Abstract
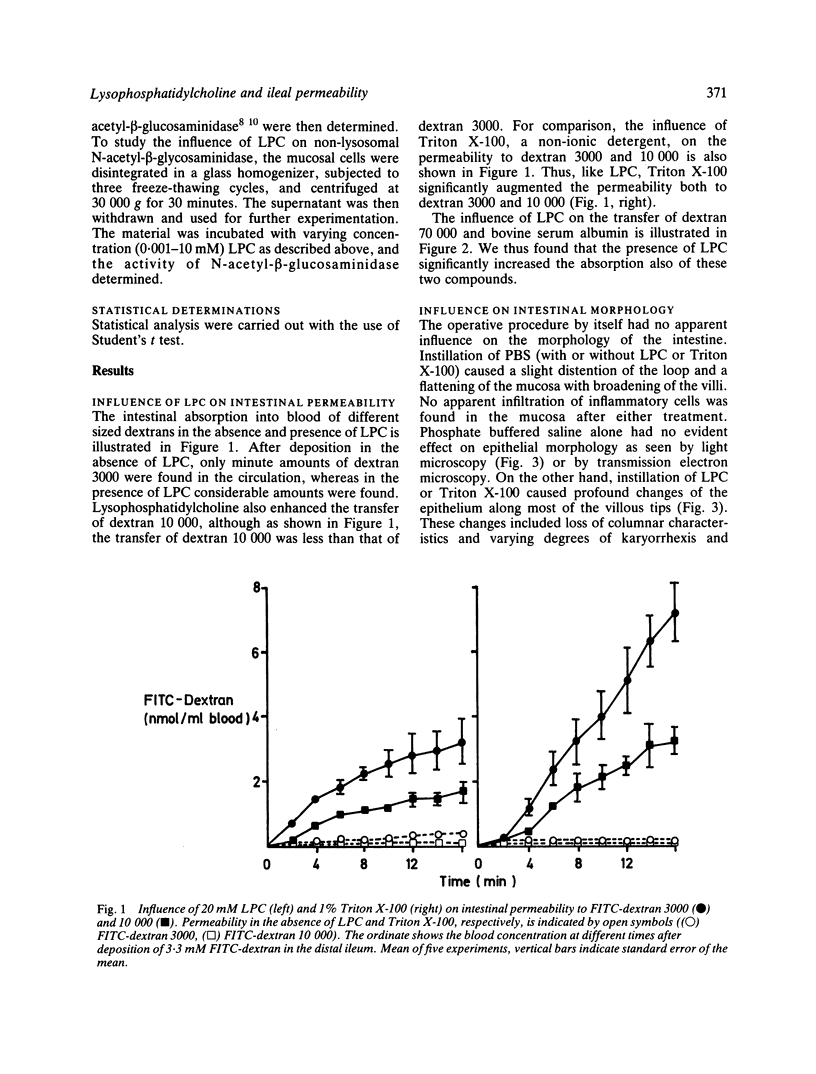
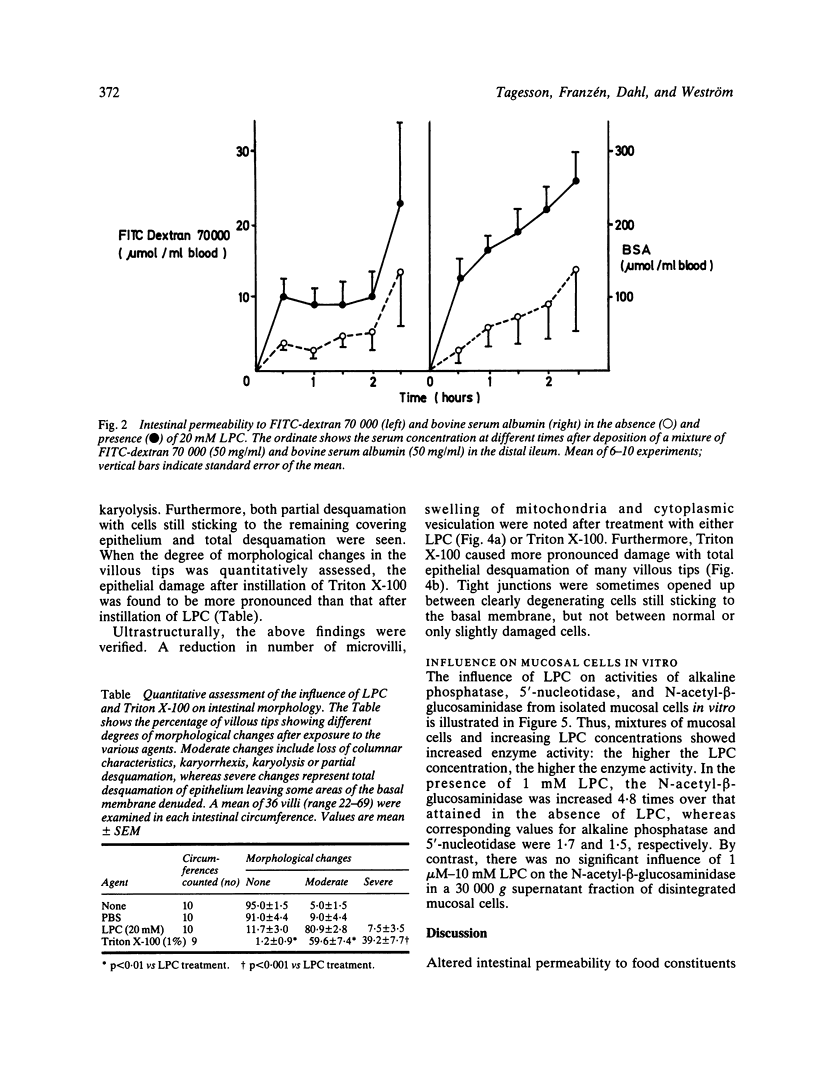
The influence of lysophosphatidylcholine (LPC) on macromolecular permeability in the distal ileum has been studied. Using a rat experimental model, we determined the intestinal permeability to different sized dextrans (3000-70 000 daltons) and bovine serum albumin (BSA) in the absence and presence of LPC. We also examined the morphology of the ileal mucosa after deposition of LPC in the gut lumen, and determined N-acetyl-beta-glucosaminidase, 5'-nucleotidase, and alkaline phosphatase activities in suspensions of isolated mucosal cells and different concentrations of LPC. We found that 20 mM LPC damaged the ileal mucosa and that it increased its permeability to all the molecules investigated. Moreover, mixtures of mucosal cells and 0.01-1 mM LPC showed increased N-acetyl-beta-glucosaminidase activity: the higher the LPC concentration, the higher the enzyme activity. These findings indicate that LPC, a naturally occurring surfactant in the intestine, might damage mucosal cells and release lysosomal enzyme activity, and that higher LPC concentrations may impair the mucosal barrier function and increase the gut permeability to macromolecules such as proteins. This could have relevance to the development of various disease states, in which increased intestinal absorption of macromolecules is of importance.
Full text
PDF

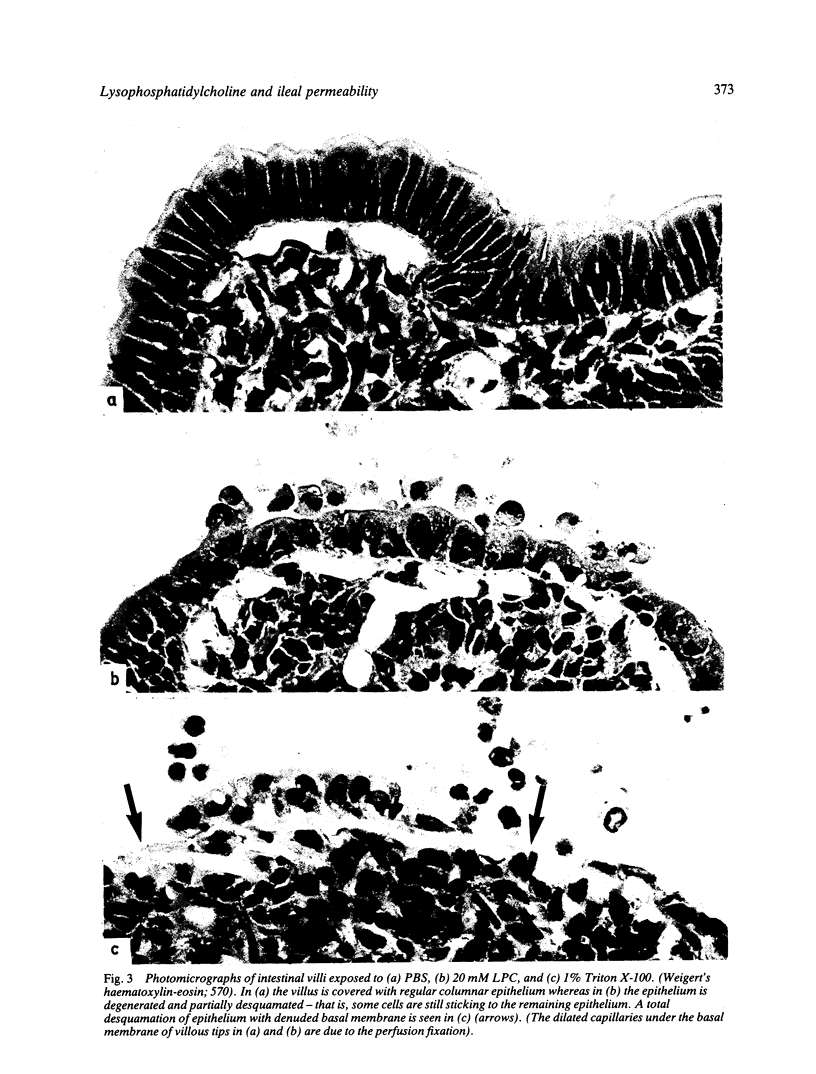
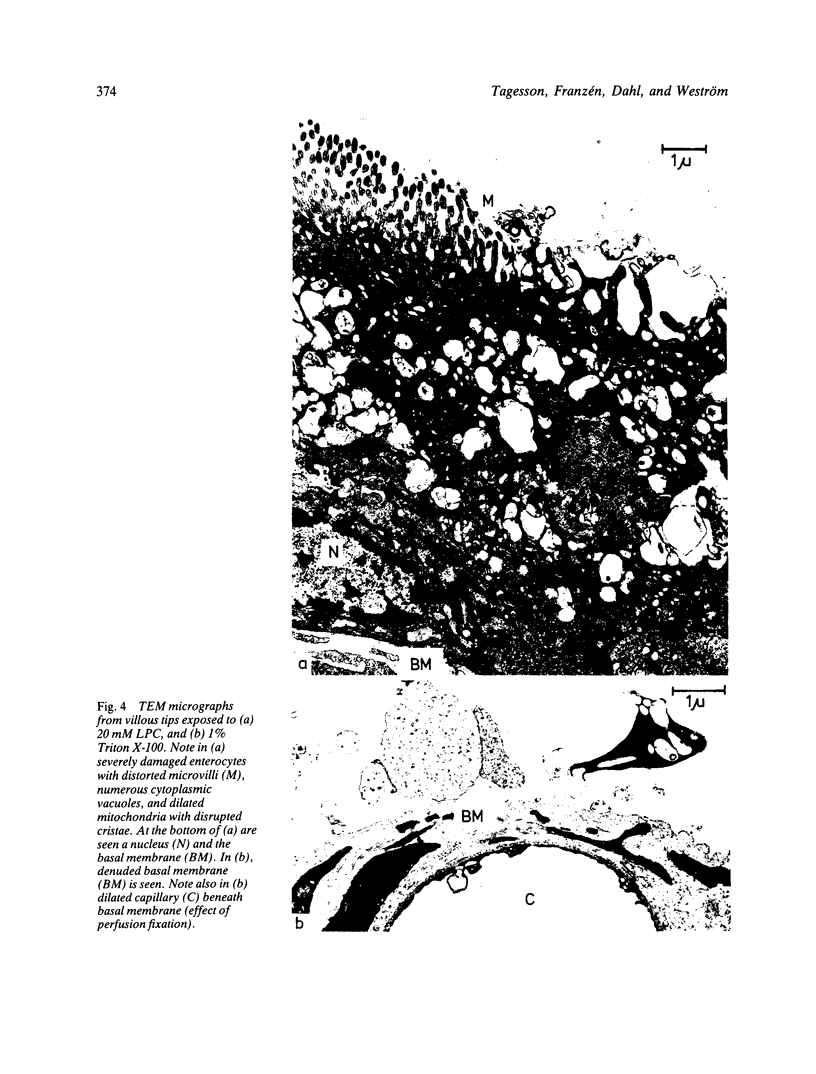
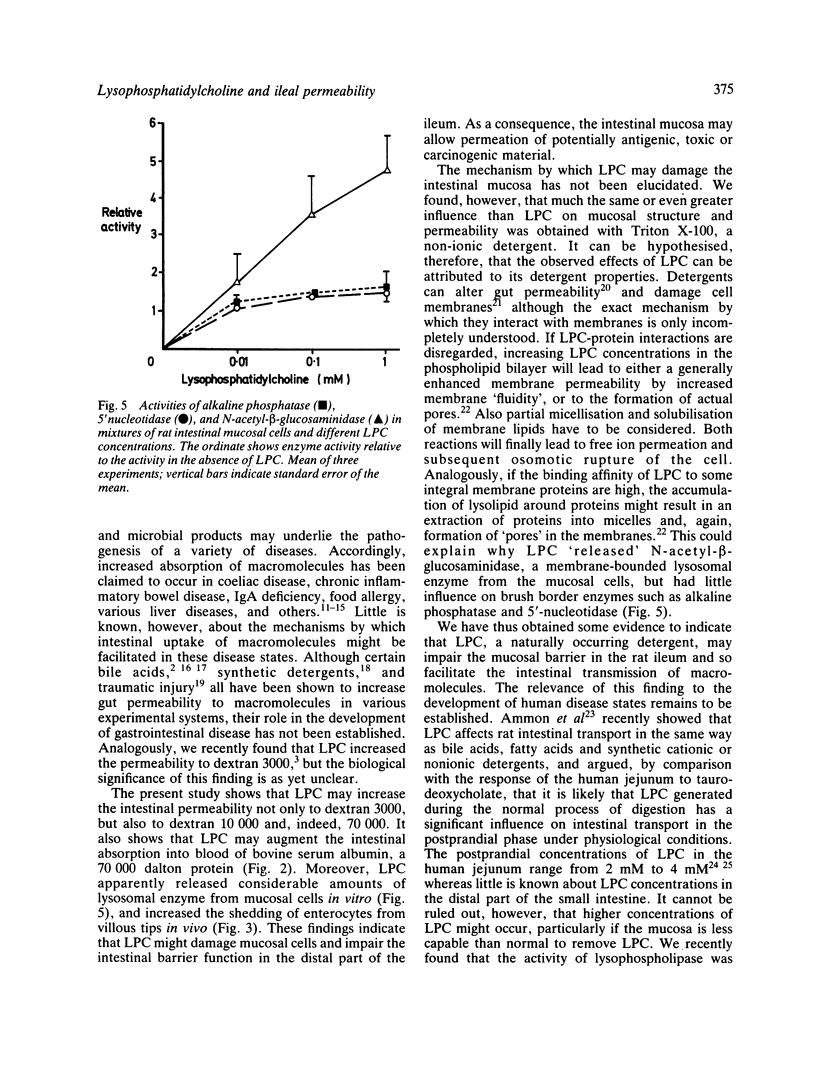


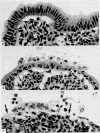
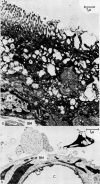
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. V., Loeffler R. E., Luedtke L. A. Effects of lysophosphatidylcholine on jejunal water and solute transport in the rat in vivo. Lipids. 1983 Jun;18(6):428–433. doi: 10.1007/BF02535429. [DOI] [PubMed] [Google Scholar]
- Anderson W. B., Jaworski C. J. Modulation of adenylate cyclase activity of fibroblasts by free fatty acids and phospholipids. Arch Biochem Biophys. 1977 Apr 30;180(2):374–383. doi: 10.1016/0003-9861(77)90051-0. [DOI] [PubMed] [Google Scholar]
- Bohman S. O., Maunsbach A. B. Effects on tissue fine structure of variations in colloid osmotic pressure of glutaraldehyde fixatives. J Ultrastruct Res. 1970 Jan;30(1):195–208. doi: 10.1016/s0022-5320(70)90073-0. [DOI] [PubMed] [Google Scholar]
- Bolin T., Heuman R., Sjödahl R., Tagesson C. Decreased lysophospholipase and increased phospholipase A2 activity in ileal mucosa from patients with Crohn's disease. Digestion. 1984;29(1):55–59. doi: 10.1159/000199009. [DOI] [PubMed] [Google Scholar]
- Bolin T., Sjödahl R., Sundqvist T., Tagesson C. Passage of molecules through the wall of the gastrointestinal tract. Increased passive permeability in rat ileum after exposure to lysolecithin. Scand J Gastroenterol. 1981;16(7):897–901. doi: 10.3109/00365528109181820. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Gaginella T. S., Carlson G. L., Debongnie J. C., Phillips S. F., Hofmann A. F. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979 Nov;94(5):661–674. [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Passive permeability in experimental intestinal damage in rats. Clin Sci (Lond) 1981 Jan;60(1):115–118. doi: 10.1042/cs0600115. [DOI] [PubMed] [Google Scholar]
- Collins V. P., Arborgh B., Brunk U. A comparison of the effects of three widely used glutaraldehyde fixatives on cellular volume and structure. A TEM, SEM, Volumetric and Cytochemical Study. Acta Pathol Microbiol Scand A. 1977 Mar;85A(2):157–168. doi: 10.1111/j.1699-0463.1977.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Douglas A. P., Kerley R., Isselbacher K. J. Preparation and characterization of the lateral and basal plasma membranes of the rat intestinal epithelial cell. Biochem J. 1972 Aug;128(5):1329–1338. doi: 10.1042/bj1281329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes-Neto U., Teichberg S., Bayne M. A., Morton B., Lifshitz F. Bile salt-enhanced rat jejunal absorption of a macromolecular tracer. Lab Invest. 1981 Jan;44(1):18–26. [PubMed] [Google Scholar]
- Feldman S., Reinhard M., Willson C. Effect of sodium taurodeoxycholate on biological membranes: release of phosphorus, phospholipid, and protein from everted rat small intestine. J Pharm Sci. 1973 Dec;62(12):1961–1964. [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Joist J. H., Dolezel G., Cucuianu M. P., Nishizawa E. E., Mustard J. F. Inhibition and potentiation of platelet function by lysolecithin. Blood. 1977 Jan;49(1):101–112. [PubMed] [Google Scholar]
- KIVEL R. M., KEARNS D. H., LIEBOWITZ D. SIGNIFICANCE OF ANTIBODIES TO DIETARY PROTEINS IN THE SERUMS OF PATIENTS WITH NONTROPICAL SPRUE. N Engl J Med. 1964 Oct 8;271:769–772. doi: 10.1056/NEJM196410082711504. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Lagunoff D. Interactions of lysophospholipids and mast cells. Nature. 1979 May 17;279(5710):250–252. doi: 10.1038/279250a0. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Moore J. D., Zatzman M. L., Overack D. E., Platner W. S. The effects of synthetic surfactants on intestinal permeability to glucose in vitro. Proc Soc Exp Biol Med. 1971 Sep;137(4):1135–1139. doi: 10.3181/00379727-137-35742. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Analytical subcellular fractionation of jejunal biopsy specimens: methodology and characterization of the organelles in normal tissue. Clin Sci Mol Med. 1976 Dec;51(6):557–574. doi: 10.1042/cs0510557. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Porter H. P., Saunders D. R. Isolation of the aqueous phase of human intestinal contents during the digestion of a fatty meal. Gastroenterology. 1971 Jun;60(6):997–1007. [PubMed] [Google Scholar]
- Reman F. C., Demel R. A., De Gier J., van Deenen L. L., Eibl H., Westphal O. Studies on the lysis of red cells and bimolecular lipid leaflets by synthetic lysolecithins, lecithins and structural analogs. Chem Phys Lipids. 1969 Sep;3(3):221–233. doi: 10.1016/0009-3084(69)90014-0. [DOI] [PubMed] [Google Scholar]
- Rhodes R. S., Karnovsky M. J. Loss of macromolecular barrier function associated with surgical trauma to the intestine. Lab Invest. 1971 Sep;25(3):220–229. [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagesson C., Bolin T., Heuman R., Magnusson K. E., Norrby K., Sjödahl R. Subcellular fractionation of human intestinal mucosa by large-scale zonal centrifugation. I. Characterization of subcellular organelles in the distal part of the ileum. Scand J Gastroenterol. 1980;15(3):353–362. doi: 10.3109/00365528009181483. [DOI] [PubMed] [Google Scholar]
- Walker W. A. Antigen absorption from the small intestine and gastrointestinal disease. Pediatr Clin North Am. 1975 Nov;22(4):731–746. doi: 10.1016/s0031-3955(16)33204-7. [DOI] [PubMed] [Google Scholar]
- Wands J. R., LaMont J. T., Mann E., Isselbacher K. J. Arthritis associated with intestinal-bypass procedure for morbid obesity. Complement activation and characterization of circulating cryoproteins. N Engl J Med. 1976 Jan 15;294(3):121–124. doi: 10.1056/NEJM197601152940301. [DOI] [PubMed] [Google Scholar]
- Ward M. The pathogenesis of Crohn's disease. Lancet. 1977 Oct 29;2(8044):903–905. doi: 10.1016/s0140-6736(77)90835-2. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]