Abstract
We expressed rod-type homotetrameric cyclic nucleotide-gated (CNGA1) channels in Xenopus oocytes and studied activation by photolysis-induced jumps of the 3′,5′-cyclic guanosine monophosphate (cGMP) concentration and by voltage steps. cGMP jumps to increasing concentrations up to the EC50 value of 46.5 μM decelerate the activation gating, indicative that even at concentrations of cGMP ≪EC50 binding is not rate limiting. Above the EC50 value, activation by cGMP jumps is again accelerated to the higher concentrations. At the same cGMP concentration, the speed of the activation gating by depolarizing voltage steps is roughly similar to that by cGMP jumps. Permeating ions passing the pore more slowly (Rb+ > K+ > Na+) slow down the activation time course. At the single-channel level, cGMP jumps to high concentrations cause openings directly to the main open level without passing sublevels. From these results it is concluded that at both low and high cGMP the gating of homotetrameric CNGA1 channels is not rate-limited by the cGMP binding but by conformational changes of the channel which are voltage dependent and include movements in the pore region.
INTRODUCTION
In the outer segment of photoreceptors, light activates an intracellular signal cascade which results in a rapid decrease of the cytosolic concentration of 3′,5′-cyclic guanosine monophosphate (cGMP). This drop of cGMP closes cyclic nucleotide-gated (CNG) channels (1–4) in the plasma membrane, resulting in a hyperpolarizing receptor potential. Native CNG channels of rod photoreceptors are heterotetramers composed of three CNGA1 and one CNGB1 subunit (5–7). Upon heterologous expression, CNGA1 subunits suffice to generate functional homotetrameric channels which develop part of the characteristic properties of native channels, as ion selectivity and activation gating by cGMP. The typical rapid flicker of the open native channels is inferred by the CNGB1 subunit (8–10). A CNGA1 subunit contains six transmembrane spanning regions (S1–S6) and a pore region between the S5 and S6 segment. The C-terminus, which is attached to the S6 segment, contains the cyclic nucleotide-binding domain (cNBD) (4). The binding of cyclic nucleotides to a cNBD is thought to trigger first conformational changes in this domain, which are then transferred via the C-linker to the S6 segment and the pore by an allosteric reaction (11,12).
Because CNG channels do not desensitize, the gating of these channels was investigated in nearly all studies under steady-state conditions in the presence of effective concentrations of an activating cyclic nucleotide. Further insight into the channel gating can be expected when studying currents under nonsteady-state conditions, i.e., by changing a gating stimulus in a step-like fashion. This type of approach has been performed for CNG channels in only a few studies. Gating in response to voltage steps has been characterized in both native channels of the rod photoreceptor (13) and homotetrameric CNGA1 channels (14). However, the low degree of voltage dependence of these channels (equivalent gating charge z = 0.23) limits interpretation of these results. More promising was to induce jumps of the cyclic nucleotide concentration. Zufall and co-workers (1993) (15) studied activation of olfactory CNG channels by producing these jumps with a liquid filament switch technique (16). In inside-out patches, however, the speed of solution exchange is limited by diffusion within the unstirred solution of the electrode tip, resulting in delays of 5 ms or even longer (17). This limitation can be overcome by photolytically liberating the cyclic nucleotides from respective caged compounds because photolysis takes place also within the pipette tip. Karpen and co-workers (1988) (13) applied this technique to native rod photoreceptor channels by using the 4,5-dimethoxy-2-nitrobenzyl ester of cGMP as caged cGMP. The result was that the activation time course became monotonically faster at increasing cGMP. Similarly, the activation time course induced by voltage became also faster at increasing cGMP concentrations. The authors concluded that at physiological cGMP (<5 μM) the activation kinetics are limited by a cGMP-binding step.
In a previous report we showed that in structurally related olfactory CNG channels the binding of cyclic nucleotides does not limit the activation gating of these channels, not even at the lowest cyclic nucleotide concentration of 0.06 μM (18). Because homotetrameric CNGA1 channels are more than 20 times less sensitive to cGMP than homotetrameric olfactory CNGA2 channels (19), we questioned whether activation of homotetrameric CNGA1 channels is rate-limited by the binding of cGMP. We therefore studied the gating of homotetrameric CNGA1 channels by cGMP jumps, induced by flash photolysis, and we compared the time courses with those induced by depolarizing voltage steps. For the cGMP jumps, we took benefit of the superior physical properties of coumarinylmethyl esters of cGMP as caged cyclic nucleotides, which allowed us to jump over the whole relevant range of channel activation (20,21). Our results show that in CNGA1 channels activation by jumps to both low and high cGMP concentrations is rate-limited by conformational changes of the channel and not by the cGMP binding. These conformational changes proceed in the transmembrane electric field and include part of the pore region.
MATERIALS AND METHODS
Oocyte preparation and cRNA injection
Oocytes were obtained surgically from adult females of Xenopus leavis. The oocytes were treated for 60–90 min with 1.2 mg/ml collagenase (Type I, Sigma, St. Louis, MO) and manually dissected. They were injected with 40–70 nl of a solution containing cRNA specific for bovine CNGA1 channels (accession No. X51604). The oocytes were incubated at 18°C in Barth medium until experimental use within 6 days after injection.
Chemicals
All chemicals were of analytical grade. cGMP was obtained from Sigma. As caged cGMP for flash photolysis, the [7-(diethylamino)coumarin-4-yl]methyl ester of cGMP (DEACMcGMP) was used in most experiments (20). For cGMP jumps to the largest concentrations, the [6,7-bis(carboxymethoxy)coumarin-4-yl]methyl ester of cGMP (BCMCMcGMP) (20) and the [7-bis(carboxymethylamino)coumarin-4-yl]methyl ester of cGMP (BCMACMcGMP) (21) were used.
The wavelengths of the light used for photolysis were 320–480, 275–355, and 320–480 nm, respectively. Synthesis and the superior physicochemical properties of these compounds for photolysis have been described elsewhere (20,21).
Recording technique
Currents were recorded in inside-out patches with the patch-clamp technique. The patch pipettes were pulled from quartz tubing (outer diameter 1.0 mm, inner diameter 0.7 mm (macroscopic currents) or 0.4 mm (single-channel experiments)) or borosilicate glass tubing (outer diameter 2.0 mm, inner diameter 1.0 mm). The pipette resistance ranged from 0.6–25 MΩ, depending on the desired number of channels in the patch. Recording was performed with an Axopatch 200A or 200B amplifier (Axon Instruments, Union City, CA). If not otherwise stated, the currents were recorded with K+ solution (in mM: 150 KCl, 1 EGTA, 5 Hepes, pH = 7.4 with KOH) in the bath and the pipette. In part of the experiments, Na+ solution (in mM: 150 NaCl, 1 EGTA, 5 Hepes, pH = 7.4 with NaOH) or Rb+ solution (in mM: 150 RbCl, 1 EGTA, 5 Hepes, pH = 7.4 with RbOH) were used. To test for possible background channel activity, each excised patch was first exposed to a solution containing no cyclic nucleotide. Then the maximum current was activated by 700 μM free cGMP. If not otherwise stated the currents were measured at the voltage of +100 mV.
The experimental chamber for flash photolysis has been described previously (18). In brief: the chamber was composed of two compartments, the main compartment and the small photolysis compartment. In the main compartment all free cyclic nucleotide concentrations were administered. The solution containing the caged cGMP passed the photolysis compartment (width 0.5 mm, height 1.0 mm) just before entering the main compartment. One wall of the photolysis compartment was formed by the end of a light guide (diameter 1.0 mm) and the opposite wall by a mirror. The bottom of the experimental chamber consisted of two parallel glass plates. Between these plates thermostated water flew to control the temperature in both the main and the photolysis compartment. If not otherwise stated the recordings were performed at 20.3 ± 0.1°C.
Flash photolysis
The technique used for flash photolysis has been described previously (18). In brief: Light flashes were generated by the flash-lamp system JML-C2 (Rapp OptoElectronic, Hamburg, Germany). Photolysis was completed within 150 μs. According to the manual of the flash-lamp system, the energy of a light pulse was 0.45–1.47 mJ. The tip of the quartz pipette was positioned in the middle of the photolysis chamber. The solution flow through the photolysis chamber was adjusted such that the concentration of the liberated cyclic nucleotide was constant for at least 1.5 s, as evaluated by the constant amplitude of the late current. The next flash was elicited only after the current induced by cGMP had dropped to the current level in the absence of cGMP. The interval between the flashes ranged from 10 to 20 s.
To determine the concentration of free cGMP liberated by flash photolysis, for each patch the ratio of the steady-state current after a flash to the steady-state current at a saturating concentration of free cGMP (I∞/Imax,) was calculated and then inserted in the equation
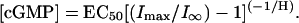 |
(1) |
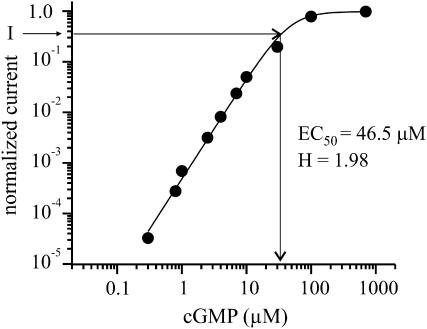
EC50 and H are the half maximum concentration and the Hill coefficient determined from the concentration-response relationship for free cGMP (Fig. 1). The current amplitude at saturating concentrations of the cyclic nucleotide was determined immediately after a light flash.
FIGURE 1.
Concentration-response relationship for CNGA1 channels with free cGMP. The steady-state current at the indicated concentrations was normalized with respect to the steady-state current at 700 μM cGMP and the data points were fitted by 1/{(1 + (EC50/[cGMP])H} yielding the indicated values for EC50 and H. To determine the free cGMP concentration in flash photolysis experiments, the actual steady-state current (I) was normalized with respect to the actual steady-state current (Imax) at 700 μM cGMP. The free cGMP concentration [cGMP] was obtained with Eq. 1 by using the values for EC50 and H.
To rule out direct effects of the light flashes on the channels, the channels were activated by free cGMP (100 μM) and the patches were exposed to 10 flashes as used for uncaging. The current amplitude was unaffected. We also examined whether the caged compounds affect the channels without uncaging. To this end the channels were activated with 100 μM free cGMP and different concentrations of caged cGMP were applied. High concentrations of the caged compounds slightly reduced the amplitude of the macroscopic current. This effect was reversible. Hence, for determination of the open probability the measured current amplitude was corrected by amplitude factors ranging from 1.007 to 1.137.
Data acquisition and analysis
Measurements were controlled and data were collected with the ISO2 and ISO3 soft- and hardware (12- or 16-bit resolution, respectively; MFK Niedernhausen, Germany) running on a pentium PC. The sampling rate was 20 kHz (filter 5 kHz). All currents were corrected for capacitive and very small leak components by subtracting corresponding averaged currents in the absence of a cyclic nucleotide.
Curves were fitted to the data with nonlinear approximation algorithms using either the ISO3 or the Origin 6.1 (OriginLab, Northampton, MA) software. Statistical data are given as mean ± SEM.
RESULTS
Channel activation after cGMP jumps is rate-limited by conformational changes and not by the cGMP binding
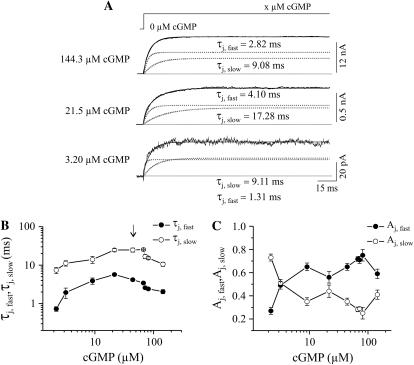
Activation of CNGA1 channels by cGMP jumps appeared in the time range of milliseconds (Fig. 2 A). If cGMP binds to equivalent sites at the subunits and the binding limits the activation process, then the activation time course must be monotonically slowed in the direction to lower cGMP concentrations. The time courses could be described by the sum of two exponentials, yielding the fast and slow time constant, τj,fast and τj,slow, and their relative contribution, Aj,fast and Aj,slow. The recordings show that activation at the intermediate concentration of 21.5 μM cGMP was slower than at the lower concentration of 3.20 μM cGMP and at the higher concentration of 144.3 μM cGMP. Fig. 2 B shows that increasing cGMP concentrations first increase both time constants until concentrations close to the EC50 value (arrow) and then decrease again to the highest concentrations. The increase of the time constants at the lowest cGMP concentrations implies that at all concentrations tested, activation can not be rate limited by the binding of cGMP but must be rate limited by conformational changes. The contribution of the fast and slow time constant also depends on the cGMP concentration: The fast exponential dominates at all cGMP concentrations apart from the lowest (Fig. 2 C) which essentially differs from the results in CNGA2 channels (18).
FIGURE 2.
Activation of CNGA1 currents by jumps of the cGMP concentration. (A) Current traces at 3 cGMP jumps from zero to the indicated concentrations by flash photolysis. The transmembrane voltage was +100 mV. At the intermediate concentration of 21.5 μM, the activation time course was slower than at both higher and lower cGMP. The traces were fitted with the sum of two exponentials yielding the indicated time constants τj,fast and τj,slow. The dotted curves show the respective two components. (B) Plot of activation time constants as function of the cGMP concentration. Each data point was obtained from 5 to 11 individual measurements. The arrow indicates the EC50 value for steady-state conditions. (C) Relative contribution of the fast and slow exponential, Aj,fast and Aj,slow, to the activation time course.
Gating by voltage is controlled by similar conformational changes as activation by cGMP jumps
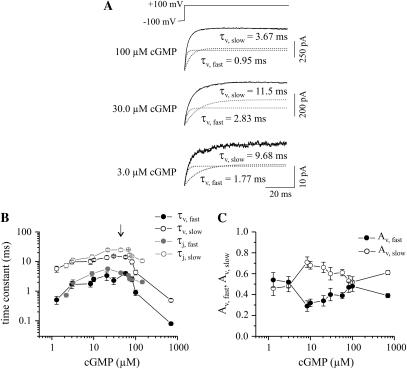
Voltage-dependent activation in CNGA1 channels was studied and compared to the time course of the cGMP-jump induced activation to investigate whether both types of activation are related. Basically, voltage-dependent activation differs from cGMP-jump induced activation by the fact that it does not start from Po = 0 but from a value at −100 mV that is only slightly below that at +100 mV (for steady-state curves at these voltages see Benndorf et al. (14)). The activating component of the current traces could be described by the sum of two exponentials, yielding the fast and slow time constant, τv,fast and τv,slow, and their relative contribution, Av,fast and Av,slow (Fig. 3 A). As for cGMP jumps, activation was slower at the intermediate concentration of 30 μM cGMP than at the lower concentration of 3 μM cGMP and at the higher concentration of 100 μM cGMP. Fig. 3 B shows that at increasing cGMP both time constants first increase until concentrations close to the EC50 value (arrow) and then decrease to the highest concentrations. At high and low cGMP concentrations, both time constants are similar to those determined for cGMP jumps, suggesting that similar molecular arrangements are involved. In contrast to cGMP jumps, however, the slow exponential dominates at all cGMP concentrations apart from the lowest (Fig. 3 C).
FIGURE 3.
Voltage-dependent activation of CNGA1 channels. (A) Activating component of the current traces when stepping from −100 to +100 mV at 3 cGMP concentrations. The time courses were fitted with the sum of two exponentials yielding the indicated time constants τv,fast and τv,slow. The dotted curves show the respective two components. (B) Plot of τv,fast and τv,slow as function of the cGMP concentration. Each data point was obtained from 3 to 14 individual measurements. τj,fast and τj,slow from Fig. 2 B are shown for comparison. The arrow indicates the EC50 value for steady-state conditions. (C) Contribution of the fast and slow exponential of voltage-dependent activation, Av,fast and Av,slow, respectively.
The results so far suggest that common conformational changes are rate limiting for activation gating induced by cGMP jumps and depolarizing voltage steps. Minor differences between the time constants would reflect specificities of these conformational changes. However, when comparing the time constants caution is needed because the contributions of the exponentials differ substantially. A rough but simple possibility to compare biexponential time courses with different contributions of the exponentials is to compare the mean time constants calculated by
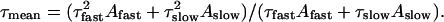 |
(2) |
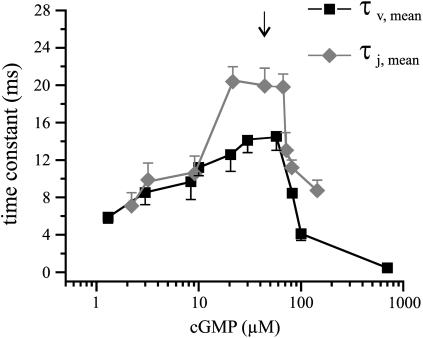
Also both mean time constants depend on the cGMP concentration in a bell-shaped fashion with a maximum in the range of the EC50 value (Fig. 4), substantiating that the gating at these cGMP concentrations is rate-limited by similar conformational changes. Whereas at cGMP ≤10 μM activation by cGMP jumps and by depolarizing voltage steps at corresponding cGMP concentrations are similarly fast, at the higher cGMP concentrations cGMP jumps generate only some slower activation than voltage steps. It is therefore likely that at cGMP concentrations >10 μM, activation by cGMP jumps involves an additional rate-limiting step compared to activation by voltage.
FIGURE 4.
Mean time constants for activation by cGMP jumps and depolarizing voltage steps as a function of the cGMP concentration. The time constants were calculated according to Eq. 2. The vertical arrow indicates the EC50 value for steady-state conditions.
The binding of cGMP affects both the forward and backward transition of the allosteric reaction
The gating of CNG channels can be interpreted by kinetic schemes which contain several sequential cGMP binding steps and an allosteric opening reaction which can proceed from all closed states (22–24). The exact number of cGMP binding steps required to fully describe activation is still unknown (4). At zero cGMP, a channel is in the equilibrium C0↔O0 and the equilibrium is strongly shifted to C0 (Scheme 1):
Figure .
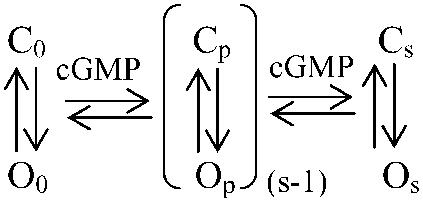
At saturating cGMP, the channel is fully liganded and the equilibrium Cs↔Os is strongly shifted to Os. At cGMP concentrations causing partial liganding, a set of reactions Cp↔Op (1 ≤ p ≤ (s − 1)) is possible. If the C↔O reaction determines the activation time course, then it would be mainly determined by the time constant 1/(kCO + kOC) corresponding to the actual number of ligands bound. Hence, at saturating cGMP the activation time constant would be 1/(kCO,s + kOC,s). At subsaturating cGMP, an individual channel with p ligands bound would be activated with the time constant 1/(kCO,p + kOC,p). In multi-channel patches, the individual channels are differently liganded, resulting in the contribution of different exponentials to the activation time course. We considered whether the progressive shift of the equilibrium from C to O at increasing cGMP is caused by an increase of kCO only, a decrease of kOC only, or the combination of both. The result that activation is faster at both saturating and low cGMP than near the EC50 value (Fig. 4) directly shows that both kCO and kOC are controlled by the cGMP concentration: at low cGMP kOC must be strongly accelerated whereas at high cGMP kCO must be strongly accelerated. Near the EC50 value, the slowest activation time course then finds an explanation by a minimum of the sum of kCO and kOC.
The conclusions that at cGMP concentrations near the EC50 value both rate constants, kCO and kOC, directly control the activation time course was substantiated by another series of experiments. We previously showed that at all cGMP concentrations the open probability is increased at depolarizing voltages with respect to hyperpolarizing voltages (14). Eyring rate theory (25) enables us to predict the interaction of voltage with the C↔O transition, kCO ∝ exp(zCOFV/RT) and kOC ∝ exp(zOCFV/RT). F is the Faraday constant, R the molar gas constant, and T the temperature. zCO and zOC are the equivalent charge movements across the membrane which occur when the channel moves from the closed and the open state to the respective transition state. Because depolarization should promote activation and retard deactivation, zCO should be >0 and zOC should be <0.
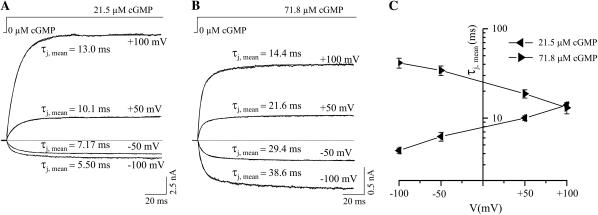
When the absolute values of zCO and zOC are similar, then cGMP jumps to concentrations above and below the EC50 value should cause an opposite voltage dependence of the activation time course: Above the EC50 value (kCO > kOC), the depolarization-induced increase of the open probability would be associated with an accelerated activation because the opening rate (kCO) dominates the activation time constant, 1/(kCO + kOC). Conversely, below the EC50 value (kCO < kOC), the depolarization-induced increase of the open probability would be associated with a decelerated activation because now the closing rate (kOC) dominates the activation time constant, 1/(kCO + kOC). Currents were elicited by cGMP jumps at the voltages −100, −50, +50, +100 mV. The traces were fitted by the sum of two exponentials and the mean activation time constant was calculated by Eq. 2. At the cGMP concentration below the EC50 value (21.5 μM; Fig. 5 A) depolarization decelerated the activation time course, whereas at the cGMP concentration above the EC50 value (71.8 μM; Fig. 5 B), depolarization accelerated the activation time course (Fig. 5 C). Hence, near the EC50 value the rate constants kCO and kOC directly control the activation time course.
FIGURE 5.
Effect of voltage on the activation by cGMP jumps. The voltage was either −100, −50, +50, or +100 mV. (A) Current traces at 21.5 μM cGMP. The traces were recorded from the same patch. The traces were fitted with the sum of two exponentials and the mean activation time constants, τj,mean, were calculated by Eq. 2. (B) Current traces at 71.8 μM cGMP. The traces were recorded from the same patch. Same analysis as in panel A. (C) Plot of τj,mean as function of voltage. Each data point was obtained from 5 to 8 individual measurements.
Ions decreasing the closing rate concomitantly decelerate the activation process
The result that the closing transition itself contributes to determining the activation time course was verified by another approach. The rate constant of the closing reaction should be proportional to the mean open time of a channel. Since the mean channel open time of CNGA1 channels is prolonged by permeating ions staying longer in the pore (Rb+ > K+ > Na+) (26), it can be expected that these ions concomitantly decelerate the activation time course and that this effect disappears at saturating cGMP where kCO is much faster than kOC.
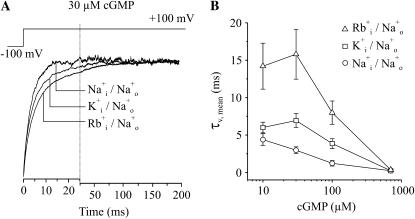
The effect of the ions was tested on voltage-dependent activation. Activation time courses were studied when stepping from −100 to +100 mV (Fig. 6 A). The ion at the extracellular side of the membrane was Na+ in all cases. The mean activation time constants, calculated by Eq. 2, were plotted as function of the cGMP concentration (Fig. 6 B). As expected, at subsaturating cGMP the permeating ions slowed the activation time course in the sequence Rb+ > K+ > Na+ and at saturating cGMP the differences disappeared. These results substantiate that gating movements of the pore region proceeding at subsaturating cGMP are involved in the rate-limiting reactions determining the activation gating.
FIGURE 6.
Modulation of voltage-dependent activation of CNGA1 channels by ions. Patches were depolarized by pulses from −100 to +100 mV. The activation time courses were quantified by τv,mean. (A) Representative current traces illustrating the effect of intracellular, permeating ions. The traces were normalized with respect to the amplitude of the late current. The pipette contained Na+ ions, the bath either Na+, K+, or Rb+ ions. (B) Plot of τv,mean as function of the cGMP concentration for the three ionic conditions in A.
Activation of single channels by cGMP jumps confirms that the cGMP binding is much faster than the allosteric reaction
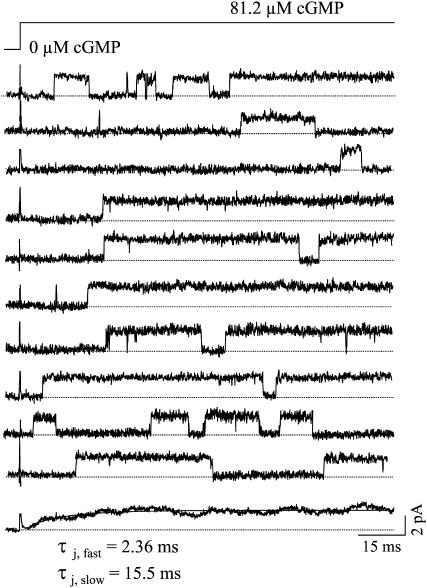
If conformational changes associated with the pore opening are rate limiting for the activation process, then the binding of cGMP, and intimately coupled reactions, must be much faster than the allosteric opening reaction. This hypothesis could be tested as follows: We took into consideration that partially liganded CNGA1 channels open sometimes to sublevels (27,28) and sometimes also to superlevels (26) whereas fully liganded channels open to a defined level. If the cGMP binding reaction plus intimately coupled reactions are much faster than the allosteric opening reaction, then cGMP jumps from zero to high concentrations should directly gate the single channels to the open level observed at high cGMP under steady-state conditions. Alternatively, if the cGMP binding reaction plus intimately coupled reactions are much slower than the allosteric opening reaction, then the single channels should frequently open by passing first sublevels and, eventually, superlevels before the steady-state level is adopted. Fig. 7 shows recordings from a single CNGA1 channel activated by cGMP jumps from zero to an approximate concentration of 81.2 μM. (The value was estimated from macroscopic currents with the same concentration of 200 μM BCMACMcGMP). The channels opened directly to the main level observed at saturating cGMP but did not develop preceding sub- or superlevels. Hence, the cGMP binding plus intimately coupled reactions are not rate limiting for the activation process.
FIGURE 7.
Representative consecutive single-channel recordings of CNGA1 channels activated by jumps of the cGMP concentration from zero to ∼81.2 μM cGMP. The cyclic nucleotide was liberated from 200 μM BCMACMcGMP. The channel gated directly from the closed to the main open level. The ensemble averaged current (bottom) was formed from 37 traces from 4 patches.
DISCUSSION
The main result of this study on homotetrameric CNGA1 channels is that cGMP jumps to increasing concentrations up to the EC50 value decelerate the activation gating, whereas above the EC50 value, activation by cGMP jumps is accelerated to the higher concentrations. The mean time constant of activation therefore depends on the cGMP concentration in a bell-shaped fashion, quite similar to the results of a previous study upon the gating of CNGA2 channels (18). In the present study, the effective range of cGMP concentrations to activate CNGA1 channels was much narrower than that for CNGA2 channels because the sensitivity of CNGA1 channels to cGMP is lower than that of CNGA2 channels. Any approach to analyze the CNGA1 data in an analog fashion by globally fitting Markovian state models was therefore not promising. Nevertheless, these data unequivocally confirm the finding in CNGA2 channels that close to the EC50 value activation is slowest. The most simple explanation for this maximally slow activation is that the sum of kCO,p and kOC,p is minimal because at higher cGMP concentrations kCO,p increases more than kOC,p decreases whereas at lower cGMP concentrations kOC,p increases more than kCO,p decreases (cf. Scheme 1).
In the previous study upon CNGA2 channels (18), we also observed that toward the lowest cyclic nucleotide concentrations the activation time course slows again. Because cAMP produced the same activation time course as cGMP, only at a ∼26 times higher concentration, whereas the diffusibility of cAMP and cGMP is presumably similar, we concluded that also at these low concentrations the binding reactions of the cyclic nucleotide are not rate limiting for the activation time course. Taking into account that the cGMP concentrations required herein to generate respective activation of CNGA1 channels were much higher than those used for CNGA2 channels, it is not surprising that the diffusional access was found to be not rate-limiting for the activation time course of CNGA1 channels. The reason why we did not observe a slowing of the activation time course for CNGA1 channels toward the lowest cGMP concentrations is certainly that at sufficiently low cGMP the open probability of these channels is too low to resolve reasonable currents at all.
Karpen and co-workers (13) observed that native channels of the rod photoreceptor are activated faster at increasing cGMP concentrations over the whole concentration range. The authors therefore concluded that at physiological cGMP (<5 μM) the activation kinetics are limited by a cGMP binding step. This result conflicts with that obtained herein because we showed that activation becomes slower at increasing cGMP concentrations up to the EC50 value. Two explanations for this discrepancy are possible. One is that Karpen and co-workers studied native channels from the rod photoreceptor, which contains three CNGA1 and one CNGB1 subunit (5–7), whereas we worked in heterologously expressed homotetrameric CNGA1 channels. The gating of native channels and expressed heterotetrameric CNGA1/CNGB1 channels essentially differs from that of homotetrameric CNGA1 channels by a fast flicker (8,9) with an opening and closing rate constant of 15,000 and 21,000 s−1, respectively. Hence, a different gating could be responsible also for the different cGMP dependency of activation.
A second explanation could arise from the different caged cyclic nucleotides used for flash photolysis. Karpen and co-workers (13) used the 4,5-dimethoxy-2-nitrobenzyl ester of cGMP whereas we used coumarinylmethyl esters of cGMP. As noted by Karpen and co-workers (13), nonideal physicochemical properties of their caged cGMP prevented a more thorough quantitative analysis of the activation kinetics. In particular, activation by cGMP jumps did not start from zero current whereas our cGMP jumps were larger and started from zero. To clarify the fundamental difference in the activation time course, it would be interesting to repeat the experiments in native rod channels and heterotetrameric CNGA1/CNGB1 channels with the technique used herein.
Further support for a much more rapid ligand binding than allosteric reaction came from the experiments in which single channels, activated by cGMP jumps, opened directly to the main level without passing sublevels (Fig. 7), which have been attributed previously to the activity of partially liganded channels (27,28). Because each cGMP jump to a saturating concentration must transiently cause a partially liganded channel, the conclusion can only be that the allosteric reaction is slower than the cGMP binding.
The result that conformational changes of the allosteric reaction are rate limiting for the gating of CNGA1 channels over the whole cGMP range was further substantiated by the similarity of the time courses of voltage-dependent activation to those of activation by cGMP jumps (Fig. 4). Consequently, for small cGMP concentrations also the voltage-dependent gating was accelerated toward the lower cGMP concentration. This result is opposite to results in native rod channels, for which a monotonical deceleration of the activation toward the lower cGMP concentration has been described not only for cGMP jumps but also for voltage steps (13). Possible explanations were discussed above.
Despite the rough similarity of the voltage-dependent and the cGMP-jump induced activation, the activation time constants following voltage steps were some slower at the higher cGMP concentrations (Fig. 3 B). This can be explained as follows: The main difference between both types of activation is that activation by voltage starts at substantial open probability, only from a more negative voltage. Hence, the slower activation after cGMP jumps than depolarizing voltage steps at low cGMP is supposed to be caused by additional conformational changes in the allosteric reaction following the binding of cGMP which have already been passed at the same cGMP concentration at −100 mV. At higher cGMP, where both types of activation are similarly rapid, these conformational changes would not be involved. Furthermore, at the higher cGMP concentrations also the weight of the slow exponential is larger for voltage steps than for cGMP jumps (Figs. 2 C and 3 C). This result can be interpreted in a related way: When activating the channels by cGMP jumps, a double exponential activation process is started from an open probability near zero. Apart from the small concentrations, the fast exponential dominates (Fig. 2 C). In contrast, at a given cGMP concentration generating considerable open probability, depolarizing voltage steps increase the open probability only gradually. Apart from the small concentrations, here the slow exponential dominates (Fig. 3 C). Hence, it is likely that at a given cGMP concentration the processes underlying the fast component of activation have mostly been passed already at −100 mV. Consequently, the voltage step to +100 mV at the same cGMP concentration would predominantly drive those processes underlying the slow component of activation and only to a lesser extent those underlying the fast component of activation.
Our results with different permeating ions (Fig. 6) support the notion that in CNGA1 channels the pore gating is involved in the rate-limiting reactions determining the activation time course in the sequence of molecular events between the cGMP binding and pore opening. These results substantiate previous studies which showed that the selectivity filter of CNG channels forms the principal gate (12,29–33). Effects of the permeating ions on the gating were also reported previously. Gamel and Torre (34) observed a slowing of the activation gating by permeating K+ ions with respect to Na+ ions, whereas Holmgren (35) observed shorter open times with permeating Na+ ions than K+ ions. Both studies were conducted with cGMP concentrations exceeding the physiological range by more than ten times. At physiologically low cGMP we previously showed in a single-channel study that ions permeating more slowly (Rb+ > K+ > Na+) prolong the mean open time (26) and in conjunction with the results of the present we conclude that the closing reaction itself is essentially involved in determining the activation time course. This interpretation also explains why at saturating cGMP the different effects of the ions on the activation gating disappear (Fig. 6 B) because the opening is so fast that the influence of the closing reaction is negligible (kCO,s ≫ kOC,s).
Acknowledgments
We are indebted to U. B. Kaupp, Jülich, for providing the CNGA1 clone. We also thank K. Schoknecht, S. Bernhardt, A. Kolchmeier, and B. Tietsch for excellent technical assistance.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to K.B.
References
- 1.Kaupp, U. B., T. Niidome, T. Tanabe, S. Terada, W. Bönigk, W. Stühmer, N. J. Cook, K. Kangawa, H. Matsuo, T. Hirose, T. Miyata, and S. Numa. 1989. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 342:762–766. [DOI] [PubMed] [Google Scholar]
- 2.Finn, J. T., M. E. Grunwald, and K.-W. Yau. 1996. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu. Rev. Physiol. 58:395–426. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman, A. L. 1995. Cyclic nucleotide gated channels. Curr. Opin. Neurobiol. 5:296–303. [DOI] [PubMed] [Google Scholar]
- 4.Kaupp, U. B., and R. Seifert. 2002. Cyclic nucleotide-gated ion channels. Physiol. Rev. 82:769–824. [DOI] [PubMed] [Google Scholar]
- 5.Zheng, J., M. C. Trudeau, and W. N. Zagotta. 2002. Gating rearrangements in cyclic nucleotide-gated channels revealed by patch-clamp fluorometry. Neuron. 28:369–374. [DOI] [PubMed] [Google Scholar]
- 6.Weitz, D., N. Ficek, E. Kremmer, P. J. Bauer, and U. B. Kaupp. 2002. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 36:881–889. [DOI] [PubMed] [Google Scholar]
- 7.Zhong, H., L. L. Molday, R. S. Molday, and K.-W. Yau. 2002. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 420:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, T.-Y., Y.-W. Peng, R. S. Dhallan, B. Ahamed, R. R. Reed, and K.-W. Yau. 1993. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 362:764–767. [DOI] [PubMed] [Google Scholar]
- 9.Körschen, H. G., M. Illing, R. Seifert, F. Sesti, A. Williams, S. Gotzes, C. Colville, F. Müller, A. Dosé, M. Godde, L. Molday, U. B. Kaupp, and R. S. Molday. 1995. A 240 kDa protein represents the complete β-subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 15:627–636. [DOI] [PubMed] [Google Scholar]
- 10.Taylor, W. R., and D. A. Baylor. 1995. Conductance and kinetics of single cGMP-activated channels in salamander rod outer segments. J. Physiol. 483:567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matulef, K., G. E. Flynn, and W. N. Zagotta. 1999. Molecular rearrangements in the ligand binding domain of cyclic nucleotide-gated channel. Neuron. 24:443–452. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, G. E., and W. N. Zagotta. 2001. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 30:689–698. [DOI] [PubMed] [Google Scholar]
- 13.Karpen, J. W., A. L. Zimmerman, L. Stryer, and D. A. Baylor. 1988. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage jump studies. Proc. Natl. Acad. Sci. USA. 85:1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benndorf, K., R. Koopmann, E. Eismann, and U. B. Kaupp. 1999. Gating by cyclic GMP and voltage in the subunit of the cyclic GMP-gated channel from rod photoreceptors. J. Gen. Physiol. 114:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zufall, F., H. Hatt, and S. Firestein. 1993. Rapid application and removal of second messengers to cyclic nucleotide-gated channels from olfactory epithelium. Proc. Natl. Acad. Sci. USA. 90:9335–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke, C., F. Hatt, and J. Dudel. 1987. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci. Lett. 77:199–204. [DOI] [PubMed] [Google Scholar]
- 17.Markwardt, F., and G. Isenberg. 1992. Gating of Maxi K+ channels studied by Ca2+ concentration jumps in excised inside-out multi-channel patches (myocytes from guinea pig urinary bladder). J. Gen. Physiol. 99:841–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nache, V., E. Schulz, T. Zimmer, J. Kusch, C. Biskup, R. Koopmann, V. Hagen, and K. Benndorf. 2005. Activation of olfactory-type cyclic nucleotide-gated channels is highly cooperative. J. Physiol. 569:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zagotta, W. N., and S. A. Siegelbaum. 1996. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 19:235–263. [DOI] [PubMed] [Google Scholar]
- 20.Hagen, V., J. Bendig, S. Frings, T. Eckardt, S. Helm, D. Reuter, and U. B. Kaupp. 2001. Highly efficient and ultrafast phototriggers for cAMP and cGMP by using long-wavelength UV/Vis-activation. Angew. Chem. Int. Ed. Engl. 40:1046–1048. [DOI] [PubMed] [Google Scholar]
- 21.Hagen, V., B. Dekowski, V. Nache, R. Schmidt, D. Geißler, D. Lorentz, J. Eichhorst, S. Keller, H. Kaneko, K. Benndorf, and B. Wiesner. 2005. Coumarinylmethyl esters for ultrafast release of high concentrations of cyclic nucleotides upon one- and two-photon flash photolysis. Angew. Chem. Int. Ed. Engl. 44:7887–7891. [DOI] [PubMed] [Google Scholar]
- 22.Goulding, E. H., G. R. Tibbs, and S. A. Siegelbaum. 1994. Molecular mechanism of cyclic-nucleotide-gated channel activation. Nature. 372:369–374. [DOI] [PubMed] [Google Scholar]
- 23.Tibbs, G. R., E. H. Goulding, and S. A. Siegelbaum. 1997. Allosteric activation and tuning of ligand efficacy in cyclic-nucleotide-gated channels. Nature. 386:612–615. [DOI] [PubMed] [Google Scholar]
- 24.Li, J., W. N. Zagotta, and H. A. Lester. 1997. Cyclic-nucleotide gated channels: structural basis of ligand efficacy and allosteric modulation. Q. Rev. Biophys. 30:177–193. [DOI] [PubMed] [Google Scholar]
- 25.Glasstone, S., K. J. Laidler, and H. Eyring. 1941. The Theory of Rate Processes. McGraw-Hill, New York.
- 26.Kusch, J., V. Nache, and K. Benndorf. 2004. Effects of permeating ions and cGMP on gating and conductance of rod-type cyclic nucleotide-gated (CNGA1) channels. J. Physiol. 560:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz, M. L., and J. W. Karpen. 1997. Single cyclic nucleotide-gated channels locked in different ligand-bound states. Nature. 389:389–392. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz, M. L., and J. W. Karpen. 1999. Opening mechanism of a cyclic nucleotide-gated channel based on analysis of single channels locked in each liganded state. J. Gen. Physiol. 113:873–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, Z. P., M. H. Akabas, E. H. Goulding, A. Karlin, and S. A. Siegelbaum. 1996. Exposure of residues in the cyclic nucleotide-gated channel pore: P region structure and function in gating. Neuron. 16:141–149. [DOI] [PubMed] [Google Scholar]
- 30.Bucossi, G., M. Nizzari, and V. Torre. 1997. Single-channel properties of ionic channels gated by cyclic nucleotides. Biophys. J. 72:1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becchetti, A., K. Gamel, and V. Torre. 1999. Cyclic nucleotide-gated channels. Pore topology studied through the accessibility of reporter cysteines. J. Gen. Physiol. 114:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, J., and S. A. Siegelbaum. 2000. Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron. 28:899–909. [DOI] [PubMed] [Google Scholar]
- 33.Tränkner, D., H. Jagle, S. Kohl, E. Apfelstedt-Sylla, L. T. Sharpe, U. B. Kaupp, E. Zrenner, R. Seifert, and B. Wissinger. 2004. Molecular basis of an inherited form of incomplete achromatopsia. J. Neurosci. 24:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamel, K., and V. Torre. 2000. The interaction of Na+ and K+ in the pore of cyclic nucleotide-gated channels. Biophys. J. 79:2475–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmgren, M. 2003. Influence of permeant ions on gating in cyclic nucleotide-gated channels. J. Gen. Physiol. 121:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]