Despite optimal treatment some wounds are slow to heal. The challenge clinically and microbiologically is to identify those wounds in which healing is impaired as a result of infection or heavy bacterial burden and in which systemic or topical antimicrobial treatment will be of benefit.
Figure 1.

A charcoal swab preserves bacteria during transport to the laboratory
Table 1.
Management of bite wounds
| • Carry out meticulous surgical debridement and cleansing of wound |
| • Send deep tissue specimens for microbiology testing |
| • Consider empirical treatment with antibiotics |
| • Consider tetanus prophylaxis |
| • Seek microbiological advice if bite was by exotic animal |
Staphylococci and streptococci are the most commonly encountered pathogenic organisms in community acquired superficial wounds. More unusual organisms may be found in bite wounds, and these reflect the source of the bite. Pathogenic organisms causing surgical wound infections vary according to the anatomical site of surgery. Antibiotic resistant organisms, such as methicillin resistant Staphylococcus aureus (MRSA), are more commonly encountered, reflecting the hospital flora.
Figure 2.
Punch biopsy for microbiological analysis
Table 2.
Signs of wound infection
| • Redness |
| • Heat |
| • Pain |
| • Swelling |
| • Exudate (purulent, serous, or serosanguinous) |
| • Odour |
| • Poor healing |
| • Contact bleeding |
| • Epithelial bridging |
| • Tissue breakdown |
| • Presence of unhealthy granulation tissue |
| • Systemic illness in the absence of other focus of infection |
When to sample
It is inappropriate to swab all wounds: swabs should be taken only from overtly infected wounds and from wounds that are deteriorating, increasing in size, or failing to make satisfactory progress despite an optimal environment for wound healing. Indicators of wound infection include redness, swelling, purulent exudate, smell, pain, and systemic illness in the absence of other foci. Subtle signs of local wound infection include unhealthy “foamy” granulation tissue, contact bleeding, tissue breakdown, and epithelial bridging.
Figure 3.
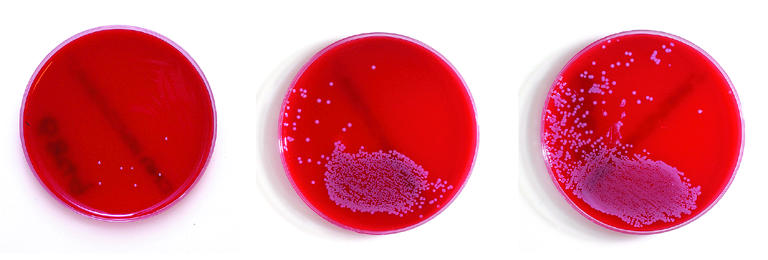
Semiquantitative analysis of swab showing light or scanty, moderate, and heavy growth of Staphylococcus aureus
Types of sample
Superficial wound swabs—The ease of obtaining and processing superficial wound swabs, combined with their relatively low cost and non-invasive nature, make them in most instances the most appropriate method for wound sampling. Organisms cultured from a superficial swab may, however, simply reflect the colonising bacterial flora and are not always representative of the pathogenic organisms invading deeper tissue. This is particularly relevant to deep surgical and deep penetrating wounds in which infection from internal sources may occur.
Table 3.
How to take a superficial wound swab
| • Removal of superficial debris followed by swabbing of the wound bed is considered to be the best way to obtain a superficial wound swab |
| • Swabs containing transport media and charcoal should be used as they help to preserve bacteria before laboratory analysis |
| • Timely delivery of the swab to the microbiology laboratory is essential |
Tissue and pus—Tissue or pus, or both, should be collected whenever possible, as growth from these samples is more representative of pathogenic flora. These are amenable to quantitative microbiological analysis and other techniques used to improve the diagnostic yield. Tissue biopsy should always be carried out when therapeutic debridement of the wound is done, in cases of osteomyelitis, and when superficial sampling methods have been ineffective.
Less invasive techniques—Less invasive sampling techniques—such as dermabrasion and various absorbent pads—have been developed. A wide range of products is available, but no single method is used routinely yet.
Microbiological analysis
Semiquantitative analysis
Most laboratories will perform a semiquantitative analysis on wound swabs. This entails grading bacterial growth as scanty, light, moderate, or heavy. Semiquantitative analysis introduces a bias towards motile and fast growing organisms.
This is the 10th in a series of 12 articles
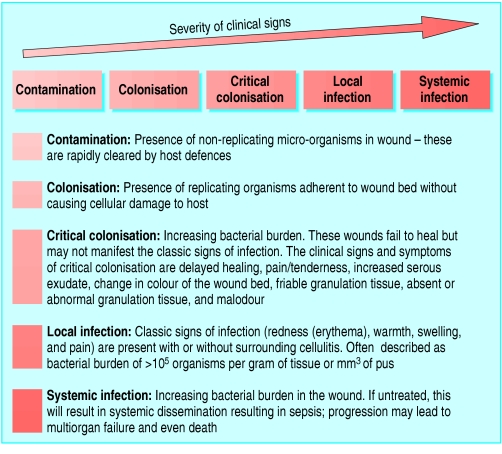
Infection is a major source of failed wound healing
Fastidious organisms such as anaerobes may be under-represented. Semiquantitative counts have been shown to correlate with quantitative tissue counts in both burn wounds and diabetic foot ulcers.
Figure 4.
Spectrum of interaction between bacteria and host
Quantitative analysis
Bacterial load greater than 100 000 organisms or colony forming units per gram of tissue or mm3 of pus is a predictor of wound infection.
Figure 5.
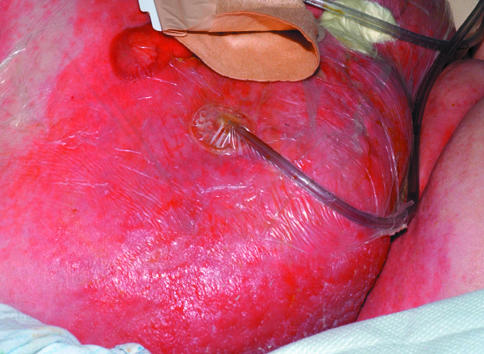
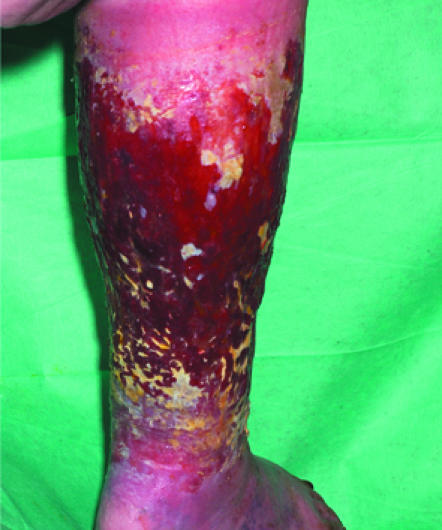
Left: Extensive cellulitis complicating laparotomy wound. Right: Severely locally infected wound showing unhealthy granulation tissue
However, some wounds that are more heavily colonised will heal spontaneously, and, conversely, some organisms are able to cause serious infection at much lower levels of colonisation. Infection depends on the pathogenicity of the organism, the type of wound, and the host response.
Table 4.
Microbiological analysis
| Type of analysis | Suitable samples | Advantages | Disadvantages |
|---|---|---|---|
| Gram stain | Tissue, pus, or swab transported immediately to laboratory | Instant results; good correlation with quantitative counts | Poor sensitivity; no antibiotic sensitivity pattern |
| Quantitative culture | Tissue, pus, dermabrasion specimens, absorbent pad specimens | Counts > 105 organisms or colony forming units per gram of tissue predict wound infection | Invasive; labour intensive; costly |
| Semiquantitative culture | All specimens | Practical; can be carried out on swab specimens; some correlation with quantitative analysis | Imprecise; bias towards motile/fast growing organisms; sampling of superficial colonising bacteria |
Interpretation of results
Most wound swabs will yield bacterial growth. Growth of bacteria from wounds is not synonymous with infection, and treatment based on microbiological results alone is not warranted.
Table 5.
Empirical antibiotic treatment of wound infection in systematically unwell patient*
| Type of wound | Antibiotic |
|---|---|
| Wound infection | Co-amoxiclav |
| Surgical wound infection | Cefuroxime and metronidazole or co-amoxiclav |
| Bite wound | Co-amoxiclav |
| Diabetic ulcer | Co-amoxiclav and ciprofloxacin |
| Osteomyelitis | Co-amoxiclav or ciprofloxacin and clindamycin |
| Necrotising fasciitis | High dose benzylpenicillin plus clindamycin (with or without ciprofloxacin) |
| MRSA infection suspected | Vancomycin or linezolid |
Rough guide only. In general, treatment should be guided by discussion with the local microbiology department. Choice of antibiotics will depend on previous microbiology where available, previous antibiotic treatment, site of surgery, and the local prevalence of MRSA.
Treatment
Wound infections in association with systemic illness, deep invasion, or cellulitis require empirical systemic antibiotic treatment while culture results are awaited. Choice of treatment will depend on factors such as the type and site of wound; previous microbiological results; and host factors such as drug allergies. Clinicians must always be alert to the possibility of necrotising fasciitis. A high level of suspicion followed by prompt aggressive surgical debridement of devitalised necrotic tissue is essential if the patient is to survive. Important clinical markers include pain disproportionate to clinical signs, anaesthesia over the infected area, and systemic illness.
Table 6.
Clinical markers of necrotising fasciitis*
| Early presentation | Late presentation |
|---|---|
| • Pain (may be disproportionate to clinical signs) | • Severe pain |
| Skin discoloration (purple or black) | |
| • Blistering | |
| • Cellulitis | • Haemorrhagic bullae |
| • Swelling of the affected region | • Crepitus |
| • Discharge of “dishwater” fluid | |
| • Induration | • Severe sepsis or systemic inflammatory response syndrome |
| • Skin anaesthesia | |
| • Fever | • Multiorgan failure |
| • Tachycardia |
From Hasham et al, 2005 (see Further Reading box)
Superficial wound swabs are not always representative of the pathogenic organisms invading deeper tissue
Treatment of locally infected wounds with topical antiseptics such as silver compounds or iodine will be sufficient in most instances. Topical treatment avoids the potential side effects of systemic antibiotics, such as Clostridium difficile diarrhoea, anaphylaxis, gastrointestinal upset, and, perhaps most importantly, selection of resistant organisms. Systemic treatment may be indicated if topical medication is unsuccessful.
Figure 6.
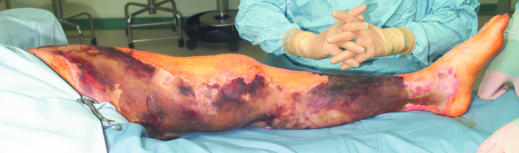
Necrotising fasciitis
In general, topical antibiotics are not recommended. Reasons for this include inadequate penetration for deep skin infections, development of antibiotic resistance, hypersensitivity reactions, systemic absorption when applied to large wounds, and local irritant effects leading to further delay in wound healing. Short courses of silver sulfadiazine or topical metronidazole can be useful, however, in certain circumstances—for example, with burns and chronic ulcers.
Figure 7.
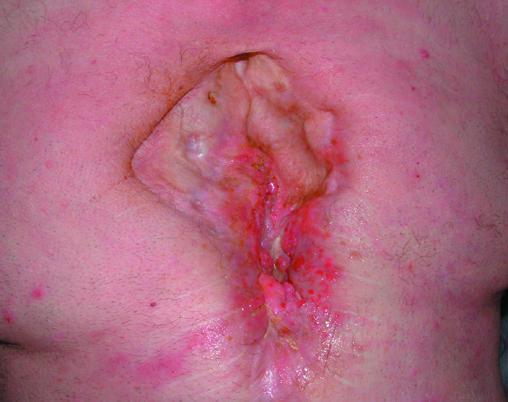
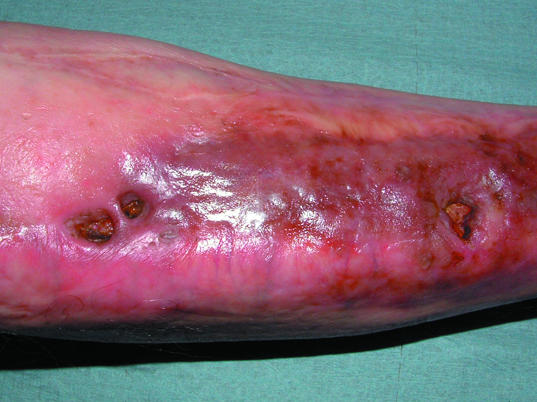
Left: Osteomyelitis in a chronic, non-healing sternotomy wound. Right: Osteomyelitis arising at the site of a previous traumatic wound to the tibia, previously healed by reconstructive surgery. The sinuses probe to bone
Osteomyelitis associated with wound infection
Osteomyelitis may develop after direct inoculation of bone from a contiguous focus of infection. This can be a devastating complication of wound infection, requiring specialist intervention and management.
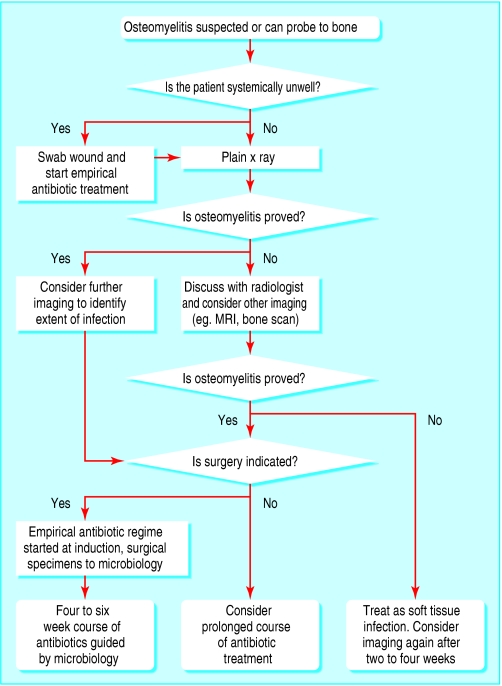
Figure 8.
Algorithm for management when osteomyelitis is suspected or if the wound can be probed to bone
Diagnosis
The diagnosis of osteomyelitis should be considered in any chronic wound that does not heal despite optimal treatment or in any wound (especially in those with diabetes) that can be probed to bone. Plain x rays of the affected area should be the first line of investigation.
Table 7.
Topical antimicrobial preparations
| • Iodine releasing agents (povidone-iodine preparations, cadexomer-iodine preparations) |
| • Potassium permanganate solution |
| • Silver releasing agents (composite silver dressings, silver sulfadiazine) |
| • Topical antibiotic (metronidazole) |
Radiographic changes, however, can lag behind the evolution of infection by at least two weeks; a single, negative plain x ray film does not, therefore, exclude osteomyelitis. Magnetic resonance imaging is more sensitive than plain radiography. Nuclear scintigraphy—either a technetium bone scan or a labelled white cell scan—may also be helpful but requires careful interpretation. It can be difficult to differentiate osteomyelitis from chronic soft tissue infection.
Management
Antibiotics penetrate poorly into devitalised bone, and long courses of antibiotics may be required. It is therefore important to define the infecting organism(s) from the outset so that antibiotic treatment can be targeted. Ideally, in the absence of systemic illness, antibiotics should not be started before microbiological sampling of the infected bone.
Surgery followed by prolonged intravenous antibiotic treatment (generally a minimum of six weeks), is indicated in selected patients. Periodic antibiotic treatment at times of wound deterioration or of systemic illness may be appropriate if cure is unachievable.
Surgery
Surgery enables debridement of all necrotic bone and tissue and provides deep samples for microbiological analysis. In some patients, surgery is not possible either because of the site of the wound or because of the patient's debility. Under these circumstances, a prolonged course of antibiotics may be warranted.
Antibiotic treatment
Choice of treatment is dependent on the antibiotic sensitivity pattern of the infecting organism(s) along with antibiotic properties, such as bone penetration, and host factors, such as drug allergy. Combination therapy is often used to gain maximal effect. Inflammatory markers (including C reactive protein and erythrocyte sedimentation rate) and radiological images can be used to monitor response.
Figure 9.
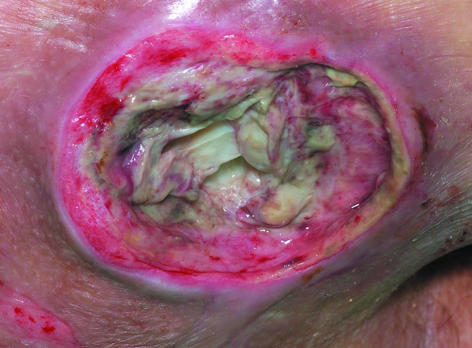
Pressure sore associated with MRSA osteomyelitis. Bone is visible at the base of the ulcer
Methicillin resistant Staphylococcus aureus
The incidence of MRSA wound infection and osteomyelitis is increasing. Isolation of MRSA from a wound, however, does not require treatment in the absence of clinical signs of infection. Topical antimicrobial agents, such as iodine and silver compounds, have activity against MRSA and may be used in localised wound infection when there is no evidence of invasion, cellulitis, or systemic upset.
In a systemically unwell individual, a glycopeptide (vancomycin or teicoplanin) should be administered. In all cases of MRSA osteomyelitis and in some MRSA wound infections a second antistaphylococcal agent with good penetration to bone and superficial skin sites should be added—for example, fusidic acid or rifampicin. Both rifampicin and fusidic acid can cause hepatitis and require regular monitoring of liver function tests.
With the exception of linezolid, evidence for the use of oral antibiotics in MRSA infections is lacking. However, when oral antibiotics are used, combinations are recommended to protect against the development of resistance. Combinations of rifampicin or fusidic acid with either trimethoprim or minocycline have been used with some success. The combination of rifampicin with fusidic acid is not advisable because of the increased risk of hepatotoxicity.
Linezolid, an oxazolidinone, is a new agent active against MRSA. It has excellent bioavailability, can be administered orally, and has good skin and bone penetration. Linezolid is generally well tolerated, but can cause bone marrow suppression, and regular haematological monitoring is therefore required. Linezolid use is currently limited by its high cost.
Agents that may be available in the near future include daptomycin, tigecycline, and dalbavancin.
Radiological improvement will often lag behind clinical improvement by up to six weeks
The ABC of wound healing is edited by Joseph E Grey (joseph.grey@cardiffandvale.wales.nhs.uk), consultant physician, University Hospital of Wales, Cardiff and Vale NHS Trust, Cardiff, and honorary consultant in wound healing at the Wound Healing Research Unit, Cardiff University, and by Keith G Harding, director of the Wound Healing Research Unit, Cardiff University, and professor of rehabilitation medicine (wound healing) at Cardiff and Vale NHS Trust. The series will be published as a book in summer 2006.
Competing interests: For series editors' competing interests, see the first article in this series.
The figure showing the spectrum of interaction between bacteria and host was supplied by J E Grey and Stuart Enoch.
Further reading and resources
- • Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005;330: 830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C, Linezolid CSSTI Study Group. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother 2005;49: 2260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA, Expert Panel on Managing Skin and Soft Tissue Infections. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother 2003;52(suppl 1): i3-17. [DOI] [PubMed] [Google Scholar]
- • Bisno AL, Cockerill FR 3rd, Bermudez CT. The initial outpatient-physician encounter in group A streptococcal necrotizing fasciitis. Clin Infect Dis 2000;31: 607-8. [DOI] [PubMed] [Google Scholar]