Abstract
Hydrogen bonds are important in determining the structure and function of biomolecules. Of particular interest are hydrogen bonds to quinones, which play an important role in the bioenergetics of respiration and photosynthesis. In this work we investigated the hydrogen bonds to the two carbonyl oxygens of the semiquinone 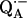 in the well-characterized reaction center from the photosynthetic bacterium Rhodobacter sphaeroides R-26. We used electron paramagnetic resonance and electron nuclear double resonance techniques at 35 GHz at a temperature of 80 K. The goal of this study was to identify and assign sets of 1H-ENDOR lines to protons hydrogen bonded to each of the two oxygens. This was accomplished by preferentially exchanging the hydrogen bond on one of the oxygens with deuterium while concomitantly monitoring the changes in the amplitudes of the 1H-ENDOR lines. The preferential deuteration of one of the oxygens was made possible by the different 1H → 2H exchange times of the protons bonded to the two oxygens. The assignment of the 1H-ENDOR lines sets the stage for the determination of the geometries of the H-bonds by a detailed field selection ENDOR study to be presented in a future article.
in the well-characterized reaction center from the photosynthetic bacterium Rhodobacter sphaeroides R-26. We used electron paramagnetic resonance and electron nuclear double resonance techniques at 35 GHz at a temperature of 80 K. The goal of this study was to identify and assign sets of 1H-ENDOR lines to protons hydrogen bonded to each of the two oxygens. This was accomplished by preferentially exchanging the hydrogen bond on one of the oxygens with deuterium while concomitantly monitoring the changes in the amplitudes of the 1H-ENDOR lines. The preferential deuteration of one of the oxygens was made possible by the different 1H → 2H exchange times of the protons bonded to the two oxygens. The assignment of the 1H-ENDOR lines sets the stage for the determination of the geometries of the H-bonds by a detailed field selection ENDOR study to be presented in a future article.
INTRODUCTION
Quinones play an important role as cofactors in many proteins (1,2). Their biological function is related to their electrochemical properties, which are influenced by their local protein environment. Quinones can be reduced in two successive reversible one-electron steps. The first step leads to an intermediate, a semiquinone (quinone anion radical); the second step, followed by protonation, leads to the fully reduced quinol (3,4). These properties make quinones ideally suited as electron and proton carriers in the bioenergetic processes of respiration and photosynthesis (5).
Two ubiquinones, QA and QB, are present in the reaction centers (RCs) of photosynthetic purple bacteria. QA accepts one-electron and is not protonated, whereas QB accepts, sequentially, two electrons. The second electron transfer is coupled with protonation leading to the formation of ubiquinol, which is weakly bound and diffuses out of the binding site to participate in the formation of the proton gradient required for ATP synthesis (5). QA and QB form hydrogen bonds to the RC protein. The bonds contribute to the binding and to the chemical properties and function of the quinones. It is, therefore, important to characterize the hydrogen bonds in detail.
Electron paramagnetic resonance (EPR) and electron nuclear double resonance (ENDOR) spectroscopies are ideally suited techniques to study the transient paramagnetic species involved in the primary events of photosynthesis, e.g., the ubiquinone anion radicals, 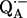 and
and  These studies provide information about the electronic and spatial structure of the transient radicals. In particular,
These studies provide information about the electronic and spatial structure of the transient radicals. In particular, 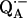 in the photosynthetic RC of Rhodobacter sphaeroides has been investigated extensively using EPR and ENDOR spectroscopies (6–12). These investigations showed that the ENDOR lines of
in the photosynthetic RC of Rhodobacter sphaeroides has been investigated extensively using EPR and ENDOR spectroscopies (6–12). These investigations showed that the ENDOR lines of  are due to three classes of protons: 1), protons associated with the protein in the vicinity of the binding site (matrix lines);2), nonexchangeable protons on the quinone, e.g., methyl, methoxy, or methylene protons; and 3), exchangeable protons, the most important of which are the protons forming hydrogen bonds with the two carbonyl oxygens of the quinone. The ENDOR lines corresponding to the exchangeable protons were identified by comparing ENDOR spectra of RCs in H2O and D2O buffer (6).
are due to three classes of protons: 1), protons associated with the protein in the vicinity of the binding site (matrix lines);2), nonexchangeable protons on the quinone, e.g., methyl, methoxy, or methylene protons; and 3), exchangeable protons, the most important of which are the protons forming hydrogen bonds with the two carbonyl oxygens of the quinone. The ENDOR lines corresponding to the exchangeable protons were identified by comparing ENDOR spectra of RCs in H2O and D2O buffer (6).
The initial ENDOR results suggested that 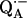 is bound to the RC protein by two nonequivalent hydrogen bonds to the carbonyl oxygens of the quinone (7). Confirming evidence of two H-bonds was obtained by electron spin echo envelope modulation (ESEEM) (13–15). Additional, independent evidence of two nonequivalent hydrogen bonds was obtained by Paddock et al. (16), who showed that there are two different proton-deuterium exchange times associated with two distinct hydrogen bonded protons.
is bound to the RC protein by two nonequivalent hydrogen bonds to the carbonyl oxygens of the quinone (7). Confirming evidence of two H-bonds was obtained by electron spin echo envelope modulation (ESEEM) (13–15). Additional, independent evidence of two nonequivalent hydrogen bonds was obtained by Paddock et al. (16), who showed that there are two different proton-deuterium exchange times associated with two distinct hydrogen bonded protons.
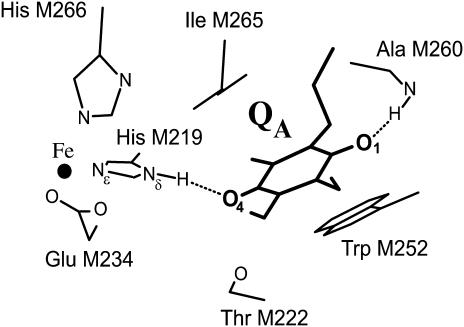
Based on the RC structure (17) the two hydrogen bonds were assigned to the imidazole nitrogen of His (M219) (Nδ-H⋯O4) and the NH group of Ala (M260) (N-H⋯O1) (see Fig. 1). However, there remained two problems: 1), in none of the previous experiments (6,7,16) could the ENDOR lines be assigned to protons associated with specific oxygens (i.e., O1 or O4, Fig. 1); and 2), there remained the puzzle why only three pairs of 1H-ENDOR lines were observed instead of the expected four pairs (in randomly oriented molecules each proton gives rise to two pairs of lines corresponding approximately to the parallel and perpendicular components of the axially symmetric hyperfine tensors). In this work we addressed both of these problems. We first determined the different 1H → 2H exchange times of the two hydrogen bonds by incubating protonated (or deuterated) RCs for different times in D2O and monitoring the amplitudes of the 1H-ENDOR lines. By using the results of these experiments we prepared RCs whose hydrogen bonds were preferentially deuterated (or protonated) at either O1 or O4. This enabled us to determine which set of ENDOR lines belong to a given oxygen. This solved the problem of the assignment of the observed three pairs of lines. Using the known structure of the RC (17) we postulated an assignment of each set of lines to a particular oxygen. A detailed simulation of the ENDOR spectra and the determination of the geometry of the H-bonds will be presented in a future article.
FIGURE 1.
Structure in the vicinity of QA in the RC of Rb. sphaeroides (Brookhaven Protein Data Bank entry 1AIG (17)). The iron is ligated to four histidines (only two are shown) and one glutamate (bidentate ligand). The isoprenoid chain of the quinone is truncated for simplicity. The two carbonyl oxygens of the quinone, labeled 1 and 4, form H-bonds shown by dotted lines.
Hamiltonian of the spin system
In this study, we focus on the interaction between the magnetic moment of the unpaired electron of 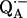 and the magnetic moments of protons or deuterons that form hydrogen bonds to the quinone oxygens O1 and O4 (see Fig. 1). The observed ENDOR spectra were interpreted using a spin Hamiltonian, ℋ, containing the electron and nuclear Zeeman interactions with the applied magnetic field Bo, the hyperfine coupling (hfc) and the nuclear quadrupole coupling (nqc) terms (e.g., Weil et al. (18)):
and the magnetic moments of protons or deuterons that form hydrogen bonds to the quinone oxygens O1 and O4 (see Fig. 1). The observed ENDOR spectra were interpreted using a spin Hamiltonian, ℋ, containing the electron and nuclear Zeeman interactions with the applied magnetic field Bo, the hyperfine coupling (hfc) and the nuclear quadrupole coupling (nqc) terms (e.g., Weil et al. (18)):
 |
(1) |
where S is the electron spin operator, I is the nuclear spin operator of protons or deuterons in H-bonds to the carbonyl oxygens; A and P are the hfc and nqc tensors in frequency units, g is the electronic g-tensor, h is Planck's constant, gN is the g-factor of the corresponding magnetic nucleus (proton or deuteron), and βe and βN are the electron and nuclear magnetons, respectively.
The first term in Eq. 1 represents the electronic Zeeman term that gives rise to the observed EPR spectrum. The other three terms represent nuclear interactions that give rise to the ENDOR spectra. These can be calculated to first order from Eq. 1 using an expansion in powers of the ratio hA/(gβeBo) (18,19). At the microwave frequency of 35 GHz used in this work, second order terms are small and can be neglected. Furthermore, at 35 GHz the ENDOR free frequency of protons (or deuterons) is much larger than its respective hfc interaction, i.e., gNβNBo/h ≫ A/2. Thus, the resonance frequency of the ENDOR transition Mγ ↔ Mγ − 1 is given to first order for a given direction of the magnetic field by (18,19):
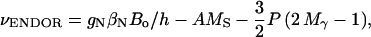 |
(2) |
where MS is the magnetic quantum number of the electron (±½) and Mγ the magnetic quantum number corresponding to the lower energy levels of the nucleus, i.e., +½ for protons and +1, 0 for deuterons.
For protons Mγ = +½ making the term containing the nuclear quadrupole interaction P zero. Thus, for a given magnetic field direction with respect to the molecular axes, one expects two ENDOR lines separated by the hfc A centered around the proton Larmor frequency gNβNBo/h. For deuterons Mγ = +1, 0 and each line is additionally split by 3P. In a sample having randomly oriented molecules, the anisotropies of A and P smear out the spectrum and sharp lines are observed only at the extrema (e.g., approximately at A// and A⊥).
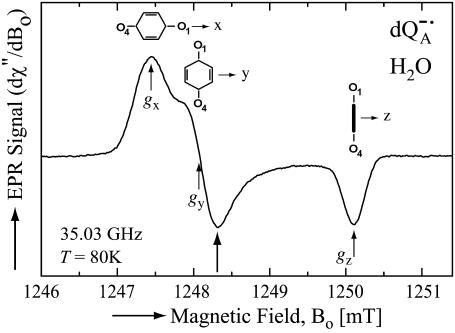
At 35 GHz (Q-band) the anisotropies of the electronic g-value gives rise to a spectrum shown in Fig. 2. At the positions labeled gx, gy, and gz, the majority of the molecules contributing to the spectrum have their molecular axes along the principal directions x, y, and z. However, it should be noted that at each position there is a significant admixture of molecules having different directions. Thus, only a partial magnetic field selection of orientations is obtained.
FIGURE 2.
EPR powder spectrum of 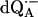 in H2O. Insets show the quinone orientations with respect to the magnetic field corresponding to gx, gy, and gz. Experimental conditions: T = 80 K, MW frequency = 35.03 GHz, MW power = 1 × 10−7 W, field modulation = 0.15 mT peak-to-peak at 270 Hz, average of 9 scans, 20 s per scan.
in H2O. Insets show the quinone orientations with respect to the magnetic field corresponding to gx, gy, and gz. Experimental conditions: T = 80 K, MW frequency = 35.03 GHz, MW power = 1 × 10−7 W, field modulation = 0.15 mT peak-to-peak at 270 Hz, average of 9 scans, 20 s per scan.
At Q-band the magnetic field Bo is ∼1.25 T and the ENDOR lines for protons and deuterons are observed in different frequency ranges, ∼53 MHz for 1H and ∼8 MHz for 2H. This enabled us to separate the signals due to protons in H-bonds from those in the quinone ring by using samples of deuterated RCs in H2O buffer. To observe deuterons in H-bonds we used samples of protonated RCs in D2O buffer.
MATERIALS AND METHODS
Preparation of reaction centers
RCs from Rb. sphaeroides R-26 were isolated and purified following the procedures of Isaacson et al. (9). The paramagnetic nonheme Fe2+ was chemically removed and replaced with diamagnetic Zn2+ to reduce the EPR line width of the semiquinone, following the procedure of Utschig et al. (20). The ratio of Zn/RC was determined by atomic absorption spectroscopy and Q-band EPR spectroscopy to be ≥0.90.
Deuteration of reaction centers
Fully deuterated RCs were obtained by growing Rb. sphaeroides R-26 bacteria in a D2O-based modified Hutner's medium using perdeuterated succinic acid (98% D, Aldrich, Milwaukee, WI) as the sole carbon source as described previously by van der Est et al. (21).
Proton-deuterium exchange
We used two sets of samples in which protons were exchanged with deuterons—one for the determination of the exchange times and the other for obtaining detailed ENDOR spectra, which required a higher signal/noise ratio, i.e., a higher RC concentration.
For the determination of the exchange times, fully protonated and fully deuterated RCs were purified and concentrated to an optical absorbance of 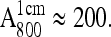 The RC samples were diluted into D2O buffer for different lengths of time, at the end of which
The RC samples were diluted into D2O buffer for different lengths of time, at the end of which  was generated photochemically (see next section) and frozen by immersing the sample in liquid nitrogen. We used a fivefold dilution for deuterated RCs and a fourfold dilution for protonated RCs. The final optical absorbance
was generated photochemically (see next section) and frozen by immersing the sample in liquid nitrogen. We used a fivefold dilution for deuterated RCs and a fourfold dilution for protonated RCs. The final optical absorbance 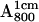 was ∼40 and ∼50, respectively.
was ∼40 and ∼50, respectively.
To obtain ENDOR spectra in which one of the H-bonds was preferentially deuterated, we prepared the following high concentration (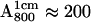 ) samples:
) samples:
Deuterated RCs in H2O buffer incubated for 27 min in D2O buffer (10-fold dilution).
Protonated RCs in H2O buffer incubated for 50 min in D2O buffer (fivefold dilution).
Protonated RCs in D2O buffer incubated for 190 min in H2O buffer (300-fold dilution).
The incubation times (see section on ENDOR results) and dilutions were chosen to maximize the preferential deuteration of one of the H-bonds to QA. After dilution the samples were concentrated by centrifuging in a Millipore (5–50k) filter to an optical absorbance  At the end of the incubation
At the end of the incubation 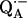 was generated chemically (see next section) and the sample frozen by immersing it in liquid nitrogen.
was generated chemically (see next section) and the sample frozen by immersing it in liquid nitrogen.
Generation of semiquinone radical anion
We used two methods to generate 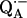 : 1), for low concentration samples (
: 1), for low concentration samples (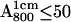 ) we illuminated the RCs with a single, saturating laser flash (Lumen-X DL2100C, λ = 590 nm) in the presence of excess cyt c2 to reduce the donor, D+, and stigmatellin to inhibit electron transfer to QB (photochemical method). After the laser flash the sample was plunged into liquid nitrogen. 2), For high concentration samples (
) we illuminated the RCs with a single, saturating laser flash (Lumen-X DL2100C, λ = 590 nm) in the presence of excess cyt c2 to reduce the donor, D+, and stigmatellin to inhibit electron transfer to QB (photochemical method). After the laser flash the sample was plunged into liquid nitrogen. 2), For high concentration samples (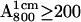 ), we used Na2S2O4 to reduce QA before freezing (chemical method) (6). The quartz sample tubes were type 705PQ (Wilmad, Buena, NJ) (OD 3 mm, ID 2 mm).
), we used Na2S2O4 to reduce QA before freezing (chemical method) (6). The quartz sample tubes were type 705PQ (Wilmad, Buena, NJ) (OD 3 mm, ID 2 mm).
Nomenclature
Since we used several combinations of protonated and deuterated RCs and buffer, we define the following notation: a), (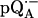 ) for
) for 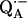 in fully protonated RCs and (
in fully protonated RCs and (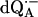 ) for
) for 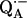 in fully deuterated RCs; b), (H2O) for fully protonated buffer and (D2O) for fully deuterated buffer; and c), (
in fully deuterated RCs; b), (H2O) for fully protonated buffer and (D2O) for fully deuterated buffer; and c), (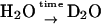 ) for RCs prepared in H2O and incubated later in D2O for a specific time (in minutes) and (
) for RCs prepared in H2O and incubated later in D2O for a specific time (in minutes) and (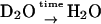 ) for the reverse.
) for the reverse.
EPR instrumentation
EPR and ENDOR measurements were performed at 35 GHz and 80 K. The spectrometer is a home-built superheterodyne-type instrument with a Varian klystron, a cylindrical TE011 brass cavity, and an immersion dewar system for temperature control. The cavity and coupler is similar to one described by Jaworski et al. (22). A Li:LiF sample was used as a primary g-value standard (g = 2.00229) (23), and P-doped Si as a secondary standard (g = 1.99891 at 80 K) (24,25). The P-Si marker was permanently attached to the bottom wall of the cavity. ENDOR experiments were performed with the EPR spectra 50% saturated. ENDOR spectra were recorded at the magnetic field positions as indicated in the corresponding traces, using frequency modulation (FM) of ±140 kHz for protons and ±30 kHz for deuterons, at FM rates as indicated in the figure captions. The output of the RF amplifier (ENI 3100L) feeding the ENDOR coils was 50 W for protons and 25 W for deuterons. To improve the signal/noise ratio of the ENDOR signal, many traces were averaged for up to 3 h for protons and 42 h for deuterons.
EXPERIMENTAL RESULTS AND DISCUSSION
EPR results
The Q-band EPR spectrum from 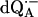 in protonated buffer (H2O), at T = 80 K, is shown in Fig. 2. The broadening of the spectrum is mainly due to protons in solution (exchangeable protons), including the H-bonds to the carbonyl oxygens. Exchanging H2O with D2O, decreases the broadening (data not shown).
in protonated buffer (H2O), at T = 80 K, is shown in Fig. 2. The broadening of the spectrum is mainly due to protons in solution (exchangeable protons), including the H-bonds to the carbonyl oxygens. Exchanging H2O with D2O, decreases the broadening (data not shown).
ENDOR results
1H ENDOR spectrum corresponding to the H-bonds
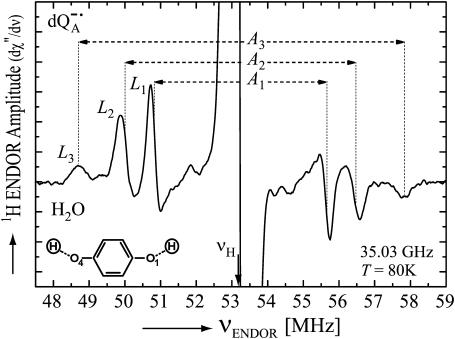
Fig. 3 shows the Q-band 1H ENDOR spectrum at T = 80 K of 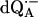 in H2O (chemically reduced), arising from 1H-bonds and from protons in the solvent. The spectrum was recorded at the magnetic field corresponding to gy (see Fig. 2). The splittings of the 1H-ENDOR peaks associated with the H-bonds are A1 (4.82 MHz), A2 (6.43 MHz), and A3 (9.07 MHz). Similar splittings were previously observed at X-band by comparing ENDOR spectra of
in H2O (chemically reduced), arising from 1H-bonds and from protons in the solvent. The spectrum was recorded at the magnetic field corresponding to gy (see Fig. 2). The splittings of the 1H-ENDOR peaks associated with the H-bonds are A1 (4.82 MHz), A2 (6.43 MHz), and A3 (9.07 MHz). Similar splittings were previously observed at X-band by comparing ENDOR spectra of  in H2O and D2O (6,12).
in H2O and D2O (6,12).
FIGURE 3.
1H ENDOR spectrum of 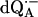 in H2O. The spectrum was taken at the field position corresponding to gy = 2.0053 (see Fig. 2). The intensities of the transitions are denoted by L1, L2, L3, and the hyperfine coupling constants by A1, A2, A3. Experimental conditions: T = 80 K, MW frequency = 35.03 GHz, MW power = 3 × 10−6 W; FM = ±140 kHz at a rate of 947 Hz. Number of scans: 500. Scan time: 4 s.
in H2O. The spectrum was taken at the field position corresponding to gy = 2.0053 (see Fig. 2). The intensities of the transitions are denoted by L1, L2, L3, and the hyperfine coupling constants by A1, A2, A3. Experimental conditions: T = 80 K, MW frequency = 35.03 GHz, MW power = 3 × 10−6 W; FM = ±140 kHz at a rate of 947 Hz. Number of scans: 500. Scan time: 4 s.
As described in the Spin Hamiltonian section, the single-crystal 1H ENDOR spectrum corresponding to a proton contains two lines (see Eq. 2). However, at the magnetic field position corresponding to gy, the spectrum corresponds to a “two-dimensional” powder-type spectrum, with weighted contributions mainly from all in-plane directions. Therefore, a powder spectrum is expected for each H-bond tensor with two sharp features, corresponding approximately to the parallel (A//) and the perpendicular (A⊥) components of an axially symmetric hfc tensor (7,26–28), i.e., each proton should give rise to two pairs of lines. For benzoquinone anion radicals in solution, the ENDOR experiments showed that the H-bond hfc tensor is approximately axially symmetric. This is typical for a proton in a normal H-bond. Deviations are expected only for very short H-bonds as described in (29). Since in reaction centers there are two nonequivalent H-bonds to 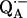 (12–16), in principle four pairs of 1H ENDOR lines should be observed in the spectrum. However, the experiment shows only three pairs, despite a thorough search for a fourth set of lines. This problem is discussed in a subsequent section.
(12–16), in principle four pairs of 1H ENDOR lines should be observed in the spectrum. However, the experiment shows only three pairs, despite a thorough search for a fourth set of lines. This problem is discussed in a subsequent section.
There are two problems to be solved in the identification of the ENDOR lines associated with the hydrogen-bonded protons: 1), which pair of lines belong to the same proton; and 2), with which oxygen (O1 or O4) is a particular set of ENDOR lines associated.
To answer the first question we make use of the selective deuteration of the oxygens as discussed in the next section.
Determination of the 1H → 2H exchange times of the two protons H-bonded to QA
To preferentially deuterate one of the H-bonds to QA, it is necessary to determine their respective 1H → 2H exchange times. We used ENDOR to measure the 1H → 2H exchange times of the two protons H-bonded to QA, in fully deuterated RCs and in fully protonated RCs (R-26). In the fully deuterated RCs in H2O we measured the amplitudes of the 1H-ENDOR lines L1 and L2 corresponding to the two protons (Fig. 3) at different times of incubation in D2O. The incubation time started with a fivefold dilution of the H2O sample in D2O. The semiquinone 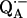 was produced photochemically (see Materials and Methods) at the end of the incubation period.
was produced photochemically (see Materials and Methods) at the end of the incubation period.
In view of the fivefold dilution of the sample, 20% of the bonds remain protonated and only 80% are subject to exchange with deuterium. Thus, the normalized ratio of amplitudes of L1 to L2 is given by:
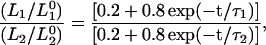 |
(3) |
where 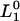 and
and 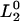 are the respective initial intensities and τ1 and τ2 are the respective 1H → 2H exchange times for the two protons. For protonated RCs, the H2O samples were diluted fourfold in D2O.
are the respective initial intensities and τ1 and τ2 are the respective 1H → 2H exchange times for the two protons. For protonated RCs, the H2O samples were diluted fourfold in D2O.
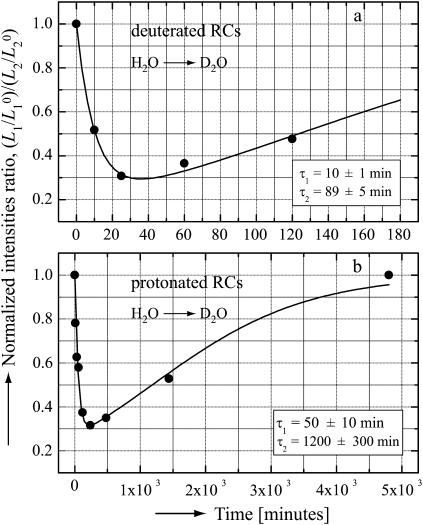
Fig. 4 shows the observed time dependence of the normalized intensity ratio of the L1 and L2 lines for fully deuterated and fully protonated RCs. The 1H → 2H exchange times were determined by fitting the experimental data with Eq. 3, with τ1 and τ2 as adjustable parameters. The resultant values of τ1 and τ2 are shown in the inserts of Fig. 4.
FIGURE 4.
The normalized intensities ratios ( ) for the low frequency 1H-ENDOR lines (see Fig. 3), as a function of the incubation time in D2O for deuterated RCs (a) and for protonated RCs (b). The 1H → 2H exchange times were obtained by fitting the experimental data (dots) with Eq. 3 (solid lines). The values of the exchange times τ1 and τ2 are shown in the inserts. Experimental conditions: Microwave frequency 35 GHz, T = 80 K, Bo at position corresponding to gy (see Fig. 2). Optical absorbance of sample
) for the low frequency 1H-ENDOR lines (see Fig. 3), as a function of the incubation time in D2O for deuterated RCs (a) and for protonated RCs (b). The 1H → 2H exchange times were obtained by fitting the experimental data (dots) with Eq. 3 (solid lines). The values of the exchange times τ1 and τ2 are shown in the inserts. Experimental conditions: Microwave frequency 35 GHz, T = 80 K, Bo at position corresponding to gy (see Fig. 2). Optical absorbance of sample 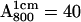 (a) and 50 (b).
(a) and 50 (b).
The different exchange times observed for protonated and deuterated RCs is believed to reflect the structural changes in the RCs when the bacteria are grown in a fully deuterated medium. The shorter exchange times in deuterated RCs suggest a less compact structure, in which the quinone oxygens are more accessible to the solvent. Paddock et al. (16), who measured the exchange times in protonated RCs of the HC (M266) mutant, found an order of magnitude different times than in the wild-type, suggesting again a structural change. Thus, the exchange times are very sensitive to the details of the structure and may provide a useful probe in investigating structural changes.
Assignment of pairs of lines belonging to the same proton
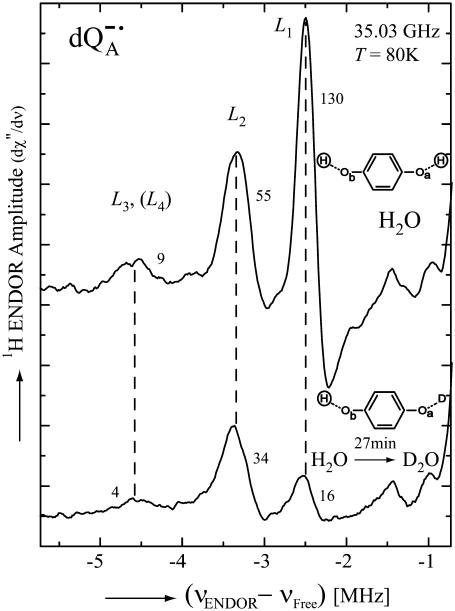
To solve this problem we used the differential exchange of one proton with respect to the other as discussed in the previous section. The lines associated with a particular proton should show the same reduction in amplitude when the proton is exchanged with deuterons. Fig. 5 shows the low frequency part of the 1H-ENDOR spectrum of  in H2O (top) and after 27 min of exchanges in D2O (bottom), both samples were produced chemically. Deuterated RCs were used to eliminate ENDOR signals from protons of the protein. Using the exchange times for the two protons of 10 min and 89 min, respectively (see Fig. 4 a), we predict that one proton should be ∼80% exchanged and the other ∼20%. The reduction in amplitudes of L1 and L2 correspond roughly to these values. Thus, the peaks L1 and L2 are associated with two different protons, as had been postulated previously (7,16).
in H2O (top) and after 27 min of exchanges in D2O (bottom), both samples were produced chemically. Deuterated RCs were used to eliminate ENDOR signals from protons of the protein. Using the exchange times for the two protons of 10 min and 89 min, respectively (see Fig. 4 a), we predict that one proton should be ∼80% exchanged and the other ∼20%. The reduction in amplitudes of L1 and L2 correspond roughly to these values. Thus, the peaks L1 and L2 are associated with two different protons, as had been postulated previously (7,16).
FIGURE 5.
Low-frequency part of the 1H ENDOR spectra of  in H2O (upper spectrum) and in
in H2O (upper spectrum) and in  (lower spectrum), at 35 GHz, taken at a magnetic field of 1248.3 mT (see arrow in Fig. 2). The H-bond ENDOR lines are labeled L1, L2, L3, and L4 in the upper spectrum. Numbers next to each line refer to their amplitudes (in arbitrary units) (see Table 1). Experimental conditions: T = 80 K, MW power = ∼3 × 10−6 W, FM = ±140 kHz at a rate of 950 Hz. Number of scans: 500 (upper spectrum) and 2860 (lower spectrum). Scan time: 4 s.
(lower spectrum), at 35 GHz, taken at a magnetic field of 1248.3 mT (see arrow in Fig. 2). The H-bond ENDOR lines are labeled L1, L2, L3, and L4 in the upper spectrum. Numbers next to each line refer to their amplitudes (in arbitrary units) (see Table 1). Experimental conditions: T = 80 K, MW power = ∼3 × 10−6 W, FM = ±140 kHz at a rate of 950 Hz. Number of scans: 500 (upper spectrum) and 2860 (lower spectrum). Scan time: 4 s.
We now turn to the assignment of the line corresponding to the largest hyperfine coupling (see left of Fig. 5). Does it represent the partner to L1 or L2? We shall show that this line is an overlap of two lines, one being a partner to L1 and the other to L2. The proof is as follows: The exchange reduced the amplitude of L1 8.1-fold (130/16; see Table 1). If the peak were only the partner of L1 we would expect it to decrease to 1.1 units. The observed peak is 4 units, i.e., significantly larger, showing that a considerable part of it is associated with another line, L4, the partner to L2, thus confirming the overlap. The effect is best observed for a magnetic field position slightly off gy (at Bo = 1248.3 mT; see Fig. 2).
TABLE 1.
Amplitudes of the 1H ENDOR lines (in arbitrary units) corresponding to the protons H-bonded to 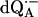 in H2O (before exchange) and in
in H2O (before exchange) and in 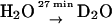 (after exchange) (see Fig. 5)
(after exchange) (see Fig. 5)
| L3 + L4 | L2 | L1 | |
|---|---|---|---|
| Before exchange | 9 | 55 | 130 |
| After exchange | 4 | 34 | 16 |
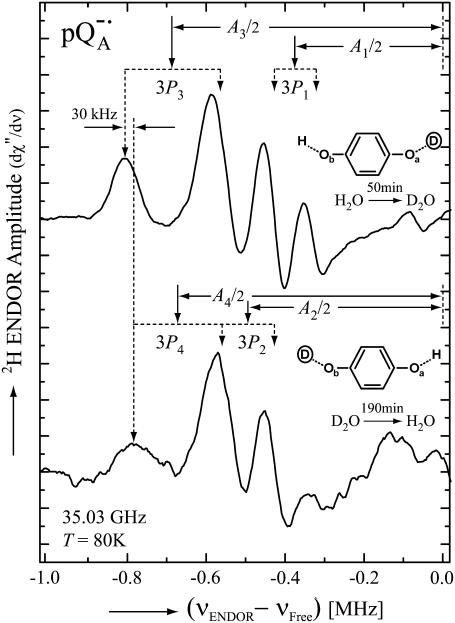
Additional evidence for the overlap is provided by the 2H-ENDOR spectra of protonated RCs in D2O shown in Fig. 6 (high concentration samples; see Materials and Methods). Each 2H-ENDOR line is split into a doublet by the nuclear quadrupole interaction, 3P (Eq. 2). The center of each doublet (solid arrows in Fig. 6) corresponds to the hfc (18). The top trace shows the low frequency 2H-ENDOR spectrum after incubating the RC for 50 min in D2O. In these experiments protonated RCs were used to eliminate signals from the protein. With the exchange times of 50 min and 1200 min (Fig. 4 b), we expect one oxygen (Oa) to be ∼50% deuterated, whereas the other (Ob) only ∼3% (see Eq. 3 and inset in Fig. 6, top). Thus, the spectrum is essentially due to deuterons on Oa. In the lower trace the deuterons were exchanged with protons giving rise to an 2H-ENDOR spectrum associated with deuterons on Ob (see inset in Fig. 6, bottom). Comparing the two spectra we see that the low frequency line (at −0.8 MHz) of P3 is shifted by ∼30 kHz from that of P4 showing that the two lines belong to different D-bonds. The shift between the two lines is smaller than the line width which explains why the two lines are not resolved when both oxygens are protonated (or deuterated). These results clearly show that the line at −0.8 MHz is associated with deuterons on both Oa and Ob.
FIGURE 6.
Low-frequency part of the 2H ENDOR spectra of 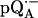 in
in  (upper spectrum) and in
(upper spectrum) and in 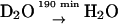 (lower spectrum), at 35 GHz, taken along the magnetic field position corresponding to gy (see Fig. 2). The position of the hfc tensor components (A1, A2, A3, and A4) are indicated by solid arrows, whereas the nqc splittings (3P1, 3P2, 3P3, and 3P4) are indicated by dotted arrows. Inserts show the preferential deuteration of the carbonyl oxygens. Experimental conditions: T = 80 K, MW power = ∼5 × 10−6 W, FM = ±30 kHz at a rate of 985 Hz. Number of scans: 8000 (upper spectrum) and 37,000 (lower spectrum). Scan time: 4 s.
(lower spectrum), at 35 GHz, taken along the magnetic field position corresponding to gy (see Fig. 2). The position of the hfc tensor components (A1, A2, A3, and A4) are indicated by solid arrows, whereas the nqc splittings (3P1, 3P2, 3P3, and 3P4) are indicated by dotted arrows. Inserts show the preferential deuteration of the carbonyl oxygens. Experimental conditions: T = 80 K, MW power = ∼5 × 10−6 W, FM = ±30 kHz at a rate of 985 Hz. Number of scans: 8000 (upper spectrum) and 37,000 (lower spectrum). Scan time: 4 s.
Assignment of the ENDOR lines of the hydrogen bonds to a particular (O1 or O4) oxygen
In the previous section we refer to Oa and Ob as being associated with a given set of ENDOR lines. Our goal is to relate these results to the specific oxygen, O1 and O4 of the quinones (see Fig. 1). This, unfortunately, cannot be accomplished from the ENDOR experiments discussed above. We know, however, from the x-ray structure (17) that Ala (M260), i.e., O1, is closer to the surface of the RC and is, therefore, expected to exchange its proton faster than the buried His (M219), i.e., O4. Furthermore, EPR (8,10,30) and FTIR (31,32) experiments on 13C substituted quinones showed that the hydrogen bond to O1 is weaker than to O4 (reviewed in Lubitz and Feher (12). We, therefore, postulate that the ENDOR line L1 and L3 (Fig. 5) are associated with O1 and L2 and L4 with O4. This assignment will be further validated in a subsequent publication.
CONCLUSION
We have identified the 1H and 2H-ENDOR lines with the hfcs of the specific protons hydrogen bonded to the two carbonyl oxygens on 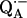 in reaction centers of Rb. sphaeroides. In a forthcoming study, these assignments will be used to determine the exact geometries of the hydrogen bonds.
in reaction centers of Rb. sphaeroides. In a forthcoming study, these assignments will be used to determine the exact geometries of the hydrogen bonds.
Acknowledgments
We thank M. Paddock (University of California, San Diego) for providing data on the 1H/2H exchange times in protonated Zn-RCs (Fig. 4 b) and for helpful discussions.
This work was supported by National Science Foundation MCB 99/82186 and National Institutes of Health GM13191 and by Max Planck Society, and Fonds der Chemischen Industrie (to W.L.).
References
- 1.Lenaz, G., editor. 1985. Coenzyme Q: Biochemistry, Bioenergetics and Clinical Applications of Ubiquinone. John Wiley & Sons, Chichester; New York.
- 2.Trumpower, B. L. 1982. Function of Quinones in Energy Conserving Systems. Academic Press, New York
- 3.Chambers, J. Q. 1988. Electrochemistry of quinones. In The Chemistry of Quinonoid Compounds. S. Patai and Z. Rappoport, editors. John Wiley & Sons, Chichester; New York. 719–757.
- 4.Morrison, L. E., J. E. Schelhorn, T. M. Cotton, C. L. Bering, and P. A. Loach. 1982. Electrochemical and spectral properties of ubiquinone and synthetic analogs: Relevance to bacterial photosynthesis. In Function of quinones in energy conserving systems. B. L. Trumpower, editor. Academic Press, New York. 35–58.
- 5.Cramer, W. A., and D. B. Knaff. 1990. The quinone connection. In EnergyTransduction in Biological Membranes. C. R. Cantor, editor. Springer-Verlag, New York. 193–238.
- 6.Lubitz, W., E. C. Abresch, R. J. Debus, R. A. Isaacson, M. Y. Okamura, and G. Feher. 1985. Electron nuclear double resonance of semiquinones in reaction centers of Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta. 808:464–469. [DOI] [PubMed] [Google Scholar]
- 7.Feher, G., R. A. Isaacson, M. Y. Okamura, and W. Lubitz. 1985. ENDOR of semiquinones in RCs from Rhodopseudomonas sphaeroides. In Antennas and Reaction Centers of Photosynthetic Bacteria-Structure, Interactions and Dynamics. M. E. Michel-Beyerle, editor. Springer-Verlag, Berlin. 174–189.
- 8.van den Brink, J. S., A. P. Spoyalov, P. Gast, W. B. S. van Liemt, J. Raap, J. Lugtenburg, and A. J. Hoff. 1994. Asymmetric binding of the primary acceptor quinone in reaction centers of the photosynthetic bacterium Rhodobacter sphaeroides R26, probed with Q-Band (35 GHz) EPR spectroscopy. FEBS Lett. 353:273–276. [DOI] [PubMed] [Google Scholar]
-
9.Isaacson, R. A., F. Lendzian, E. C. Abresch, W. Lubitz, and G. Feher. 1995. Electronic structure of
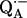 in reaction centers from Rhodobacter sphaeroides. 1. Electron paramagnetic resonance in single crystals. Biophys. J. 69:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
in reaction centers from Rhodobacter sphaeroides. 1. Electron paramagnetic resonance in single crystals. Biophys. J. 69:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar] -
10.Isaacson, R., E. C. Abresch, F. Lendzian, C. Boullais, M. Paddock, C. Mioskowski, W. Lubitz, and G. Feher. 1996. Asymmetry of the binding sites of
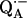 and
and 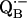 in reaction centers of Rb. sphaeroides probed by Q-band EPR with 13C-labeled quinones. In The Reaction Center of Photosynthetic Bacteria: Structure and Dynamics. M. E. Michel-Beyerle, editor. Springer-Verlag, Berlin. 353–367.
in reaction centers of Rb. sphaeroides probed by Q-band EPR with 13C-labeled quinones. In The Reaction Center of Photosynthetic Bacteria: Structure and Dynamics. M. E. Michel-Beyerle, editor. Springer-Verlag, Berlin. 353–367.
-
11.Rohrer, M., F. MacMillan, T. F. Prisner, A. T. Gardiner, K. Mobius, and W. Lubitz. 1998. Pulsed ENDOR at 95 GHz on the primary acceptor ubisemiquinone
 in photosynthetic bacterial reaction centers and related model systems. J. Phys. Chem. B. 102:4648–4657. [Google Scholar]
in photosynthetic bacterial reaction centers and related model systems. J. Phys. Chem. B. 102:4648–4657. [Google Scholar] -
12.Lubitz, W., and G. Feher. 1999. The primary and secondary accepters in bacterial photosynthesis III. Characterization of the quinone radicals
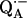 and
and  by EPR and ENDOR. Appl. Magn. Reson. 17:1–48. [Google Scholar]
by EPR and ENDOR. Appl. Magn. Reson. 17:1–48. [Google Scholar] -
13.Bosch, M. K., P. Gast, A. J. Hoff, A. P. Spoyalov, and Y. D. Tsvetkov. 1995. The primary acceptor quinone QA in reaction centers of Rhodobacter-sphaeroides R26 is hydrogen-bonded to the Nδ(1)-H of His M219. An electron-spin echo study of
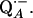 Chem. Phys. Lett. 239:306–312. [Google Scholar]
Chem. Phys. Lett. 239:306–312. [Google Scholar] - 14.Lendzian, F., J. Rautter, H. Kass, A. Gardiner, and W. Lubitz. 1996. ENDOR and pulsed EPR studies of photosynthetic reaction centers: protein-cofactor interactions. Ber. Bunsenges. Phys. Chem. 100:2036–2040. [Google Scholar]
-
15.Spoyalov, A. P., R. J. Hulsebosch, S. Shochat, P. Gast, and A. J. Hoff. 1996. Evidence that Ala M260 is hydrogen-bonded to the reduced primary acceptor quinone
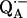 in reaction centers of Rb. sphaeroides. Chem. Phys. Lett. 263:715–720. [Google Scholar]
in reaction centers of Rb. sphaeroides. Chem. Phys. Lett. 263:715–720. [Google Scholar] -
16.Paddock, M. L., E. C. Abresch, R. A. Isaacson, W. Lubitz, M. Y. Okamura, and G. Feher. 1999. Identification of hydrogen bonds to
 in RCs of Rb. sphaeroides by ENDOR spectroscopy. Biophys. J. 76:A141–A141. [Google Scholar]
in RCs of Rb. sphaeroides by ENDOR spectroscopy. Biophys. J. 76:A141–A141. [Google Scholar] - 17.Stowell, M. H. B., T. M. McPhillips, D. C. Rees, S. M. Soltis, E. Abresch, and G. Feher. 1997. Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron-proton transfer. Science. 276:812–816. [DOI] [PubMed] [Google Scholar]
- 18.Weil, J. A., J. R. Bolton, and J. E. Wertz. 1994. Electron Paramagnetic Resonance. Elementary Theory and Practical Applications. Wiley, New York.
- 19.Weil, J. A. 1975. Comments on second-order spin Hamiltonian energies. J. Magn. Reson. 18:113–116. [Google Scholar]
- 20.Utschig, L. M., S. R. Greenfield, J. Tang, P. D. Laible, and M. C. Thurnauer. 1997. Influence of iron-removal procedures on sequential electron transfer in photosynthetic bacterial reaction centers studied by transient EPR spectroscopy. Biochemistry. 36:8548–8558. [DOI] [PubMed] [Google Scholar]
- 21.van der Est, A., R. Bittl, E. C. Abresch, W. Lubitz, and D. Stehlik. 1993. Transient EPR spectroscopy of perdeuterated Zn-substituted reaction centers of Rhodobacter sphaeroides R-26. Chem. Phys. Lett. 212:561–568. [Google Scholar]
- 22.Jaworski, M., A. Sienkiewicz, and C. P. Scholes. 1997. Double-stacked dielectric resonator for sensitive EPR measurements. J. Magn. Reson. 124:87–96. [DOI] [PubMed] [Google Scholar]
- 23.Stesmans, A., and G. Vangorp. 1989. Novel method for accurate g-measurements in electron-spin resonance. Rev. Sci. Instrum. 60:2949–2952. [Google Scholar]
- 24.Feher, G., and E. Gere. 1959. Electron spin resonance experiments on donors in silicon. II. Electron spin relaxation effects. Phys. Rev. 114:1245–1256. [Google Scholar]
- 25.Stesmans, A., and G. Devos. 1986. Electron-spin-resonance observation of temperature dependent g shifts in submetallic P doped Si at low temperatures. Phys. Rev. B. 34:6499–6502. [DOI] [PubMed] [Google Scholar]
- 26.O'Malley, P. J., and G. T. Babcock. 1986. Powder ENDOR spectra of para-benzoquinone anion radical principal hyperfine tensor components for ring protons and for hydrogen-bonded protons. J. Am. Chem. Soc. 108:3995–4001. [Google Scholar]
- 27.MacMillan, F., F. Lendzian, and W. Lubitz. 1995. EPR and ENDOR characterization of semiquinone anion radicals related to photosynthesis. Magn. Reson. Chem. 33:S81–S93. [Google Scholar]
- 28.Flores, M., R. A. Isaacson, R. Calvo, G. Feher, and W. Lubitz. 2003. Probing hydrogen bonding to quinone anion radicals by 1H and 2H ENDOR spectroscopy at 35 GHz. Chem. Phys. 294:401–413. [Google Scholar]
- 29.Sinnecker, S., E. Reijerse, F. Neese, and W. Lubitz. 2004. Hydrogen bond geometries from electron paramagnetic resonance and electron-nuclear double resonance parameters: density functional study of quinone radical anion-solvent interactions. J. Am. Chem. Soc. 126:3280–3290. [DOI] [PubMed] [Google Scholar]
- 30.Hoff, A. J., T. N. Kropacheva, R. I. Samoilova, N. P. Gritzan, J. Raap, J. S. van den Brink, P. Gast, and J. Lugtenburg. 1996. Site-directed isotope labelling as a tool in spectroscopy of photosynthetic preparations. Investigations on quinone binding in bacterial reaction centers. In The Reaction Center of Photosynthetic Bacteria: Structure and Dynamics. M. E. Michel-Beyerle, editor. Springer-Verlag, Berlin. 405–420.
- 31.Breton, J., C. Boullais, J. R. Burie, E. Nabedryk, and C. Mioskowski. 1994. Binding sites of quinones in photosynthetic bacterial reaction centers investigated by light-induced FTIR difference spectroscopy: assignment of the interactions of each carbonyl of QA in Rhodobacter sphaeroides using site-specific 13C-labeled ubiquinone. Biochemistry. 33:14378–14386. [DOI] [PubMed] [Google Scholar]
- 32.Brudler, R., H. J. M. de Groot, W. B. S. van Liemt, W. F. Steggerda, R. Esmeijer, P. Gast, A. J. Hoff, J. Lugtenburg, and K. Gerwert. 1994. Asymmetric binding of the 1- and 4-C=O groups of QA in Rhodobacter sphaeroides R26 reaction centres monitored by Fourier transform infra-red spectroscopy using site-specific isotopically labelled ubiquinone-10. EMBO J. 13:5523–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]