Abstract
We report here that a cubane-like europium-L-aspartic acid complex at physiological pH can discriminate between DNA structures as judged by the comparison of thermal denaturation, binding stoichiometry, temperature-dependent fluorescence enhancement, and circular dichroism and gel electrophoresis studies. This complex can selectively stabilize non-B-form DNA polydApolydT but destabilize polydGdCpolydGdC and polydAdTpolydAdT. Further studies show that this complex can convert B-form polydGdCpolydGdC to Z-form under the low salt condition at physiological temperature 37°C, and the transition is reversible, similar to RNA polymerase, which turns unwound DNA into Z-DNA and converts it back to B-DNA after transcription. The potential uses of a left-handed helix-selective probe in biology are obvious. Z-DNA is a transient structure and does not exist as a stable feature of the double helix. Therefore, probing this transient structure with a metal-amino acid complex under the low salt condition at physiological temperature would provide insights into their transitions in vivo and are of great interest.
INTRODUCTION
It is well known that amino acids play an essential role at the catalytic site as cofactors of many natural enzymes, and lanthanide complexes have been widely used as probes in luminescent resonance energy transfer for bioassays and as reagents for diagnosis in magnetic resonance imaging (1,2). Small molecules that selectively target specific genes to inhibit the biological function in which particular DNA structures participate have shown great potential application in biotechnology (3–5). We report here that a cubane-like europium-L-aspartic acid complex Eu-(-L-Asp) at physiological pH can recognize non-B-form DNA (6,7) and convert B-form poly(dGC)2 to Z-form (4) under the low salt condition. The B-Z transition is reversible, similar to RNA polymerase, which turns unwound DNA into Z-DNA and converts it back to B-DNA after transcription. The Z-DNA conformation has been difficult to study because it does not exist as a stable feature of the double helix. Instead, it is a transient structure that is occasionally induced by biological activity and then quickly disappears. Therefore, probing this transient structure with metal natural amino acid complex under the low salt condition at physiological temperature is of great interest. To the best of our knowledge, there is no report yet to show that metal-amino acid complexes can discriminate between DNA structures.
MATERIALS AND METHODS
Synthesis and crystallization of [Eu4(OH)4(Asp)5 (H2O)7]Cl·15.5H2O
L-aspartic acid (0.26 g, ∼2 mmol) was added as a solid in an aqueous solution of EuCl3 (0.1 M, 20 ml) prepared by dissolution of Eu2O3. The amount of L-aspartic acid was slightly less than that of EuCl3. With stirring, an aqueous NaOH solution (0.5 M) was added dropwise to the above solution until pH ≈ 7.0. After being stirred continuously in a thermostat (333 K) for 6 h, the mixture was filtered and the filtrate was allowed to stand at room temperature. Colorless crystals appeared in ∼8 weeks. The crystalline product was collected by filtration, washed with a mixture of tetrahydrofuran/ether (1:1 v/v), and dried in a desiccator charged with silica gel. Analysis calculated. (%) for C20H69ClEu4N5O42.50: Eu, 35.70; C, 14.00; H, 4.05; N, 4.11. Found.: Eu, 35.96; C, 14.01; H, 3.91; N, 4.08. IR: ν3394 s, 3212 s, 1593 s, 1421 s, 1354 w, 1315 w, 1232 w, 1143 w, 659 m, 542 m cm−1.
Crystal data for C20H69ClEu4N5O42.50: M = 1703.09; Orthorhombic; Space group: P212121; a = 11.559(5) Å, b = 20.748(9) Å, c = 23.684(10) Å, V = 5680(4) Å3; T = 293 K, Z = 4, Absorption coefficient: 4.509 mm−1; Reflections collected: 32108; Independent reflections: 11117 (Rint = 0.0269); Data/restraints/parameters: 11117/9/654; Final R indices [I > 2σ(I)]: R1 = 0.0351, wR2 = 0.0851. Intensity data were collected on a Siemens (Madison, WI) SMART charge-coupled device diffractometer with graphite-monochromatic MoKα(λ = 0.710 73 Å) radiation at a temperature of 298 ± 2 K. The structure was solved by direct methods using the SHELXTL-97 crystallographic software package and refined by full matrix least-squares on F2. The details of the crystal data, refinement, and final statistics are summarized in Table 1.
TABLE 1.
Crystal data and structure refinement parameters for complexes
| [Eu4(OH)4(Asp)5 (H2O)7]Cl · 15.5H2O (2) | |
|---|---|
| Empirical formula | C20H69ClEu4N5O42.50 |
| Formula weight | 1703.09 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| Unit cell dimensions | a = 11.559(5) Å α = 90° |
| b = 20.748(9) Å β = 90° | |
| c = 23.684(10) Å γ = 90° | |
| Volume | 5680(4) Å3 |
| Z | 4 |
| Density (calculated) | 1.992 mg/m3 |
| Absorption coefficient | 4.509 mm−1 |
| F (000) | 3332 |
| Crystal size | 0.21 × 0.10 × 0.05 mm |
| θ range for data collection | 1.96° to 26.00° |
| Limiting indices | −13 ≤ h ≤ 14, −25 ≤ k ≤ 25, −29 ≤ l ≤22 |
| Reflections collected | 32108 |
| Independent reflections | 11117 (Rint = 0.0269) |
| Completeness to θ = 25.02° | 99.4% |
| Refinement method | Full matrix least-squares on F2 |
| Data/restraints/parameters | 11117/9/654 |
| Goodness-of-fit on F2 | 1.072 |
| Final R indices [I > 2σ(I)] | R1 = 0.0315, wR2 = 0.0834 |
| R indices (all data) | R1 = 0.0351, wR2 = 0.0851 |
| Absolute structure parameter | −0.013(14) |
| Largest difference peak and hole | 1.440 and −0.559 eÅ−3 |
Bioassay
The various DNA oligonucleotides were synthesized by Sangon and were used without further purification. The DNA extinction coefficient was calculated through the nearest neighbor method (8). Experiments were carried out in aqueous Tris buffer (10 mM Tris, 100 mM NaCl, pH = 7.1) unless stated otherwise. Absorbance measurements were made on a Jasco (Tokyo, Japan) V-550 ultraviolet (UV)-Vis spectrophotometer, equipped with a Peltier (Tokyo, Japan) temperature control accessory. Temperature-dependent fluorescence measurements and circular dichroism (CD) spectra and CD melting experiments were measured on a JASCO F-6500 spectrofluorometer and a JASCO J-810 spectropolarimeter equipped with a temperature-controlled water bath.
Determination of binding constants
DNA binding constants were determined by absorption titration as described previously (4). Titration data were fitted directly by nonlinear least-squares methods to get binding constants, using a fitting function incorporated into the program FitAll (MTR Software, Toronto, Canada). Errors were evaluated by a Monte Carlo analysis, using a routine that has been added to the FitAll package (MTR Software).
RESULTS AND DISCUSSION
The crystal structure of our first synthesized cationic complex is shown in Fig. 1. The complex exists as a tetranuclear species with four Eu (III) ions at the four apexes, and each of the four triangular faces of the metal tetrahedron is capped by a μ3-OH group, forming a cubane-like [Eu4(μ3-OH)4]8+ cluster structure (Eu-O(μ3-OH) = 2.339 Å − 2.447 Å, average 2.396 Å). Table 1 summarizes the details of the final structure of the complex. These features should be related to the two carboxyl groups in the aspartic acid molecule, which makes the complex stable at physiological pH solution without further hydrolysis. These may also be related to its ability for DNA recognition.
FIGURE 1.
(A) Crystal structure of [Eu4(OH)4(Asp)5 (H2O)7]Cl. Carbon, cyan; nitrogen, blue; oxygen, red; and europium, green. Lattice water, hydrogen, and chloride ions are omitted for clarity. The Eu atoms are highlighted with green dots. (B) (Left) Temperature-dependent CD spectra of 22-mer duplex DNA polydGdCpolydGdC (60 μM in basepair) in the absence (all in black at 15°C, 37°C, 60°C, and 85°C) or presence of the complex in Tris buffer (10 mM Tris, 100 mM NaCl, pH = 7.1): 15°C (red); 37°C (green); 60°C (blue); and 85°C (cyan); (Right) Double-stranded GC homopolymer, B-DNA, and Z-DNA. Phosphate, yellow; carbon, cyan; nitrogen, blue; and oxygen, red. Hydrogen and water molecules are omitted for clarity.
In the presence of the complex, a marked hypochromism and bathrochromic shift was observed in the UV-Vis spectra when the complex bound to 22-mer duplex DNA polydGdCpolydGdC, polydAdTpolydAdT, and polydApolydT (Fig. 2). The binding constants of this complex bound to DNA were determined by absorption titration as described previously (4). Their DNA binding constants were 1.7 × 105 M−1; 2.3 × 105 M−1; and 1.5 × 105 M−1 for polydGdCpolydGdC, polydAdTpolydAdT, and polydApolydT, respectively. CD spectra showed that DNA ellipticity (6–8) decreased following the order polydGdCpolydGdC > polydAdTpolydAdT ≫ polydApolydT (Fig. 3, A–C). It is well known that polydGdCpolydGdC and polydAdTpolydAdT are in the B-form, whereas polydApolydT has distinct structural and functional properties and adopts a non-B conformation (6,7). As shown in Fig. 2, bottom, the CD spectrum of polydApolydT displays an unusual shape with split bands in the region of 260–300 nm as a result of the stacking of bases with relatively large propeller twist (18–24°) (6,7). This is also the reason polydApolydT (Fig. 4) exhibits retardation in gel electrophoresis (6,7).
FIGURE 2.
UV spectra of 22-mer duplex DNA (top) polydGdCpolydGdC; (middle) polydAdTpolydAdT; and (bottom) polydApolydT in the presence (dashed line) or absence (solid line) of the europium-L-Asp complex in Tris buffer (10 mM Tris, 100 mM NaCl, pH = 7.1). 1:2 ratio of [complex]/[DNA] was used in the experiments. [DNA] = 60 μM in basepair.
FIGURE 3.
(Left) CD spectra of 22-mer duplex DNA in the presence (dashed line) or absence (solid line) of the europium complex in Tris buffer (10 mM Tris, 100 mM NaCl, pH = 7.1). (A) polydAdTpolydAdT, (B) polydGdCpolydGdC, and (C) polydApolydT. DNA concentration was 60 μM in basepair; 1:2 ratio of [Eu]/[DNA] was used in the experiments. (Middle) DNA CD melting profiles measured in the presence (dashed line) or absence (solid line) of the europium complex at (D) 269 nm poly(dAT)2, (E) 252 nm polydGdCpolydGdC, and (F) 248 nm polydApolydT, respectively. (Right) Job plot of the europium complex with 22-mer DNA: (G) polydAdTpolydAdT, (H) polydGdCpolydGdC, and (I) polydApolydT. Total concentration of the complex (in units of Eu) and DNA (in units of basepair) was held constant at 60 μM over the course of the titration.
FIGURE 4.
Gel electrophoresis of 22-mer duplex DNA in the absence or presence of the complex. (Lane 1) DNA marker, (lane 2) polydAploydT, (lane 3) polydAploydT/Eu-Asp (2:1), (lane 4) polydGdCpolydGdC, (lane 5) polydGdCpolydGdC/Eu-Asp (2:1), (lane 6) polydAdTpolydAdT, and (lane 7) polydAdTpolydAdT/Eu-Asp (2:1); 15% polyacrylamide gel electrophoresis and Tris-borate running buffer were used in the experiments at 20°C.
The most intriguing property of this complex is its ability to discriminate between DNA structures. In the presence of the Eu-Asp complex (Fig. 3, D and E), both polydAdTpolydAdT and polydGdCpolydGdC became less stable but without precipitation or DNA condensation because the spectra all had flat signals outside the absorption region (9). Melting temperature was decreased by 6°C for polydAdTpolydAdT. Two melting transitions were observed for polydGdCpolydGdC. The first broad transition was the melting of Z-DNA and the second one was the melting of B-DNA (Fig. 3 E). The details of the B-Z transition will be discussed in the next section.
In the presence of the complex, as the temperature increased, B-Z transition was observed under the low salt condition at 37°C (Fig. 1 B) and the complex fluorescence was greatly enhanced (Fig. 5) because unwound DNA is well known to enhance the emission of europium (10). No such transition occurred (Fig. 1 B, left panel) in the absence of the complex, and no fluorescence increase was observed for the complex alone at elevated temperature (data not shown). The transition enthalpy between the B and Z polymorphs of GC-rich sequences is small (2 kcal mol−1) and within the range of thermal energies available from the environment (11,12). Much has been learned about Z-DNA since it was first discovered (13,14). It turns out that Z-DNA is found only transiently when genes are actively being transcribed (4,15). When the RNA polymerase stops moving, Z-DNA reverts to its normal right-handed form (15). The B-Z transition in the presence of the complex is reversible: after cooling down the sample to 15°C, Z-DNA reverts to B-DNA (Fig. 5). Interestingly, if the temperature was over 60°C, the Z-DNA spectrum was replaced by the B-DNA spectrum (Fig. 1), indicating that Z-DNA was melted before B-DNA (9). DNA melting studies are clearly suggestive of a biphasic melting profile (Fig. 3 E). This is consistent with previous reports on [Co(NH3)5H2O]3+ induced temperature-dependent B-Z transition (9).
FIGURE 5.
(A) Temperature-dependent fluorescence spectra of the complex in the presence of 22-mer duplex DNA polydGdCpolydGdC in Tris buffer (10 mM Tris, 100 mM NaCl, pH = 7.1). Excitation wavelength was 270 nm. DNA concentration was 60 μM in basepair; 1:2 ratio of [Eu]/[DNA] was used in the experiments. (B) CD spectra of polydGdCpolydGdC in the presence of the complex at 15°C (solid line), 37°C (dotted line), and 15°C (dashed line) after cooling down the sample from 37°C to 15°C. DNA concentration was 60 μM in basepair; 1:2 ratio of [Eu]/[DNA] was used in the experiment.
Z-DNA and its biological function have been extensively investigated in relation to transcription and significant progress has been made (15), such as the discovery that double-stranded RNA adenosine deaminase (ADAR1) and the tumor-associated protein, DLM-1, can specifically bind to Z-DNA, and the first evidence that Z-DNA-forming sequences are required for chromatin-dependent activation of the CSF1 promoter (15). The potential uses of a left-handed helix-selective probe in biology are obvious (16). To be able to use such compounds as tools to investigate the significance and function of left-handed DNA in vivo and even to modulate biological properties by shifting the balance in favor of left-handed helices would be of great interest to cell biologists. Detailed studies on their transition mechanism (4,17,18) and sequence length effect have been reported.
A binding of 1:1 was observed for the complex bound to polydGdCpolydGdC and polydAdTpolydAdT (Fig. 3, G–I), indicating that one Eu-Asp complex molecule bound to four AT or GC basepairs because there are four Eu (III) ions in the Eu-Asp complex. This complex can tightly bind to polydGdCpolydGdC, and the DNA binding constant is 1.7 × 105 M−1 determined by absorption titration (4). Besides the electrostatic interaction between the phosphate backbone and the free amino groups (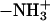 ) of aspartic acid and Eu ions, an important issue concerning interactions between DNA and the Eu-Asp complex is the relative energetic preference of the various Ione-pair sites in the bases (19,20). Based on the mechanism for the interaction of the aluminum-Asp complex with DNA (21), Eu can bind to the N7 of purines (pu) and the exocyclic C2 of pyrimidines (py), unwinding the helix and leading to destabilization of double-stranded DNA. There seems to be a general consensus that the N7 site in guanine is the most favored one among all the Ione-pair sites (19), which has been identified by crystal structure (22) and by molecular electrostatic potential calculation (19). This may be the main reason the Eu-Asp complex can drive B-Z transition under the low salt condition (17,18).
) of aspartic acid and Eu ions, an important issue concerning interactions between DNA and the Eu-Asp complex is the relative energetic preference of the various Ione-pair sites in the bases (19,20). Based on the mechanism for the interaction of the aluminum-Asp complex with DNA (21), Eu can bind to the N7 of purines (pu) and the exocyclic C2 of pyrimidines (py), unwinding the helix and leading to destabilization of double-stranded DNA. There seems to be a general consensus that the N7 site in guanine is the most favored one among all the Ione-pair sites (19), which has been identified by crystal structure (22) and by molecular electrostatic potential calculation (19). This may be the main reason the Eu-Asp complex can drive B-Z transition under the low salt condition (17,18).
Another feature of this cubane-like complex is non-B-form polydApolydT selectivity. Unlike polydGdCpolydGdC and polydAdTpolydAdT, polydApolydT stability was increased by 4°C in the presence of the complex, showing that this complex bound differently to non-B-form polydApolydT, which was observed in the binding stoichiometry experiments. In contrast to the 1:1 binding of polydAdTpolydAdT and polydGdCpolydGdC, a 1:2 binding mode (Fig. 3 I) was observed for non-B-form polydApolydT in which the stacking of bases with a relatively large propeller twist (18–24°) (6,7), demonstrating that one Eu-Asp complex molecule bound to two AT basepairs with a binding constant of 1.5 × 105 M−1 as determined by absorption titration. As proposed, the binding mechanism (21) for pu-py nonalternating DNA, polydApolydT, the complex can bind to pu-py basepairs of the two strands and leads to greater basepair overlap and, therefore, enhances duplex stability. Agents that can recognize non-B-form polydApolydT and selectively stabilize AnTn (n > 20) tracts within a positioned nucleosome can facilitate transcription (23). Currently we are extending our studies on the design and selection of compounds at physiological conditions with improved selectivity toward A- and Z-form DNA.
Acknowledgments
We thank Drs. J. B. Chaires and X. Ji for their helpful comments and suggestions on this research. The authors are grateful for the referees' helpful comments on the manuscript.
This project was supported by the National Natural Science Foundation of China (20225102, 20331020, 20325101, 20473084) Fund from Jilin Province, and the Hundred People Program from the Chinese Academy of Sciences.
References
- 1.Cha, A., G. E. Snyder, P. R. Selvin, and F. Bezanilla. 1999. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 402:809–813. [DOI] [PubMed] [Google Scholar]
- 2.Caravan, P., J. J. Ellison, T. J. McMurry, and R. B. Lauffer. 1999. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 99:2293–2352. [DOI] [PubMed] [Google Scholar]
- 3.Mergny, J. L., and C. Helene. 1998. G-quadruplex DNA: a target for drug design. Nat. Med. 4:1366–1367. [DOI] [PubMed] [Google Scholar]
- 4.Qu, X., J. O. Trent, I. Fokt, W. Priebe, and J. B. Chaires. 2000. Allosteric, chiral-selective drug binding to DNA. Proc. Natl. Acad. Sci. USA. 97:12032–12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu, X., C. Wan, H. C. Becker, D. Zhong, and A. H. Zewail. 2001. The anticancer drug-DNA complex: femtosecond primary dynamics for anthracycline antibiotics function. Proc. Natl. Acad. Sci. USA. 98:14212–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Holde, K. E., W. C. Johnson, and P. S. Ho. 1998. Principles of Physical Biochemistry. Prentice Hall, Upper Saddle River, NJ.
- 7.Brahms, S., and J. G. Brahms. 1990. DNA with adenine tracts contains poly(dA).poly(dT) conformational features in solution. Nucleic Acids Res. 18:1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu, X., J. Ren, P. V. Riccelli, A. S. Benight, and J. B. Chaires. 2003. Enthalpy/entropy compensation: influence of DNA flanking sequence on the binding of 7-amino actinomycin D to its primary binding site in short DNA duplexes. Biochemistry. 42:11960–11967. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson, A., M. Hawken, M. Hall, K. J. Sanders, and A. Rodger. 2000. Amine induced Z-DNA in poly (dG-dC) poly (dG-dC): circular dichroism and gel electrophoresis study. Phys. Chem. Chem. Phys. 2:5469–5478. [Google Scholar]
- 10.Fu, P. K.-L., and C. Turro. 1999. Energy transfer from nucleic acids to Tb(III): selective emission enhancement by single DNA mismatches. J. Am. Chem. Soc. 121:1–7. [Google Scholar]
- 11.Jovin, T. M., D. M. Soumpasis, and L. P. McIntosh. 1987. The transition between B-DNA and Z-DNA. Annu. Rev. Phys. Chem. 38:521–560. [Google Scholar]
- 12.Rich, A., A. Nordheim, and A. H. Wang. 1984. The chemistry and biology of left-handed Z-DNA. Annu. Rev. Biochem. 53:791–846. [DOI] [PubMed] [Google Scholar]
- 13.Pohl, F. M., and T. M. Jovin. 1972. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J. Mol. Biol. 7:375–396. [DOI] [PubMed] [Google Scholar]
- 14.Thamann, T. J., R. C. Lord, A. H. Wang, and A. Rich. 1981. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 9:5443–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich, A., and S. Zhang. 2003. Timeline: Z-DNA: the long road to biological function. Nat. Rev. Genet. 4:566–572. [DOI] [PubMed] [Google Scholar]
- 16.Waring, M. J. 2000. Facilitating structural transitions in DNA. Proc. Natl. Acad. Sci. USA. 97:11685–11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoagan, M., N. Daggagupta, and D. M. Crothers. 1979. Transmission of allosteric effects in DNA. Nature. 278:521–524. [DOI] [PubMed] [Google Scholar]
- 18.Suh, D., R. D. Sheardy, and J. B. Chaires. 1991. Unusual binding of ethidium to a deoxyoligonucleotide containing a BZ junction. Biochemistry. 30:8722–8726. [DOI] [PubMed] [Google Scholar]
- 19.Gadre, S. R., S. S. Pundlik, A. C. Limaye, and A. P. Rendell. 1998. Electrostatic investigation of metal cation binding to DNA bases and base pairs. Chem. Commun. 5:573–574. [Google Scholar]
- 20.Knobloch, B., W. Linert, and H. Sigel. 2005. Metal ion-binding properties of (N3)-deprotonated uridine, thymidine, and related pyrimidine nucleosides in aqueous solution. Proc. Natl. Acad. Sci. USA. 102:7459–7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharathi, S. Kosagi, J. Rao, and R. Stein. 2003. First evidence on induced topological changes in supercoiled DNA by an aluminium D-aspartate complex. J. Biol. Inorg. Chem. 8:823–830. [DOI] [PubMed] [Google Scholar]
- 22.Abrescia, N. G. A., T. Huynh-Dinh, and J. A. Subirana. 2002. Nickel-guanine interactions in DNA: crystal structure of Nickel-d[CGTGTACACG]2. J. Biol. Inorg. Chem. 7:195–199. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu, M., T. Mori, T. Sakurai, and H. Shindo. 2000. Destabilization of nucleosomes by an unusual DNA conformation adopted by poly(dA) · poly(dT) tracts in vivo. EMBO J. 19:3358–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]







