar]
- 17.Andruchova, O., M. C. Hopflinger, O. Andruchov, and S. Galler. 2005. No effect of twitchin phosphorylation on the rate of myosin head detachment in molluscan catch muscle: are myosin heads involved in the catch state? Pflugers Arch. 450:326–334. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, W. H. 1962. Tonic mechanisms in smooth muscles. Physiol. Rev. 42 Supp.5:113–159. [PubMed] [Google Scholar]
- 19.Butler, T. M., S. R. Narayan, S. U. Mooers, D. J. Hartshorne, and M. J. Siegman. 2001. The myosin cross-bridge cycle and its control by twitchin phosphorylation in catch muscle. Biophys. J. 80:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegman, M. J., T. M. Butler, S. U. Mooers, and A. Michalek. 1984. Ca2+ can affect Vmax without changes in myosin light chain phosphorylation in smooth muscle. Pflugers Arch. 401:385–390. [DOI] [PubMed] [Google Scholar]
- 21.Vyas, T. B., S. U. Mooers, S. R. Narayan, M. J. Siegman, and T. M. Butler. 1994. Cross-bridge cycling at rest and during activation: turnover of myosin-bound ADP in permeabilized smooth muscle. J. Biol. Chem. 269:7316–7322. [PubMed] [Google Scholar]
- 22.Butler, T. M., S. U. Mooers, and M. J. Siegman. 2003. Phosphorylation of twitchin modulates the effect of inorganic phosphate on force in catch muscle. Biophys. J. 84:103a (Abstr.) [Google Scholar]
- 23.Brandt, P. W., R. N. Cox, M. Kawai, and T. Robinson. 1982. Regulation of tension in skinned muscle fibers. Effect of crossbridge kinetics on apparent Ca2+ sensitivity. J. Gen. Physiol. 79:991–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke, R., and E. Pate. 1985. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys. J. 48:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kentish, J. C. 1986. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J. Physiol. 370:585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebus, J. P., G. J. Stienen, and G. Elzinga. 1994. Influence of phosphate and pH on myofibrillar ATPase activity and force in skinned cardiac trabeculae from rat. J. Physiol. 476:501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osterman, A., and A. Arner. 1995. Effects of inorganic phosphate on cross-bridge kinetics at different activation levels in skinned guinea-pig smooth muscle. J. Physiol. 484:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takagi, Y., H. Shuman, and Y. E. Goldman. 2004. Coupling between phosphate release and force generation in muscle actomyosin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359:1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higuchi, H., and S. Takemori. 1989. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J. Biochem. (Tokyo). 105:638–643. [DOI] [PubMed] [Google Scholar]
- 30.McKillop, D. F. A., N. S. Fortune, K. W. Ranatunga, and M. A. Geeves. 1994. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. J. Muscle Res. Cell Motil. 15:309–318. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann, C., J. Wray, F. Travers, and T. Barman. 1992. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 31:12227–12232. [DOI] [PubMed] [Google Scholar]
- 32.Tesi, C., F. Colomo, N. Piroddi, and C. Poggesi. 2002. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J. Physiol. 541:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel, H., S. S. Margossian, and P. D. Chantler. 2000. Locking regulatory myosin in the off-state with trifluoperazine. J. Biol. Chem. 275:4880–4888. [DOI] [PubMed] [Google Scholar]
- 34.Sellers, J. R., F. Wang, and P. D. Chantler. 2003. Trifluoperazine inhibits the MgATPase activity and in vitro motility of conventional and unconventional myosins. J. Muscle Res. Cell Motil. 24:579–585. [DOI] [PubMed] [Google Scholar]
- 35.Straight, A. F., A. Cheung, J. Limouze, I. Chen, N. J. Westwood, J. R. Sellers, and T. J. Mitchison. 2003. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 299:1743–1747. [DOI] [PubMed] [Google Scholar]
- 36.Limouze, J., A. F. Straight, T. Mitchison, and J. R. Sellers. 2004. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25:337–341. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs, M., J. Toth, C. Hetenyi, A. Malnasi-Csizmadia, and J. R. Sellers. 2004. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279:35557–35563. [DOI] [PubMed] [Google Scholar]
- 38.Allingham, J. S., R. Smith, and I. Rayment. 2005. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 12:378–379. [DOI] [PubMed] [Google Scholar]
- 39.Shelud'ko, N. S., G. G. Matusovskaya, T. V. Permyakova, and O. S. Matusovsky. 2004. Twitchin, a thick-filament protein from molluscan catch muscle, interacts with F-actin in a phosphorylation-dependent way. Arch. Biochem. Biophys. 432:269–277. [DOI] [PubMed] [Google Scholar]
- 40.Yamada, A., M. Yoshio, H. Kojima, and K. Oiwa. 2001. An in vitro assay reveals essential protein components for the “catch” state of invertebrate smooth muscle. Proc. Natl. Acad. Sci. USA. 98:6635–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]