Abstract
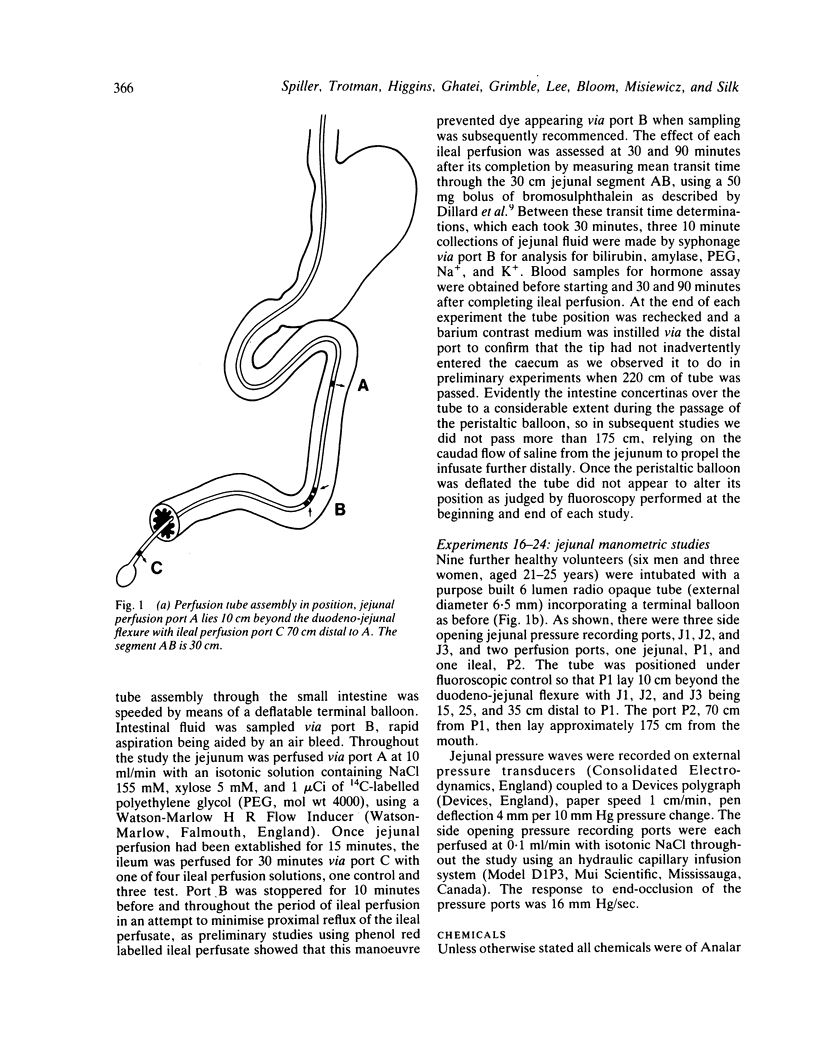
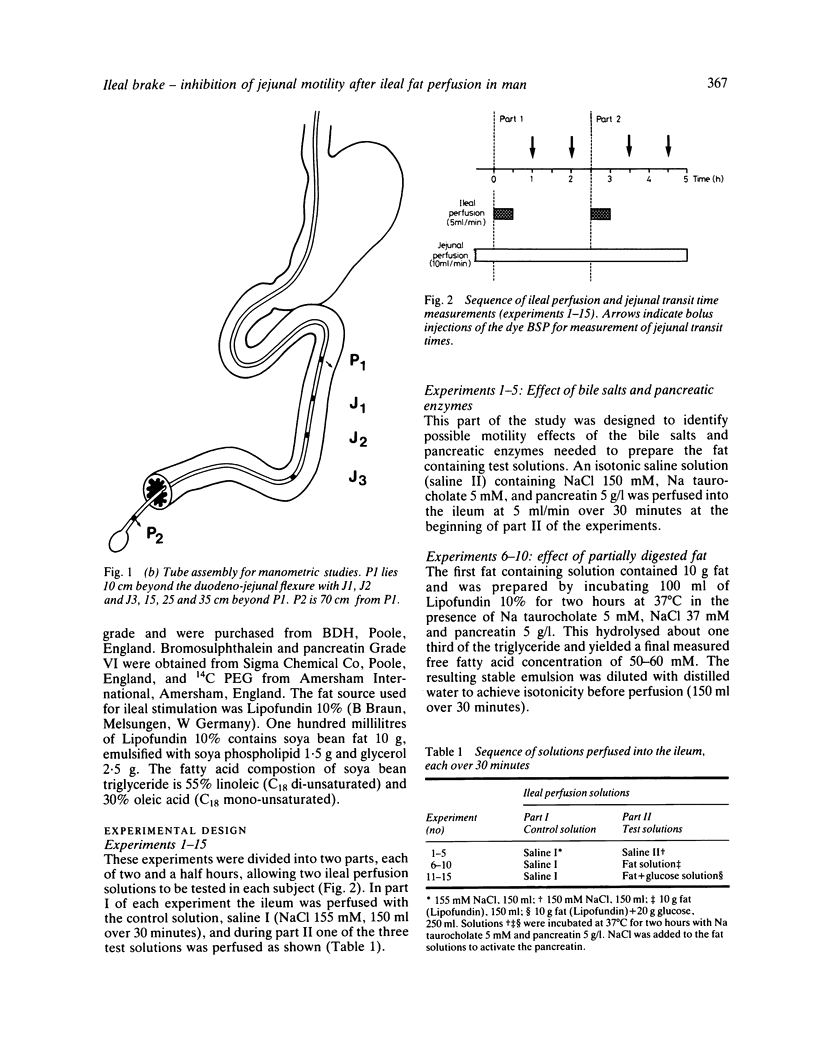
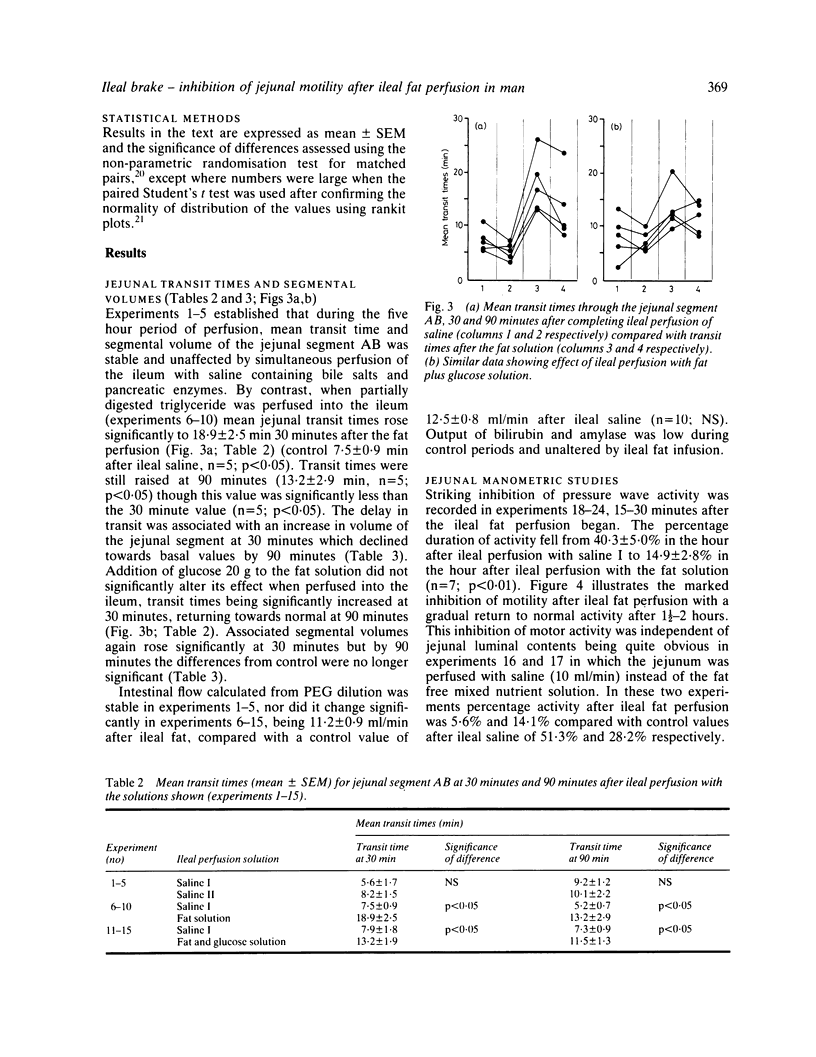
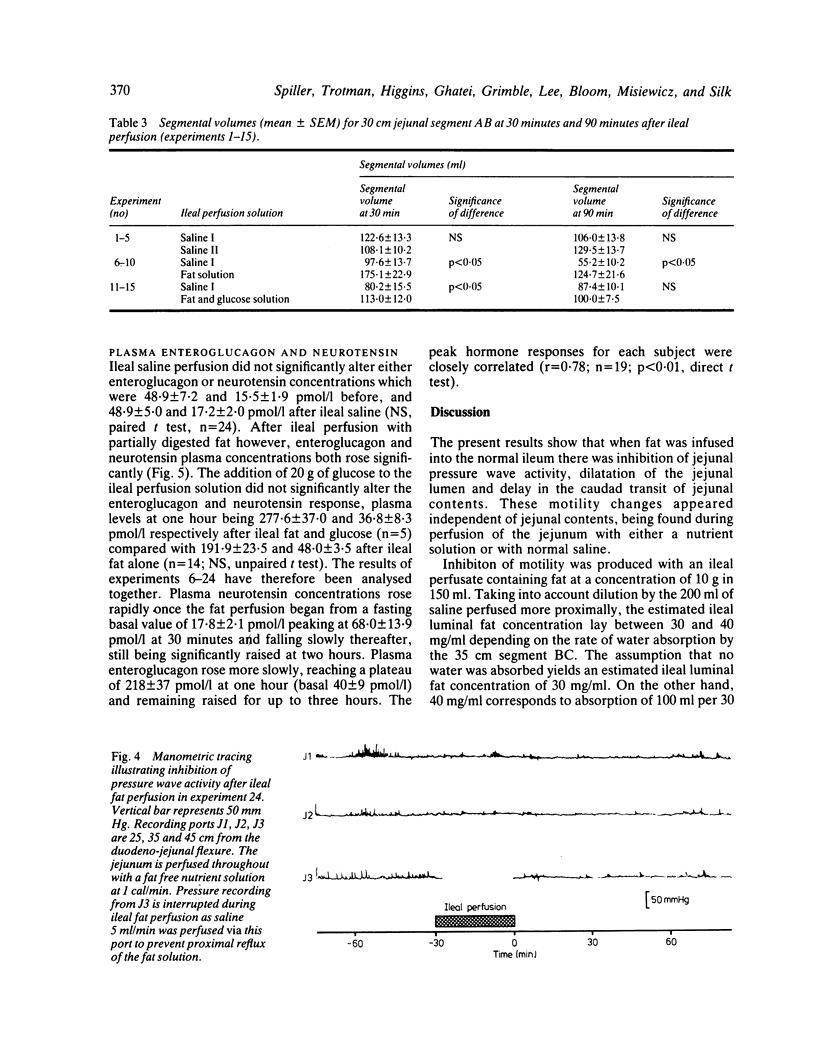
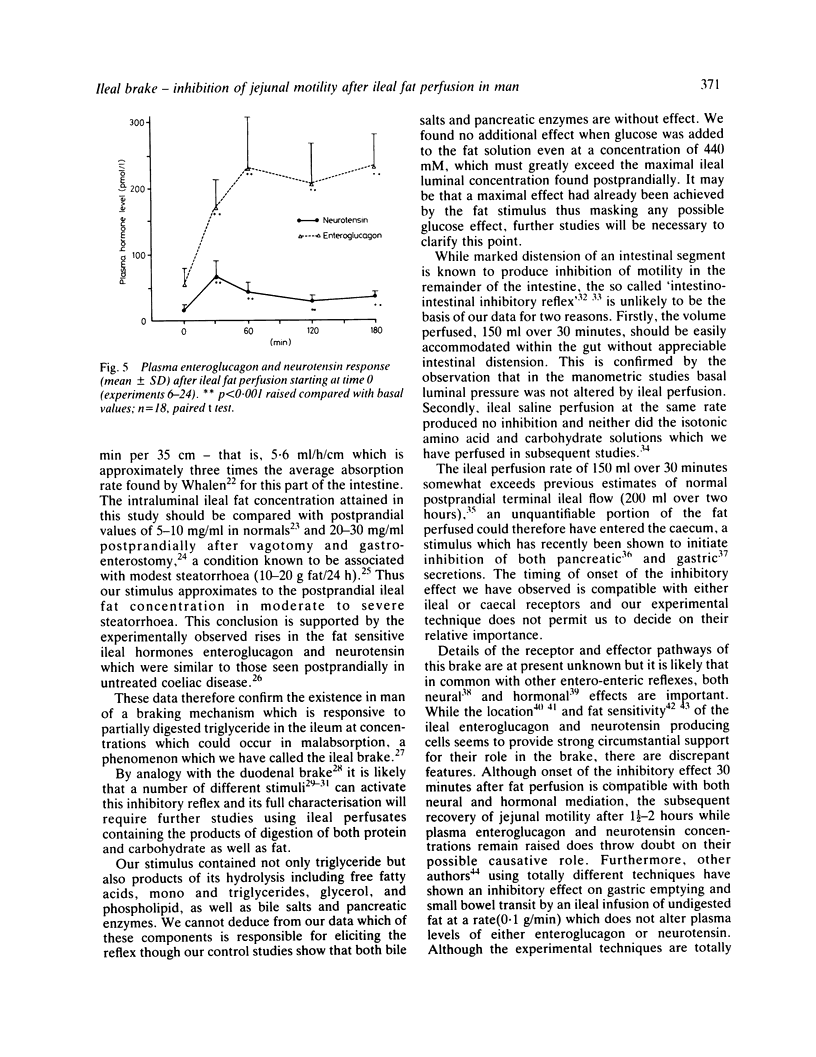
The possibility that malabsorbed fat passing through the human ileum exerts an inhibitory feedback control on jejunal motility has been investigated in 24 normal subjects by perfusing the ileum with a fat containing solution designed to produce ileal luminal fat concentrations similar to those in steatorrhoea (30-40 mg/ml). Mean transit times through a 30 cm saline perfused jejunal segment were measured by a dye dilution technique. Thirty minutes after ileal fat perfusion, mean transit times rose markedly to 18.9 +/- 2.5 minutes from a control value of 7.5 +/- 0.9 minutes (n = 5; p less than 0.05). This was associated with an increase in volume of the perfused segment which rose to 175.1 +/- 22.9 ml (control 97.6 +/- 10.3 ml, n = 5; p less than 0.05). Transit times and segmental volumes had returned towards basal values 90 minutes after completing the fat perfusion. Further studies showed that ileal fat perfusion produced a pronounced inhibition of jejunal pressure wave activity, percentage duration of activity falling from a control level of 40.3 +/- 5.0% to 14.9 +/- 2.8% in the hour after ileal perfusion (p less than 0.01). Ileal fat perfusion was associated with marked rises in plasma enteroglucagon and neurotensin, the peak values (218 +/- 37 and 68 +/- 13.1 pmol/l) being comparable with those observed postprandially in coeliac disease. These observations show the existence in man of an inhibitory intestinal control mechanism, whereby ileal fat perfusion inhibits jejunal motility and delays caudal transit of jejunal contents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Saffar A., Rosell S. Effects of neurotensin and neurotensin analogues on the migrating myoelectrical complexes in the small intestine of rats. Acta Physiol Scand. 1981 Jun;112(2):203–208. doi: 10.1111/j.1748-1716.1981.tb06805.x. [DOI] [PubMed] [Google Scholar]
- Andersson S., Rosell S., Hjelmquist U., Chang D., Folkers K. Inhibition of gastric and intestinal motor activity in dogs by (Gln4) neurotensin. Acta Physiol Scand. 1977 Jun;100(2):231–235. doi: 10.1111/j.1748-1716.1977.tb05941.x. [DOI] [PubMed] [Google Scholar]
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro M. A., McKenna R. D., Beck I. T. Determination of transit time in the human jejunum by the single-injection indicator-dilution technic. Am J Dig Dis. 1968 Mar;13(3):222–233. doi: 10.1007/BF02236597. [DOI] [PubMed] [Google Scholar]
- Blackburn A. M., Fletcher D. R., Bloom S. R., Christofides N. D., Long R. G., Fitzpatrick M. L., Baron J. H. Effect of neurotensin on gastric function in man. Lancet. 1980 May 10;1(8176):987–989. doi: 10.1016/s0140-6736(80)91434-8. [DOI] [PubMed] [Google Scholar]
- Booth C. C., Alldis D., Read A. E. Studies on the site of fat absorption: 2 Fat balances after resection of varying amounts of the small intestine in man. Gut. 1961 Jun;2(2):168–174. doi: 10.1136/gut.2.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Cook G. C. Delayed small-intestinal transit in tropical malabsorption. Br Med J. 1978 Jul 22;2(6132):238–240. doi: 10.1136/bmj.2.6132.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Functional compensation after small-bowel resection in man. Demonstration by direct measurement. Lancet. 1966 Jul 16;2(7455):146–147. doi: 10.1016/s0140-6736(66)92426-3. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Fields M., Duthie H. L. Effect of vagotomy on intraluminal digestion of fat in man. Gut. 1965 Jun;6(3):301–310. doi: 10.1136/gut.6.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Sanford P. A. Effect of actively transported hexoses on afferent nerve discharge from rat small intestine. J Physiol. 1978 Dec;285:71–84. doi: 10.1113/jphysiol.1978.sp012558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J., Christiansen J., Kühl C. The enteroglucagon response to intrajejunal infusion of glucose, triglycerides, and sodium chloride, and its relation to jejunal inhibition of gastric acid secretion in man. Scand J Gastroenterol. 1976;11(3):297–304. [PubMed] [Google Scholar]
- Holst J. J., Christiansen J., Kühl C. The enteroglucagon response to intrajejunal infusion of glucose, triglycerides, and sodium chloride, and its relation to jejunal inhibition of gastric acid secretion in man. Scand J Gastroenterol. 1976;11(3):297–304. [PubMed] [Google Scholar]
- Hunt J. N., Knox M. T. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968 Feb;194(2):327–336. doi: 10.1113/jphysiol.1968.sp008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelfinger F. J., Moss R. E. THE MOTILITY OF THE SMALL INTESTINE IN SPRUE. J Clin Invest. 1943 May;22(3):345–352. doi: 10.1172/JCI101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON B., JONSSON O., LJUNG B. SUPRASPINAL CONTROL OF THE INTESTINO-INTESTINAL INHIBITORY REFLEX. Acta Physiol Scand. 1965 Apr;63:442–449. doi: 10.1111/j.1748-1716.1965.tb04087.x. [DOI] [PubMed] [Google Scholar]
- Johansson C. Studies of gastrointestinal interactions. VII. Characteristics of the absorption pattern of sugar, fat and protein from composite meals in man. A quantitative study. Scand J Gastroenterol. 1975;10(1):33–42. [PubMed] [Google Scholar]
- Johnston D., Duthie H. L. Effect of fat in the duodenum on gastric acid secretion before and after vagotomy in man. Scand J Gastroenterol. 1969;4(7):561–567. [PubMed] [Google Scholar]
- Jorgensen K. H., Larsen U. D. Purification of 125 I-glucagon by anion exchange chromatography. Horm Metab Res. 1972 May;4(3):223–224. doi: 10.1055/s-0028-1097092. [DOI] [PubMed] [Google Scholar]
- Logan R. F., Tucker G., Rifkind E. A., Heading R. C., Ferguson A. Changes in clinical features of coeliac disease in adults in Edinburgh and the Lothians 1960-79. Br Med J (Clin Res Ed) 1983 Jan 8;286(6359):95–97. doi: 10.1136/bmj.286.6359.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD W. C., BRANDBORG L. L., FLICK A. L., TRIER J. S., RUBIN C. E. STUDIES OF CELIAC SPRUE. IV. THE RESPONSE OF THE WHOLE LENGTH OF THE SMALL BOWEL TO A GLUTEN-FREE DIET. Gastroenterology. 1964 Dec;47:573–589. [PubMed] [Google Scholar]
- Meeroff J. C., Go V. L., Phillips S. F. Control of gastric emptying by osmolality of duodenal contents in man. Gastroenterology. 1975 May;68(5 Pt 1):1144–1151. [PubMed] [Google Scholar]
- Moberg S., Carlberger G. The effect on gastric emptying of test meals with various fat and osmolar concentrations. Scand J Gastroenterol. 1974;9(1):29–32. [PubMed] [Google Scholar]
- Nygaard K. Resection of the small intestine in rats. IV. Adaptation of gastro-intestinal motility. Acta Chir Scand. 1967;133(5):407–416. [PubMed] [Google Scholar]
- Owyang C., Miller L. J., Malagelada J. R., Go V. L. Nutrient and bowel segment dependency of human intestinal control of gastric secretion. Am J Physiol. 1982 Nov;243(5):G372–G376. doi: 10.1152/ajpgi.1982.243.5.G372. [DOI] [PubMed] [Google Scholar]
- PERMAN G., MATTSSON O. The small intestine transit time in steatorrhoea. Acta Med Scand. 1962 Mar;171:273–281. doi: 10.1111/j.0954-6820.1962.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Pirk F. Changes in the motility of the small intestine in digestive disorders. Gut. 1967 Oct;8(5):486–490. doi: 10.1136/gut.8.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Sullivan S. N., Bloom S. R., Buchan A. M., Facer P., Brown M. R., Pearse A. G. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977 Nov 10;270(5633):183–184. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- Ravazzola M., Siperstein A., Moody A. J., Sundby F., Jacobsen H., Orci L. Glicentin immunoreactive cells: their relationship to glucagon-producing cells. Endocrinology. 1979 Aug;105(2):499–508. doi: 10.1210/endo-105-2-499. [DOI] [PubMed] [Google Scholar]
- Rosell S., Rökaeus A. The effect of ingestion of amino acids, glucose and fat on circulating neurotensin-like immunoreactivity (NTLI) in man. Acta Physiol Scand. 1979 Nov;107(3):263–267. doi: 10.1111/j.1748-1716.1979.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Rosell S., Rökaeus A. The effect of ingestion of amino acids, glucose and fat on circulating neurotensin-like immunoreactivity (NTLI) in man. Acta Physiol Scand. 1979 Nov;107(3):263–267. doi: 10.1111/j.1748-1716.1979.tb06472.x. [DOI] [PubMed] [Google Scholar]
- STAHLGREN L. H., UMANA G., ROY R., DONNELLY J. A study of intestinal absorption in dogs following massive small intestinal resection and insertion of an antiperistaltic segment. Ann Surg. 1962 Sep;156:483–492. doi: 10.1097/00000658-196209000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagor G. R., Al-Mukhtar M. Y., Ghatei M. A., Wright N. A., Bloom S. R. The effect of altered luminal nutrition on cellular proliferation and plasma concentrations of enteroglucagon and gastrin after small bowel resection in the rat. Br J Surg. 1982 Jan;69(1):14–18. doi: 10.1002/bjs.1800690106. [DOI] [PubMed] [Google Scholar]
- Sladen G. E., Dawson A. M. Interrelationships between the absorptions of glucose, sodium and water by the normal human jejunum. Clin Sci. 1969 Feb;36(1):119–132. [PubMed] [Google Scholar]
- Theodorsson-Norheim E., Rosell S. The effect of duodenal administration of fatty acids, triolein, liquid paraffin and lecithin on plasma neurotensin-like immunoreactivity (p-NTLI) in the rat. Acta Physiol Scand. 1983 Mar;117(3):439–443. doi: 10.1111/j.1748-1716.1983.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Wingate D. L., Sandberg R. J., Phillips S. F. A comparison of stable and 14 C-labelled polyethylene glycol as volume indicators in the human jejunum. Gut. 1972 Oct;13(10):812–815. doi: 10.1136/gut.13.10.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERLER K. L. A simplified explanation of the theory of indicator-dilution for measurement of fluid flow and volume and other distributive phenomena. Bull Johns Hopkins Hosp. 1958 Oct;103(4):199–217. [PubMed] [Google Scholar]


