Abstract
Freeze-induced perturbations of the protein native fold are poorly understood owing to the difficulty of monitoring their structure in ice. Here, we report that binding of the fluorescence probe 1-anilino-8-naphthalene sulfonate (ANS) to proteins in ice can provide a general monitor of ice-induced alterations of their tertiary structure. Experiments conducted with copper-free azurin from Pseudomonas aeruginosa and mutants I7S, F110S, and C3A/C26A correlate the magnitude of the ice-induced perturbation, as inferred from the extent of ANS binding, to the plasticity of the globular fold, increasing with less stable globular folds as well as when the flexibility of the macromolecule is enhanced. The distortion of the native structure inferred from ANS binding was found to draw a parallel with the extent of irreversible denaturation by freeze-thawing, suggesting that these altered conformations play a direct role on freeze damage. ANS binding experiments, extended to a set of proteins including serum albumin, α-amylase, β-galactosidase, alcohol dehydrogenase from horse liver, alcohol dehydrogenase from yeast, lactic dehydrogenase, and aldolase, confirmed that a stressed condition of the native fold in the frozen state appears to be general to most proteins and pointed out that oligomers tend to be more labile than monomers presumably because the globular fold can be further destabilized by subunit dissociation. The results of this study suggest that the ANS binding method may find practical utility in testing the effectiveness of various additives employed in protein formulations as well as to devise safer freeze-drying protocols of pharmaceutical proteins.
INTRODUCTION
Freezing of protein solutions may result in irreversible protein aggregation and severe loss of catalytic activity of enzymes (1), a phenomenon of basic concern and of growing commercial importance for the expanding polypeptide-based pharmaceutical industry. Notwithstanding the relevance of the phenomenon, little is known of the structure of proteins in ice owing primarily to the inapplicability or the poor sensitivity of usual spectroscopic techniques in this highly scattering and anisotropic medium. For example, Fourier transform infrared spectroscopy experiments when conducted on freeze labile proteins have not detected significant changes in their secondary structure (2) presumably because they are carried out at relatively large protein concentrations, a condition in which the perturbation is often attenuated as if the protein itself acted as a stabilizer. Lately, a study by Trp phosphorescence did provide direct evidence that the solidification of water causes important alterations of the native globular fold in all protein examined (3). From the often drastic shortening of the phosphorescence lifetime of Trp residues buried within the rigid core of these macromolecules, it was deduced that their internal flexibility is greatly enhanced in ice, a response to freezing similar to that observed when the polypeptide loses much of its tertiary structure as in the formation of molten globule states.
The aromatic chromophore 1-anilino-8-naphthalene sulfonate (ANS) is feebly fluorescent in water, but its spectrum is blue shifted and its intensity is dramatically increased in nonpolar solvents or when it binds to nonpolar sites of proteins (4,5; for a general review, see Slavik (6)). In the last decade, strong binding of ANS to molten globule states of proteins has been linked to the loss of tertiary structure (7), and since then the method has been applied to characterize transient states in protein denaturation (8–11). Recently, a study conducted in our laboratory investigated the possibility to unveil potential alterations of the protein tertiary structure in ice from binding of ANS (12). In these exploratory experiments conducted with copper-free azurin from Pseudomonas aeruginosa as a model protein system, it was shown that upon freezing of the protein solution the fluorescence of ANS is enhanced severalfold and becomes blue shifted relative to free ANS. Furthermore, the extent of ANS binding was sharply modulated by conditions that affect the thermodynamic stability of the protein. It was sharply enhanced in the presence of the destabilizing salt KSCN or on lowering the temperature, whereas no fluorescence enhancement was found when azurin is stabilized by the addition of glycerol or by adding Cd2+ to the metal binding site. From these findings it was deduced that the solidification of water affects the tertiary structure of azurin and it was suggested that binding of the fluorescence probe could represent a general approach to monitor ice-induced strains on the protein native fold.
This investigation aims to complement that initial study by addressing the following issues: i), establish whether the perturbation of protein structure reported for azurin is general to other proteins; ii), inquire on potential correlations between the susceptibility to the ice-induced perturbation and specific features of the macromolecule such as thermodynamic stability, internal flexibility, internal cavities/hydration, and covalent links between strands; and finally iii), test for a specific vulnerability of multimeric proteins to freeze damage that may be linked to the disruption of quaternary structure. To look at the generality of the phenomenon, ANS binding experiments were carried out on seven new proteins, namely, monomers (bovine serum albumin (BSA), α-amylase, and β-galactosidase (β-gal)), a dimer (alcohol dehydrogenase from horse liver (LADH)), and tetramers (alcohol dehydrogenase from yeast (YADH), lactic dehydrogenase (LDH), and aldolase (ALD)). Among them LDH, α-amylase, and β-gal have in the past been the subject of freeze-thawing inactivation studies (13–17). The second objective was addressed by monitoring the ANS binding capacity of a protein, azurin, mutated to alter its stability and internal dynamics through the creation of internal cavities and the elimination of a disulfide linkage. We examined two single point cavity-forming mutants, I7S and F110S, whose internal cavities of 40 and 100 Ǻ, respectively, appear to be fully hydrated (18), and the double mutant C3A/C26A in which the disulfide bridge linking strands β1and β2 has been severed. These azurins maintain an intact native fold (x-ray structure) but are ∼6 kcal/mole less stable relative to the wild-type (WT) (18–22). Further, their internal flexibility increases drastically from compact WT and C3A/C26A to the cavity-forming mutants I7S and F110S. The results of this investigation indicate that the magnitude of the ice-induced perturbation among the four azurins, deduced from the capacity to bind ANS, increases directly with the plasticity of the globular structure. Further, as the different extent of ANS binding correlates with the amount of irreversible denaturation upon freeze-thawing, it appears that these altered conformations play a direct role on the lability of proteins to freezing. A stressed condition of the native structure in ice appears to be common to most proteins even if oligomers seem to be more susceptible than monomers presumably because the globular fold can be further destabilized by subunit dissociation.
MATERIALS AND METHODS
Materials
All chemicals were of the highest purity grade available from commercial sources and were used without further purification. NaCl suprapur was purchased from Merck (Darmstadt, Germany) and ANS from Sigma (St. Louis, MO). Lyophilized BSA, β-gal from Aspergillus oryzae and yeast alcohol dehydrogenase (YADH), and crystalline suspensions of α-amylase from porcine pancreas, ALD from rabbit muscle and LDH from rabbit muscle (LDH) were purchased from Sigma-Aldrich Co. (St. Louis, MO). LADH was purchased as crystalline suspension from Fluka Chemie GmbH (Buchs SG, Switzerland).
Azurin WT and mutants I7S, F110S, and C3A/C26A were prepared following published protocols. The plasmid carrying the WT sequence was a generous gift from Prof. A. Desideri (Università di Roma, “Tor Vergata”). The procedure for isolation and purification of WT has been described by van de Kamp et al. (23). Details about site-directed mutagenesis, protein expression, isolation, and purification of mutants I7S, F110S, and C3A/C26A have been described elsewhere (22,24). Copper-free azurins (apo-azurins) were prepared from holo-azurins by adding potassium cyanide and EDTA to final concentrations of 0.1 M potassium cyanide and 1 mM EDTA in 0.15 M Tris-HCl, pH 8, followed by column chromatography (23). Azurins stocks were dialyzed and stored in Tris HCl, 10 mM, pH 7.5. Doubly distilled milliQ water was used throughout.
Sample preparation
Before sample preparation, all protein stocks were dialyzed against 2 mM Hepes buffer, pH 7.5. In all fluorescence experiments the samples were prepared from ANS and protein stocks to a final constant protein concentration of 2 μM in 2 mM Hepes buffer, pH 7.5, containing 25 mM NaCl and 0.5 mM EDTA. In the case of azurin, to be consistent with the previous study (12), the protein concentration was raised to 2.5 μM. The concentration of ANS, as determined by absorbance at 370 nm, using ɛ370 = 6800 M−1cm−1 (in methanol), ranged between 10 and 450 μM although in most experiments the upper limit was 320 μM. Fluorescence measurements in ice were conducted as previously described (12). Briefly, 0.5 mL samples were placed in cylindrical spectrosil cuvettes, 4 mm inner diameter, and rapidly frozen in liquid nitrogen. Subsequently, the ice was melted, leaving a small ice seed that was used for controlled freezing in a bath at −10°C. The newly frozen samples were then allowed to anneal for 12 h at −5°C, followed by a final equilibration of 2 h at the temperature selected for fluorescence measurements (−5 and −13°C). Following this procedure, reproducible (<20% variability) and stable results, if not equilibrium conditions, were obtained. Protein concentrations were calculated by using the following molar absorptivities: ɛ280 = 0.66 cm2mg−1 (BSA), 0.92 cm2mg−1 (ALD), 2.21 cm2mg−1 (α-amylase), 1.49 cm2mg−1 (LDH), 1.99 × 105 M−1cm−1 (β-gal), 1.78 × 105 M−1cm−1 (YADH), 3.5 × 104 M−1cm−1 (LADH), 9.8 × 103 M−1cm−1 (azurin).
Fluorescence measurements
Fluorescence measurements were conducted on a homemade fluorometer. The excitation light, provided by a Cermax xenon lamp (LX150UV, ILC Technology, Sunnyvale, CA), is selected (λex = 350 nm for ANS and 285 nm for azurin fluorescence, respectively, 6 nm bandwidth) by a 0.23 m double grating monochromator (SPEX, model 1680, Spex Industries, Edison, NJ) and modulated by a light chopper. The fluorescence emission, collected at 90° from the excitation, is dispersed by a 0.25 m grating monochromator (H-25, Jobin-Yvon, Longjumeau, France) set to a bandwidth of 3 nm. In the case of ANS fluorescence in ice, a 420 nm long-pass filter is inserted to cut the strong scatter of the exciting light. The photomultiplier (EMI 9635QB) current was amplified by a low-noise current preamplifier (Model SR570, Stanford, Sunnyvale, CA) followed by a lock-in amplifier (Model 393, ITHACO, Ithaca, NY) operating at the chopper frequency. The output signal was digitized and stored by a multi-function board (PCI-20428W, Intelligent Instrumentation, Tucson, AZ) utilizing visual Designer software (PCI-20901S version 3.0, Intelligent Instrumentation). To reduce the variability of fluorescence intensity in frozen samples, due to the poor homogeneity of the medium, the sample was rotated (3 Hz) during the measurement and the signal intensity was integrated for 10 s. Optically dense samples were corrected for inner filter attenuation of the intensity, which was typically <15%. Comparisons of fluorescence intensities between liquid and frozen states required a correction of ∼20% to account for the lower transmission of ice, as determined by the decrease in the fluorescence intensity of buried Trps in proteins, such as azurin, between supercooled liquid and frozen states. All experiments were carried out at least in triplicate and the data reported represent average values.
RESULTS AND DISCUSSION
Ice-induced enhancement of ANS fluorescence by WT and mutant apo-azurins
Azurin is a 14 kDa copper-binding protein with an eight-stranded β-sandwich structure about a highly hydrophobic core (25). Among the several azurin mutants available, I7S, F110S, and C3A/C26A maintain a native-like fold (19,22) and will therefore present the same surface topology/chemical composition to the ice-water interface. I7S and F110S are characterized by an internal cavity caused by the substitution of bulky Ile and Phe with smaller Ser, whereas in C3A/C26A the disulfide bridge linking strands β1and β2 has been severed. The stability of these mutants is ∼3 kcal/mole lower relatively to the WT (20–22), and the internal flexibility of the polypeptide is considerably larger, particularly with I7S and F110S (18). Here, we compare the ANS binding capacity of the four azurin species to inquire how these modified features modulate the ice-induced perturbation of tertiary structure.
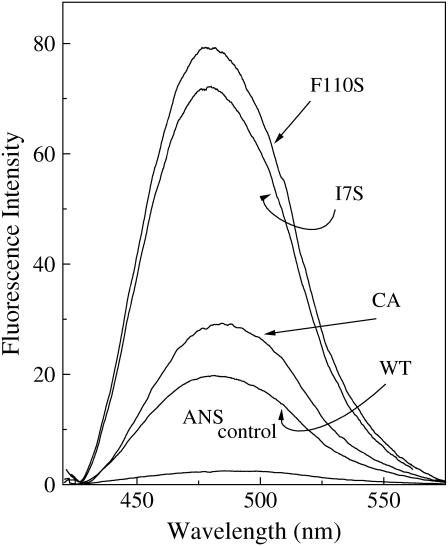
In the liquid state the fluorescence of ANS is not affected by the addition of azurin, WT, and mutants. On freezing of the solution, however, the protein sharply enhances the probe fluorescence and shifts its spectrum to the blue. Fig. 1 shows the change in ANS fluorescence induced by the four azurin species upon freezing of the sample at −13°C (200 μM ANS, 2.5 μM azurin in 2 mM NaP, pH 7.5, 25 mM NaCl). In agreement with the previous report (12) for WT azurin, the intensity is enhanced ∼7-fold and the spectrum is blue shifted by 13 nm relative to the ANS control. For the mutants the spectral blue shift is comparable or slightly larger than the WT but the intensity enhancement is significantly greater: 10.6-fold for C3A/C26A, 26-fold for I7S, and 30-fold for F110S. The preceding study (12) has demonstrated that the creation of hydrophobic ANS binding sites in ice, accounting for the spectral blue shift and intensity enhancement, is owed to partial disruption of the protein tertiary structure triggered by the solidification of water. Here, the much greater intensity enhancement observed for the mutants, compared to the WT, stands to indicate that the number of ANS binding sites and, consequently, the magnitude of the structural alteration are sensibly larger with these less stable azurins.
FIGURE 1.
Enhancement of ANS (200 μM) fluorescence intensity in ice (2 mM Hepes, pH 7.5, plus 25 mM NaCl), at −13°C, upon the addition of azurin (2.5 μM), WT, and mutants. λex = 350 nm.
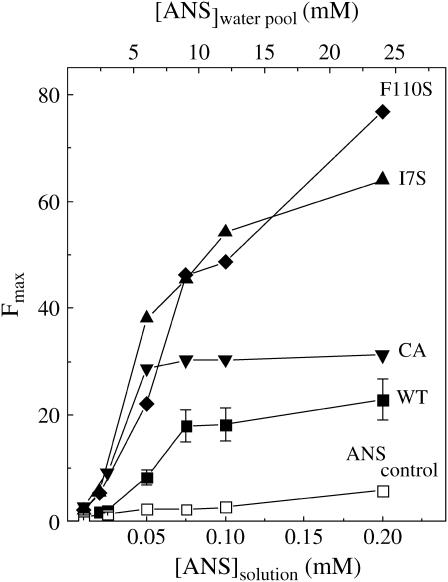
The dependence of the ANS fluorescence intensity, in ice at −13°C, on the starting ANS concentration in solution (10–200 μM) is shown in Fig. 2. These intensity profiles are reminiscent of binding curves except for assuming a slightly sigmoidal shape due to a more gradual increase in the signal in the low concentration range. It was pointed out that this trend probably reflects multiple binding sites of varying affinity, likely a consequence of the broad distribution of protein conformations in ice (3). The profiles of Fig. 2 show that, relative to the WT, onset of ANS binding occurs at lower concentrations with the mutants and in the case of I7S and F110S saturation is not achieved up to 200 μm ANS. Thus, weakening the azurin fold by the mutations leads to the creation of additional binding sites characterized by a broad distribution of binding affinities, a large fraction of which are of stronger affinity than in the WT protein. Unlike simple hyperbolic binding curves, profiles such as those of Fig. 2 can at best provide a rough indication of the thermodynamic parameters governing ANS binding. Their main utility is to compare differences in the ANS binding propensity among proteins or brought about by changing experimental conditions such as ice temperature, freezing protocols, or the addition of solutes. Having stressed that the data of Fig. 2 should not be considered a binding curve, it may still be instructive to estimate an apparent average affinity of ANS for the four azurin species. By assuming equilibrium conditions and an ANS activity in ice equal to the concentration in solution multiplied by a freeze concentration factor of 120 (12), one derives ANS dissociation constants in the range of 6–8 mM for WT, 2–4 mM for C3A/C26A, 2.5–5 mM for I7S, and 1.5–9 mM for F110S. Compared to affinities of 0.1–0.4 mM observed for protein molten globule states (7), these estimates suggest that the binding sites created in ice are intrinsically weaker. The decreased strength of hydrophobic interactions at low temperature and the peculiarly high solute concentration (∼5 M) and ionic strength of the liquid water pool may be factors that contribute to the lower affinity of ANS for proteins in ice.
FIGURE 2.
ANS fluorescence intensity (Fmax) profiles of various azurin species (2.5 μM) in ice at −13°C as a function of the starting ANS concentration in solution: WT (▪), C3A/C26A (▾), I7S (▴), and F110S (♦). The control refers to a protein-free sample. Indicated on the top horizontal axis is the concentration of ANS in the water pools of ice, [ANS](water pool), estimated from a freeze concentration factor fc = 120 (12). Other conditions are as in Fig. 1.
An estimate of the number of binding sites requires knowledge of the average fluorescence quantum yield (ϕF) of ANS bound to azurin. As this is not available one can at best calculate the minimum average number of binding sites per macromolecule, n, by assuming the maximum fluorescence intensity increment, ∼110-fold, ever observed between free and protein-bound ANS (ϕFprotein/ϕFice = 0.9/0.008 ∼110; (12)). If R is the ANS/azurin molar ratio near or above the saturation point and M the corresponding fluorescence enhancement factor, M = Fmax/Fcontrol, then n = 0.009 × R × (M − 1) ([(R − n) × 0.008 + n × 0.9]/R × 0.008 = M). Accordingly, the minimum number of bound ANS molecules (n) increases from 1.6 for WT to 2.9 for C3A/C26A, 6.1 for I7S, and >7.4 for F110S. The true number is probably larger because on average protein sites exhibit ANS fluorescence yields much lower than 0.9 (26).
Both a greater affinity and a larger number of binding sites attest to a significantly greater perturbation of tertiary structure in the azurin mutants than in the WT. With each mutant azurin, a sizable fraction of binding sites exhibits affinities in the 1–2 mm range, corresponding to a dissociation constant 3–4 times lower than that of WT. The individual response to freezing differs considerably, on the other hand, with regard to the number of binding sites n increasing two and three times relative to C3A/C26A in I7S and F110S, respectively. In an attempt to correlate affinities and n values to specific aspects of the macromolecule, we observe that all mutants are less stable than the WT by a comparable amount, the denaturation-free energy change, ΔG° at 20°C, decreasing from 6–7 to ∼3 kcal/mole (20,27). Thus, it is tempting to associate the higher affinity of the mutant binding sites to a less stable globular fold and conclude that their creation is favored by, or requires, structural plasticity. On the other hand, different n values seem to correlate with the large disparity in internal hydration and structural flexibility among the three mutant azurins. In C3A/C26A the elimination of the superficial C3-C26 disulfide bridge, linking the ends of strands β1and β2, has little effect on the compactness of the protein core and, judging from the phosphorescence properties of W48 embedded in it (18), internal motions remain restrained. On the contrary, an important consequence of creating an internal cavity of 40 in I7S and 100 Å3 in F110S, presumably filled with water (18,19), is the onset of large amplitude structural fluctuations that increase remarkably the flexibility of the globular fold, as evidenced by the three to four orders of magnitude increase in permeability to acrylamide (18). A possible explanation for the above correlation is that the drastic drop in water activity on ice formation causes the cavities to dehydrate and collapse with consequent rearrangement of the internal peptide structure. This process may ensue in widespread deformations of the native fold with the creation of a large number of ANS binding pockets. All together the above considerations suggest that the ice-induced capacity to bind ANS, and presumably the magnitude of the underlying structural perturbation, will be greatest with deformable, flexible, and internally hydrated globular folds.
To test whether there is a relationship between the extent of ANS binding induced by freezing and the instability of azurin to the freeze-thawing process, we have determined the degree of irreversible denaturation of WT and mutant species when subjected to repeated freeze-thawing cycles. Thanks to an unusually blue shifted Trp fluorescence spectrum of native azurin, a measure of the protein fraction remaining native after freezing-thawing can be obtained directly from the residual fluorescence intensity at 300 nm (λex = 292 nm), a wavelength where denatured-aggregated proteins were found to contribute little to the overall intensity. For this test the samples were rapidly frozen in liquid nitrogen and then thawed at ambient temperature. On repeating the process we found a consistent decrease in fluorescence intensity at 300 nm accompanied by an emerging red component in the spectrum and enhanced scattering from the solution, the latter response revealing partial unfolding of the macromolecule with exposure of buried W48 to the solvent and concomitant formation of protein aggregates. The remaining native fraction after 15 freeze-thawing cycles is listed in Table 1 where it is compared to the respective values of n and average dissociation constant Kd. The results clearly indicate that the extent of azurin denaturation-aggregation is correlated to the value of n increasing from WT to mutant proteins and that among the latter species it is significantly larger with the cavity-forming mutants I7S and F110S relative to compact C3A/C26A. Such a link between the instability to freeze-thawing and propensity to bind ANS in ice supports the notion that irreversible damage to freeze labile proteins, often resulting in enzyme inactivation and or protein aggregation, is likely to have originated from partial unfolding of the globular structure in ice.
TABLE 1.
Comparison between the propensity of various azurin species to binding ANS in ice at −13°C and the instability of the native fold to freeze-thawing
| Azurin | Kd (mM)* | n† | (1 − fN)‡ |
|---|---|---|---|
| WT | 7.0 | 1.6 | 0.28 |
| C3A/C26A | 3.0 | 2.9 | 0.46 |
| I7S | 3.2 | 6.1 | 0.64 |
| F110S | 5.2 | 7.4 | 0.62 |
Kd, average apparent dissociation constant for ANS binding to azurin.
n, minimum number of bound, highly fluorescent ANS molecules.
(1 − fN), nonnative fraction of azurin after 15 freeze-thawing cycles.
Ice-induced ANS binding to other proteins
To examine whether the ice-induced perturbation observed for azurin is common to proteins in general, ANS binding experiments were extended to a set of seven other proteins including monomeric BSA, α-amylase and β-gal, dimeric LADH and tetrameric ALD, YADH, and LDH. We anticipate here that every protein was found to enhance ANS fluorescence in ice, with distinct spectral blue shifts and intensity increments. This suggests that perturbations of the tertiary structure are widespread and implies that the frozen medium is a harsh environment for many globular folds. Before comparing the behavior of individual proteins it is convenient to divide the group into two classes: one (β-gal, α-amylase, and ALD), which binds ANS essentially only in the frozen state, and the rest, which bind a fair amount of ANS also in liquid solution (4,5,28). We first analyze the latter class and demonstrate that additional binding of ANS in ice is due to the creation of new binding sites rather than to a more complete saturation of preexisting weak sites after the large freeze-induced concentration of the ligand.
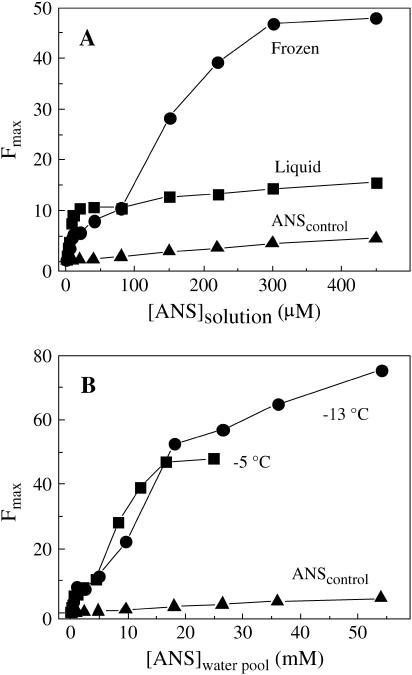
The fluorescence intensity profiles of BSA, shown in Fig. 3, A and B, are characteristic of the latter protein class. We anticipate that the observations made below for BSA apply equally to LDH, YADH, and LADH. Fig. 3 A compares the effect of 2 μM BSA on the ANS intensity in supercooled liquid and frozen states at −5°C. In supercooled solutions ANS binds with a dissociation constant in the μM range (∼5 μM) and no further intensity increment beyond the control is observed up to 450 μM, practically the maximum ANS concentration before inner filter artifacts and declining sensitivity become prohibitive. Upon freezing the sample, the ANS intensity first decreases in the low concentration range, below 80 μM, but then rises sharply above this threshold. The initial decrease in fluorescence intensity takes place despite the solidification of water raised by ∼55-fold the concentration of ANS in the liquid water pool of ice (12). Indeed, if one compares liquid and frozen state profiles at the same effective ANS concentration in the liquid phase (bulk solution versus liquid water pools formed at intergranular spaces), we see that strong ANS binding is largely abolished in ice. For example, at 20 μm ANS where binding sites are fully saturated in solution and the intensity has reached its maximum value, the corresponding intensity in ice is merely 3%–5% of the value in the liquid water. Thus, in ice either the high affinity ANS binding sites present in solution are no longer available to the aromatic probe or the fluorescence yield of ANS bound to these sites is drastically reduced. In either case the conclusion is that in the frozen state the polypeptide structure of the putative binding sites has changed, becoming unable to host ANS or to enhance its fluorescence yield, as when the probe surrounding changes from rigid to mobile (26). In the higher concentration end of the profile the intensity in ice is up to four times larger than in the liquid state. A rough estimate of the minimum number of binding sites, as done above with azurin, yields n ∼20, which is four times larger than the corresponding value in liquid solution (5). Thus, the solidification of water alters the structure of BSA, creating a looser fold able to bind numerous ANS molecules in its interior that exhibit large fluorescence yields. Fig. 3 B emphasizes that the value both of n and of the apparent dissociation constant are considerably affected by temperature. As for azurin (12), lowering the freezing temperature from −5°C to −13°C increases the number of binding sites but the average affinity is lowered. Such modulation of ANS binding upon changing the characteristics of ice (by temperature but also by stabilizing cosolutes; G. B. Strambini, unpublished data) provides additional evidence that the creation of these low affinity ANS binding sites is owed to perturbations of the protein fold associated with the frozen medium.
FIGURE 3.
(A) Effect of freezing on the ANS fluorescence intensity (λmax = 465 nm) profile of 2 μM BSA (2 mM Hepes, pH 7.5, plus 25 mM NaCl); comparison between supercooled solution (▪) and ice (•) at −5°C. Intensities in ice were corrected (increased by 20%) for the lower transmittance of the frozen state. (B) Effect of temperature on the enhancement of ANS fluorescence in ice by BSA (2 μM). The [ANS](water pool) is derived by multiplying the ANS concentration in solution by the corresponding freeze concentration factors: 55 (−5°C) and 120 (−13°C).
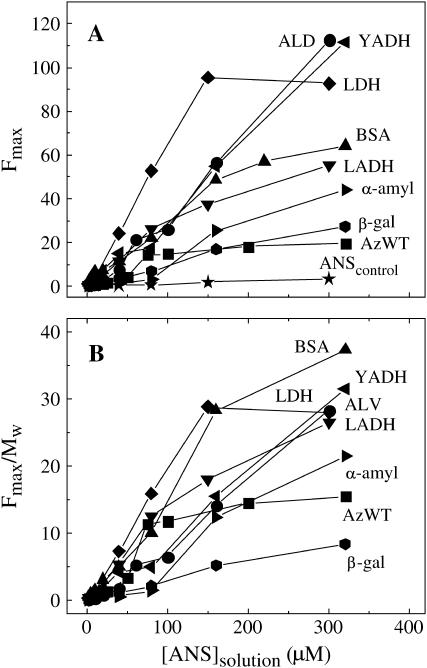
The fluorescence profiles of all seven proteins in ice, at −13°C, are compared in Fig. 4. The data establish that every protein exhibits enhanced ANS fluorescence and therefore significant ice-induced ANS binding. Relative to azurin (WT), which is a small tightly packed polypeptide, the intensity enhancement is generally larger (Fig. 4 A), up to sixfold, whereas the onset of binding occurs at either smaller or higher ANS concentrations, indicating larger and smaller binding affinities, respectively. For some proteins (BSA, YADH, ALD) the profile is distinctly Multiphasic, and in many cases (all but LDH and azurin) saturation is not reached up to 300 μM ANS. The latter feature shows that weak binding sites, similar to those induced in azurin by the destabilizing salt KSCN (12), are common with proteins under these conditions. To aid comparing the ANS binding propensity among the various proteins Table 2 lists the minimum number of binding sites (n) as well as the ratio n/Mw, normalization by the molecular weight taking into account that larger macromolecules, such as oligomers, have a greater probability to bind ANS simply for their larger mass. Mass normalized fluorescence profiles are shown in Fig. 4 B and the relative area under these curves, which depending on both Kd and n is a convenient empirical indicator of the ANS binding capacity, is given in Table 2.
FIGURE 4.
(A) ANS fluorescence intensity (Fmax) profiles of various proteins (2.0 μM) in ice, at −13°C, as a function of the starting ANS concentration in solution: ALD (•), YADH (◂), LDH (♦), BSA (▴), LADH (▾), α-amylase (▸), β-gal ( ), and azurin WT (▪). Other conditions are as in Fig. 1. (B) The same ANS fluorescence profiles of Fig. 4 A normalized by the protein molecular weight (Table 2).
), and azurin WT (▪). Other conditions are as in Fig. 1. (B) The same ANS fluorescence profiles of Fig. 4 A normalized by the protein molecular weight (Table 2).
TABLE 2.
List of ANS fluorescence maxima (λmax) and minimum number of binding sites (n) for proteins in ice at −13°C
| Protein | λmax(nm) | n | Mw · 10−3 | 10−5 · n/Mw | Rel. area* |
|---|---|---|---|---|---|
| Monomers | |||||
| Azurin WT | 480 | 1.6 | 14 | 11.4 | 2.5 |
| BSA | 465 | >25.0 | 67 | >37.3 | 4.9 |
| α-amylase | 491 | >14.4 | 45 | >21.5 | 2.2 |
| β-gal | 488 | 9.7 | 115 | 8.4 | 1.0 |
| Dimers | |||||
| LADH | 475 | >21.2 | 80 | >26.5 | 3.5 |
| Tetramers | |||||
| YADH | 486 | >44.4 | 141 | >28.3 | 3.3 |
| ALD | 475 | >44.8 | 158 | >31.5 | 2.9 |
| LDH | 470 | 37.6 | 130.5 | 28.8 | 4.7 |
Relative area under the plots of Fig. 4 B.
On a molar basis, the greatest apparent affinity and the largest values of n are exhibited by the tetramers LDH, YADH, and ALD, the smallest by the monomers azurin, β-gal, and α-amylase. The high response of tetrameric proteins may suggest a particular destabilization of the native structure associated to the oligomeric state, a perturbation probably related to dissociation of the subunits and exposure of previously buried hydrophobic regions. Both Fig. 4 B and comparison of the relative areas confirm that even on a mass basis the propensity to bind ANS, with the exception of BSA, is significantly larger for oligomers than for monomers. Although for BSA a high binding capacity may be expected from its intrinsic tendency to bind nonpolar and aromatic moieties also in the liquid state, the above ANS binding data do suggest that beyond specific trends of individual polypeptides, multi-subunit proteins are more susceptible than monomers to destabilization and partial unfolding in the frozen state. Although suitable data are not available to establish if there is a direct correlation between the above ANS binding capacity and the actual instability to freeze-thawing (depending on the protein, irreversible damage has been estimated by diverse criteria such as loss of enzymatic activity, degree of aggregation, and loss of secondary or tertiary structure and, further, the outcome is generally influenced by the rates of freezing and thawing), it is a common observation that multimeric enzymes tend to be less stable to freezing than their monomeric counterpart (17). Also, LDH, which is notoriously unstable to freezing (14–16), was found to exhibit the largest binding capacity among the proteins examined in this study. It may also be possible that a smaller degree of subunit dissociation in part explains the sharp increase in the residual enzymatic activity, retained after freeze-thawing, with increasing protein concentration (14,16,29).
As a final remark we point out the close association found between ANS binding capacity (relative area, Table 2) and the peak wavelength of the fluorescence spectrum, λmax, the latter shifting further to the blue as the binding capacity increases. Because the spectrum reflects the hydrophobicity of the binding site, shifting to the blue in less polar environments (30,31), the above correlation suggests that extensive binding of ANS is associated with the exposure of hydrophobic regions of the macromolecule that are generally buried and inaccessible to the probe. This trend is consistent with a pronounced binding capacity of oligomers whose subunits tend to dissociate in frozen aqueous media thereby making accessible large hydrophobic patches of the subunit interface.
In conclusion, from examining azurin mutants of lower thermodynamic stability and greater internal flexibility than the WT, it was found that the perturbation of the native fold in the frozen state, evidenced by the capacity to bind ANS, is enhanced by the plasticity of the globular structure and correlates with the lability to freeze-thawing. The ANS binding test extended to other proteins confirmed a widespread stressed condition of the globular structure in ice, indicating that oligomers' proteins are more susceptible to unfolding than monomers', presumably because the macromolecule can be further destabilized by subunit dissociation. The sensitivity of the ANS binding method to the tertiary structure of proteins in ice should find practical utility in devising safer freeze-drying protocols of pharmaceutical proteins and testing the effectiveness of various stabilizing/destabilizing agents employed in protein formulations.
References
- 1.Franks, F. 1985. Biophysics and Biochemistry at Low Temperature. Cambridge University Press, London.
- 2.Allison, S. D., A. Dong, and J. F. Carpenter. 1996. Counteracting effects of thiocyanate and sucrose on chymotrypsinogen secondary structure and aggregation during freezing, drying, and rehydration. Biophys. J. 71:2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strambini, G. B., and E. Gabellieri. 1996. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys. J. 70:971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stryer, L. 1965. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of nonpolar binding sites. J. Mol. Biol. 13:482–495. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, E., and G. Weber. 1966. Cooperative effects in binding by bovine serum albumin. I. The binding of 1-anilino-8-naphthalenesulfonate. Fluorimetric titrations. Biochemistry. 5:1893–1900. [DOI] [PubMed] [Google Scholar]
- 6.Slavik, J. 1982. Anilinonaphtalene sulfonate as a probe of membrane composition and function. Biochim. Biophys. Acta. 694:1–25. [DOI] [PubMed] [Google Scholar]
- 7.Semisotonov, G. V., N. A. Rodionova, O. I. Razgulyaev, V. N. Uversky, A. F. Gripas, and R. I. Gilmanshin. 1991. Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers. 31:119–128. [DOI] [PubMed] [Google Scholar]
- 8.Das, B. K., T. Bhattacharyya, and S. Roy. 1995. Characterization of a urea induced molten globule intermediate state of glutaminyl-tRNA synthetase from Escherichia coli. Biochemistry. 34:5242–5247. [DOI] [PubMed] [Google Scholar]
- 9.Guha, S., and B. Bhattacharyya. 1995. A partially folded intermediate during tubulin unfolding: its detection and spectroscopic characterization. Biochemistry. 34:6925–6931. [DOI] [PubMed] [Google Scholar]
- 10.Uversky, V. N., S. Winter, and G. Löber. 1996. Use of fluorescence decay times of 8-ANS-protein complexes to study the conformational transitions in proteins which unfold through the molten globule state. Biophys. Chem. 60:79–88. [DOI] [PubMed] [Google Scholar]
- 11.Bismuto, E., E. Gratton, and D. C. Lamb. 2001. Dynamics of ANS binding to tuna apomyoglobin measured with fluorescence correlation spectroscopy. Biophys. J. 81:3510–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabellieri, E., and G. B. Strambini. 2003. Perturbation of protein tertiary structure in frozen solutions revealed by 1-anilino-8-naphthalene sulfonate fluorescence. Biophys. J. 85:3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittam, J. H., and H. L. Rosano. 1973. Effects of the freeze-thaw process on α-amylase. Cryobiology. 10:240–243. [DOI] [PubMed] [Google Scholar]
- 14.Tamiya, T., N. Okahashi, R. Sakuma, T. Aoyama, T. Akahane, and J. J. Matsumoto. 1985. Freeze denaturation of enzymes and its prevention with additives. Cryobiology. 22:446–456. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter, J. F., and J. H. Crowe. 1988. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 25:244–255. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, S., and S. L. Nail. 1998. Effect of process conditions on recovery of protein activity after freezing and freeze-drying. Eur. J. Pharm. Biopharm. 45:249–257. [DOI] [PubMed] [Google Scholar]
- 17.Pikal-Cleland, K. A., N. Rodriguez-Hornedo, G. L. Amidon, and J. F. Carpenter. 2000. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric β-galactosidase. Arch. Biochem. Biophys. 384:398–406. [DOI] [PubMed] [Google Scholar]
- 18.Cioni, P., E. de Waal, G. W. Canters, and G. B. Strambini. 2004. Effects of cavity-forming mutations on the internal dynamics of Azurin. Biophys. J. 86:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammann, C., A. Messerschmidt, R. Huber, H. Nar, G. Gilardi, and G. W. Canters. 1996. X-ray crystal structure of the two site-specific mutants Ile7Ser and Phe110Ser of Azurin from Pseudomonas aeruginosa. J. Mol. Biol. 255:362–366. [DOI] [PubMed] [Google Scholar]
- 20.Mei, G., A. Di Venere, F. Malvezzi Campeggi, G. Gilardi, N. Rosato, F. De Matteis, and A. Finazzi-Agrò. 1999. The effect of pressure and guanidine hydrochloride on azurins mutated in the hydrophobic core. Eur. J. Biochem. 265:619–626. [DOI] [PubMed] [Google Scholar]
- 21.Guzzi, R., L. Sportelli, C. La Rosa, D. Milardi, D. Grasso, M. P. Verbeet, and G. W. Canters. 1999. A spectroscopic and calorimetric investigation on the thermal stability of the Cys3Ala/Cys26Ala azurin mutant. Biophys. J. 77:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonander, N., J. Leckner, H. Guo, B. G. Karlsson, and L. Sjolin. 2000. Crystal structure of the disulfide bond-deficient Azurin mutant C3A/C26A. How important is the S-S bond for folding and stability? Eur. J. Biochem. 267:4511–4519. [DOI] [PubMed] [Google Scholar]
- 23.Van de Kamp, M., M. C. Silvestrini, M. Brunori, J. van Beeumen, F. C. Hali, and G. W. Canters. 1990. Involvement of the hydrophobic patch of azurin in the electron-transfer reactions with cytochrome-C551 and nitrite reductase. Eur. J. Biochem. 194:109–118. [DOI] [PubMed] [Google Scholar]
- 24.Gilardi, G., G. Mei, N. Rosato, G. W. Canters, and A. Finazzi-Agrò. 1994. Unique environment of Trp48 in Pseudomonas aeruginosa azurin as probed by site-directed mutagenesis and dynamic fluorescence spectroscopy. Biochemistry. 33:1425–1432. [DOI] [PubMed] [Google Scholar]
- 25.Nar, H., A. Messerschimdt, R. Huber, M. van de Kamp, and G. W. Canters. 1991. Crystal structure analysis of oxidized Pseudomonas aeruginosa azurin at pH 5.5 and pH 9.0. J. Mol. Biol. 221:765–772. [DOI] [PubMed] [Google Scholar]
- 26.Kirk, W. R., E. Kurian, and F. G. Prendergast. 1996. Characterization of the sources of protein-ligand affinity: 1-sulfonato-8-(1′)anilinonaphthalene binding to intestinal fatty acid binding protein. Biophys. J. 70:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cioni, P., E. Bramanti, and G. B. Strambini. 2005. Effects of sucrose on the internal dynamics of azurin. Biophys. J. 88:4213–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand, L., J. R. Gohlke, and D. S. Rao. 1977. Evidence for binding of rose bengal and anilinonaphthalenesulfonates at the active site regions of liver alcohol dehydrogenase. Biochemistry. 6:3510–3518. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter, J. F., J. H. Crowe, and T. Arakawa. 1990. Comparison of solute-induced protein stabilization in aqueous solution and in the frozen and dried states. J. Dairy Sci. 73:3627–3636. [Google Scholar]
- 30.Turner, D. C., and L. Brand. 1968. Quantitative estimation of protein binding site polarity. Fluorescence of N-arylaminonaphthalenesulfonates. Biochemistry. 7:3381–3390. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, G. W., R. J. Robbins, G. R. Fleming, J. M. Morris, A. E. W. Knight, and R. J. S. Morrison. 1978. Picosecond studies of the fluorescence probe molecule 8-anilino-1-naphthalenesulfonic acid. J. Am. Chem. Soc. 100:7145–7150. [Google Scholar]