Abstract
It has been reported that Neisseria gonorrhoeae possesses a very high level of catalase activity, but the regulation of catalase expression has not been investigated extensively. In Escherichia coli and Salmonella enterica serovar Typhimurium, it has been demonstrated that OxyR is a positive regulator of hydrogen peroxide-inducible genes, including the gene encoding catalase. The oxyR gene from N. gonorrhoeae was cloned and used to complement an E. coli oxyR mutant, confirming its identity and function. The gene was inactivated by inserting a kanamycin resistance cassette and used to make a knockout allele on the chromosome of N. gonorrhoeae strain 1291. In contrast to E. coli, the N. gonorrhoeae oxyR::kan mutant expressed ninefold-more catalase activity and was more resistant to hydrogen peroxide killing than the wild type. These data are consistent with OxyR in N. gonorrhoeae acting as a repressor of catalase expression.
Neisseria gonorrhoeae is a facultative aerobe with a high iron requirement and a highly active aerobic respiratory chain. These factors suggest that this bacterium would require defense systems to respond to toxic oxygen species. Furthermore, N. gonorrhoeae is usually associated with inflamed urogenital tissues and activated polymorphonuclear leukocytes evolving substantial amounts of superoxide anion (O2˙−) and hydrogen peroxide (H2O2) as part of their oxygen-dependent bactericidal mechanisms. Despite the presence of this toxic environment, N. gonorrhoeae can be isolated from purulent exudates laden with polymorphonuclear leukocytes (3).
Hydrogen peroxide is a potent bactericidal agent. It can react with Fe(II) to generate the hydroxyl radical (OḢ), a particularly potent oxidizing agent that causes damage to DNA, lipids, and proteins (14, 40). Thus, the removal of hydrogen peroxide is a critical step in the bacterial defense against oxidative killing. N. gonorrhoeae possesses very high levels of catalase and peroxidase (3, 13). It has been demonstrated that the presence of catalase significantly increased the ability of N. gonorrhoeae to resist in vitro killing by exposure to H2O2 and human neutrophils (16, 44). Zheng et al. (44) identified a single source of catalase activity in N. gonorrhoeae extracts. The amino acid sequence of the catalase of N. gonorrhoeae is 83% identical to that of H. influenzae catalase HktE (17). It was demonstrated that catalase activities were induced, increasing threefold when subjected to 1 mM H2O2 (43). However, the molecular aspects of the regulation of catalase expression in N. gonorrhoeae were not investigated.
In contrast, in Escherichia coli and Salmonella enterica serovar Typhimurium, it is well established that catalase is part of a global system regulated by oxyR (5). OxyR is a positive regulator of the expression of nine hydrogen peroxide-inducible genes, including those encoding catalase (katG), glutathione reductase (gor), glutaredoxin (grxA), alkyl hydroperoxide reductase (ahpCF), OxyS (a regulatory RNA), Fur (ferric uptake regulation), and Dps (a nonspecific DNA-binding protein) (5, 11, 15, 25). Mutations in oxyR or oxyR-regulated enzymes involved in the defense against reduced-oxygen compounds result in a dramatic increase in the sensitivity of E. coli to H2O2, leading to the classic phenotype of increased levels of spontaneous mutagenesis during aerobic growth (5, 20-22). OxyR is a member of the LysR-NodD family of bacterial regulatory proteins and binds to the promoters of oxyR-regulated genes (4, 6, 37, 42). The oxidized, but not the reduced, form of the OxyR protein activates transcription of oxyR-regulated genes in vitro (36). Recent studies showed that oxidation by peroxide leads to the formation of an intramolecular disulfide bond in OxyR that triggers the activation of the transcription factor (45). OxyR activation and deactivation are consequences of the C199-C208 disulfide bond formation and reduction (45).
Recently, we demonstrated that N. gonorrhoeae possesses only a Fe-dependent SodB but uses Mn(II) ions to protect against superoxide killing via a Sod-independent mechanism (41). Since Mn(II) is known to quench hydrogen peroxide (35), we were also interested in determining whether it contributes to defense against this oxidant and whether it exerts any regulatory effects on catalase expression. Since N. gonorrhoeae is a gram-negative bacterium, the question of the role of OxyR must be raised since it might be expected to have a pattern of regulation of catalase expression similar to those of the enteric bacteria. In this paper we report the unusual phenotype of the oxyR mutant in N. gonorrhoeae. We present data indicating that the mechanism of oxidative stress gene regulation by OxyR in N. gonorrhoeae may be quite different from the E. coli paradigm.
Identification and mutagenesis of the oxyR gene in N. gonorrhoeae.
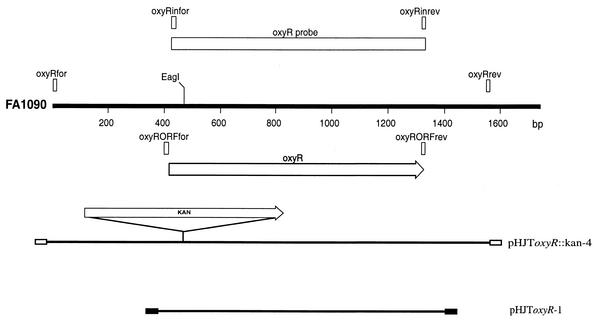
Using E. coli OxyR as the search sequence query, we identified a potential homolog in the N. gonorrhoeae FA1090 genome (nucleotide positions 1791204 to 1790284 in GenBank accession number AE004969 [this study] and AF514857 from strain 1291 [this study]). The deduced amino acid sequence of N. gonorrhoeae OxyR has 37% sequence identity and 59% sequence similarity at the protein level with E. coli OxyR. To determine whether this locus in N. gonorrhoeae was involved in gene regulation, we constructed an insertional mutation in the oxyR gene in N. gonorrhoeae strain 1291 (9). The method of construction of a knockout mutant in N. gonorrhoeae was described previously (41). Briefly, the oxyR fragment was amplified by PCR (30) using the oxyRfor and oxyRrev primers (Table 1 and Fig. 1), and a kanamycin resistance cassette (pUC4Kan; Pharmacia) was inserted into the EagI site in the coding region of the gene. This plasmid was designated pHJToxyR::kan-4 for the oxyR::kan mutant. This plasmid was linearized with EcoRI and transformed into N. gonorrhoeae strain 1291, and recombinant kanamycin-resistant colonies were selected. Both PCR (using the oxyRfor, oxyRrev, kanfor, and kanrev primers [Table 1 and Fig. 1]) and Southern blotting (34) using the oxyR gene labeled with digoxigenin (Boehringer Mannheim) as the probe showed that the oxyR::kan mutants of strain 1291 had the expected genomic organization (results not shown).
TABLE 1.
Primers used for PCR of the oxyR gene
| Primer | Sequence | Nucleotide positionsa |
|---|---|---|
| oxyRfor | 5′-CGGAGAACCGGTCATCCA-3′ | 1791602-1791619 |
| oxyRrev | 5′-GACGAATCTATCCATACG-3′ | 1790052-1790069 |
| oxyRinfor | 5′-CCGAATTGCGGTACATCG-3′ | 1791177-1791194 |
| oxyRinrev | 5′-CCTAGTCGTGGATAAAAC-3′ | 1790283-1790300 |
| oxyRORFfor | 5′-CCGGAATTCCAGAAAAAGGTATAGGAC-3′ | 1791205-1791222 |
| oxyRORFrev | 5′-CGCGGATTCCTAGTCGTGGATAAAACT-3′ | 1790284-1790301 |
| kanfor | 5′-TTATCGGCCGAAGCCACGTTGTGTCTCA-3′ | |
| kanrev | 5′-GCTGAGATCTGCCTCGTGAAGAAGG-3′ |
FIG. 1.
Maps of oxyR region, probes, and plasmids. The thick black line labeled FA1090 represents restriction endonuclease map of a region of the N. gonorrhoeae strain FA1090 genome sequencing project (GenBank accession number AE004969). The white arrow beneath the FA1090 line indicates the orientation and location of the ORF identified in the sequence. Below the ORF, the thin black lines represent the plasmids constructed during this work. The vectors of these plasmids are represented by boxes: white for pT7Blue (Novagen) and black for pGEM T-Easy (Promega). The restriction endonuclease site shown indicates where the Kanr cassette was inserted. Rectangular boxes above the FA1090 line represent the PCR products used as probes in this study. The oxyR probe was constructed from PCR products utilizing primers oxyRinfor and oxyRinrev.
Level of catalase in the oxyR mutant.
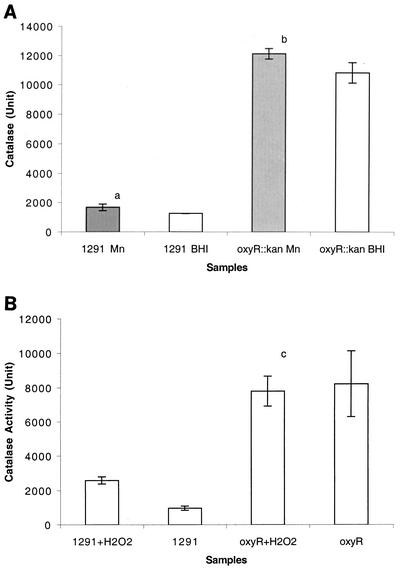
In E. coli and S. enterica serovar Typhimurium, OxyR is established as a positive regulator of catalase expression (5). Therefore, we expected that in N. gonorrhoeae the catalase level in the oxyR::kan mutant of strain 1291 would be lower than that in the wild type. It was also expected that when cells were exposed to H2O2, catalase would not be induced in the mutant in contrast to the wild type. The level of catalase activity present in cell extracts of bacteria grown overnight on brain heart infusion (BHI) agar containing 10% levinthal (2) and 1% supplex (Microdiagnostics) in the presence and absence of 100 μM Mn was measured by the method of Aebi (1). The reason we tested Mn in this assay is because we previously demonstrated that Mn is involved in resistance to oxidative stress (41), we were interested in further investigating a potential role of Mn in modulation of catalase activity and consequent resistance to H2O2 killing. The decomposition of H2O2 can be measured directly by the decrease in absorbance at 240 nm (ɛ240 = 0.0394 mmol−1 cm−1) (26). Surprisingly, the oxyR::kan mutant of N. gonorrhoeae had an approximately ninefold-higher catalase activity than the wild type (strain 1291) (Fig. 2A). This suggested that OxyR in N. gonorrhoeae works as a repressor, which is opposite to the action of its homolog in E. coli and S. enterica serovar Typhimurium. When the cells were grown on Mn-supplemented agar media, both the wild type and the oxyR::kan mutant had slightly higher catalase activities than those cells grown on BHI agar only (Fig. 2A, P = 0.04135 and 0.06356, respectively).
FIG. 2.
Catalase activities of N. gonorrhoeae 1291 (wild type) and oxyR::kan mutant during growth overnight (A) and H2O2 treatment (B). In these assays, cells were grown on BHI agar containing 10% levinthal (2) and 1% supplex (Biomerieux) (white columns) or BHI agar containing levinthal, supplex, and 100 μM MnSO4 (shaded columns). Experiments were performed in triplicate. The y-axis error bars each represent ±1 standard deviation of the mean. Student's t test was performed to determine the statistical significance between different samples. The unit of catalase is reported the concentration of H2O2 decomposed per minute per milligram of total protein. In the wild type, Mn increased the catalase activities slightly (P = 0.04135 [a]). Mn supplementation also increased the catalase activities slightly in the oxyR::kan mutant (P = 0.06356 [b]). H2O2 did not induce the catalase activity significantly in the oxyR::kan mutant (P = 0.6417 [c]).
When log-phase cells were challenged with 1 mM H2O2, the wild type grown on normal media showed a 2.7-fold increase in catalase activity level (Fig. 2B). However, the oxyR::kan mutant strain showed no increase in catalase activity when challenged with H2O2 (Fig. 2B, P = 0.6417).
The oxyR mutant is resistant to hydrogen peroxide killing.
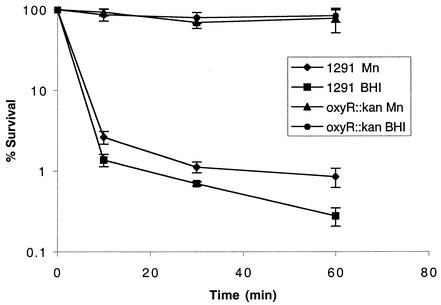
The above experiment showed that the oxyR::kan mutant of N. gonorrhoeae expressed much higher catalase activity than the wild type. This suggested that the oxyR::kan mutant might be more resistant to H2O2 than wild-type cells. Figure 3 shows that the oxyR::kan mutant was indeed more resistant to H2O2 than the wild-type cells, and under the conditions tested, there was only a slight loss of viability in the killing experiment. Again, this is opposite to oxyR-dependent regulation of catalase expression in E. coli and S. enterica serovar Typhimurium (5). Mn(II) supplementation conferred only slightly increased resistance to H2O2 in the wild-type cells, but not in the oxyR::kan mutant (Fig. 3).
FIG. 3.
H2O2 survival test of N. gonorrhoeae 1291 (wild type) and oxyR::kan mutant. In the assay, cells were grown on BHI agar containing levinthal and supplex with or without 100 μM MnSO4. Experiments were performed in triplicate. The y-axis error bars each indicate ±1 standard deviation of the mean.
An oxyR mutant is more resistant to xanthine-xanthine oxidase killing.
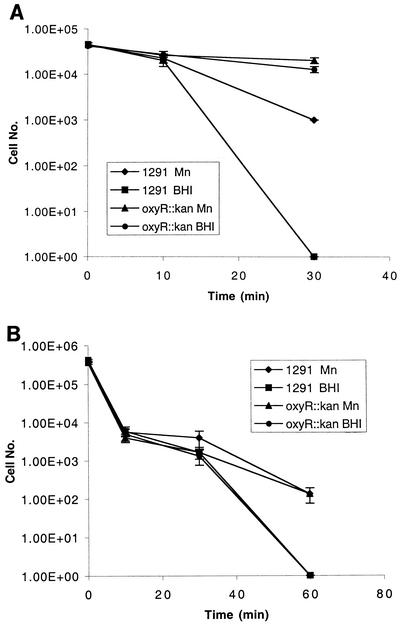
We were also interested in determining whether the oxyR::kan mutant was also resistant to killing by oxygen radicals produced external to the cells. The xanthine/xanthine oxidase system (described previously in reference 41) produces superoxide anion and also hydrogen peroxide (43). Using this system, it was observed that the oxyR::kan mutant was significantly more resistant to killing than the wild-type cells (Fig. 4A). The results shown in Fig. 4A indicate that the significant difference in protection seen in the oxyR::kan mutant results from the very high levels of catalase activity expressed by this strain (Fig. 2). The addition of Mn resulted in increased protection in both strains, presumably due to Mn-mediated dismutation of superoxide anion (41).
FIG. 4.
(A) Xanthine-xanthine oxidase and (B) paraquat killing assays of N. gonorrhoeae (wild type) and oxyR::kan mutant. Cells were grown on BHI agar containing levinthal and supplex with or without 100 μM MnSO4. Experiments were performed in triplicate. The y-axis error bars each indicate ± 1 standard deviation of the mean. 1.00E+05, 1.0 × 105.
Paraquat killing assays (12) were used to determine the effects of the oxyR::kan mutations of defense against intracellular superoxide anion. Figure 4B shows that the sensitivity to paraquat killing of the oxyR::kan mutant is similar to that of the wild type in both unsupplemented and Mn-supplemented media. This result suggests that the increased catalase levels in the oxyR::kan mutant do not provide increased protection from superoxide killing. Although in some systems the removal of hydrogen peroxide from superoxide anion dismutation could be a rate-limiting step, the difference in catalase levels between the wild-type and oxyR::kan strains was not sufficient to reveal a difference in protection in these assays.
Sequence alignment and analysis of the oxyR gene.
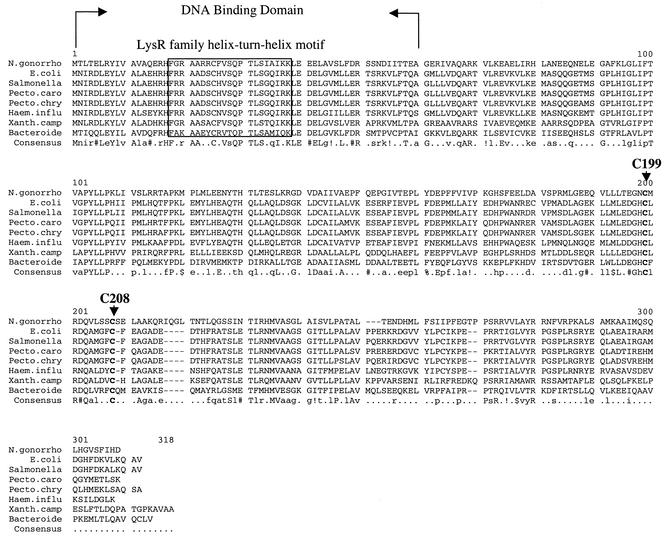
A number of oxyR homologs have been identified and characterized in many different bacteria, such as Mycobacterium marinum (28), Mycobacterium tuberculosis (33), Brucella abortus (18), Haemophilus influenzae (24), Xanthomonas campestris (23), and Bacteroides fragilis (29). Alignment of these amino acid sequence was performed by MultAlin (http://prodes.toulouse.inra.fr/multalin/multalin.html [7]) and is shown in Fig. 5. Analysis of the amino acid sequence of OxyR of N. gonorrhoeae revealed that it contains all the typical features of the OxyR proteins. OxyR belongs to the LysR family which is composed of autoregulatory transcriptional regulators (LTTRs) (32). The region of greatest amino acid sequence identity between LTTRs is the 66 N-terminal residues. The central portion of this conserved region (residues 23 to 42) is nearly 40% identical in all LTTRs; secondary structure predictions (10) and other methods (8) predict that it contains a helix-turn-helix DNA-binding motif. By comparison with the LysR family, the helix-turn-helix of OxyR is from residues 19 to 37 and the DNA-binding domain is near residues 1 to 60 (Fig. 5). Furthermore, the C199 and C208 residues that form the disulfide bond when OxyR is oxidized are strongly conserved in these OxyR homologs.
FIG. 5.
Sequence comparison of OxyR homologs from different organisms. N. gonorrhoeae (N. gonorrho) (GenBank accession number AF514857), E. coli (GenBank accession number NP418396), Salmonella enterica (GenBank accession number NP457935), Pectobacterium carotovorum (Pecto.caro) (GenBank accession number AAC72242), Pectobacterium chrysanthemi (Pecto.chry) (GenBank accession number CAB40388), Haemophilus influenzae (Haem.influ) (GenBank accession number NP438728), Xanthomonas campestris (Xanth.camp) (GenBank accession number AAC45427), and Bacteroides fragilis (Bacteriode) (GenBank accession number AAG02619) sequences were used. The DNA-binding domain is shown between the two arrows, and the helix-turn-helix motif is boxed. Gaps introduced to maximize alignment are indicated by hyphens. The conserved residues are shown in the consensus sequence below the sequences. The lowercase letters in the consensus sequence indicate a low consensus residue while the periods indicate no consensus. Symbols: !, I or V; $, L or M; %, F or Y; #, N, D, Q, E, B, or Z.
Complementation of an oxyR mutation in E. coli.
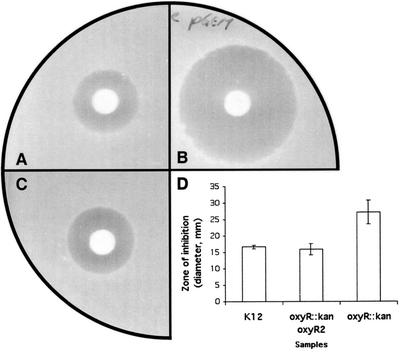
Unexpectedly, OxyR in N. gonorrhoeae acts as a repressor of gene expression, rather than an activator as seen in E. coli and S. enterica serovar Typhimurium. The ability of the N. gonorrhoeae oxyR gene to complement the E. coli oxyR mutant was therefore investigated. The oxyR open reading frame (ORF) of N. gonorrhoeae 1291 was amplified by PCR using oxyRORFfor and oxyRORFrev primers (Table 1) and cloned into pGEM-T Easy (Promega) (Fig. 1). This construct, designated pHJToxyR-1, and the vector control (pGEM-T Easy vector only) were then transformed to E. coli K-12 and GS09 (oxyR::kan mutant) kindly provided by Gisela Storz (National Institutes of Health) to see if the oxyR gene of N. gonorrhoeae could complement the defect in the E. coli GS09 mutant. Multiple independent E. coli clones were obtained and analyzed via a H2O2 disk assay by a modified version of the method of Christman et al. (5). In this assay, E. coli cells were grown in Luria-Bertani (LB) broth at 37°C with shaking overnight. Portions (100 μl) of the cell cultures grown overnight were added to LB top agar and spread onto LB agar. Then, 10 μl of 3% H2O2 (Riedel-de Haën) was pipetted onto 3MM Whatman paper disks (0.5-cm diameter), and these disks were placed on top agar and incubated at 37°C overnight. The assay was repeated on three separate occasions. The result showed that E. coli GS09 was more sensitive to H2O2 killing than the wild type (Fig. 6A and B), as shown by a larger zone of growth inhibition. However, it was demonstrated that the oxyR gene from N. gonorrhoeae could complement the defect in the E. coli GS09 mutant (Fig. 6C and D show representative results). The complemented strain was significantly more resistant to H2O2 killing than the oxyR::kan mutant (GS09) (P = 0.01757) and was similar to the wild type (K-12) (P = 0.5813) (Fig. 6D). Therefore, OxyR from N. gonorrhoeae does function as an activator of gene expression in E. coli.
FIG. 6.
H2O2 disk assay. Photos showing the zones of inhibition by H2O2 in E. coli K-12 (wild type) (A), GS09 (oxyR::kan mutant) (B), and E. coli K-12 strain GS09 complemented with the oxyR gene (pHJToxyR-1) from N. gonorrhoeae (C). (D) Histogram showing the results of H2O2 disk assay in E. coli. In the assay, cells were grown on LB agar in the presence or absence of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Sensitivity to H2O2 was measured by the zone of inhibition (diameter). The zone of inhibition was measured in three dimensions, and the mean values and standard deviations were calculated. The y-axis error bars each indicate ±1 standard deviation of the mean. Student's t test was performed to determine the statistical significance among different samples. The E. coli K-12 oxyR::kan mutant was significantly more sensitive to H2O2 than the wild type and the complemented strain (P = 0.03315 and 0.01757, respectively).
A search for the OxyR-binding site of the catalase gene in N. gonorrhoeae.
A consensus sequence for OxyR binding has been proposed and is comprised of four ATAG nucleotide elements spaced at regular intervals (27, 39). The 287-bp region between the kat gene-coding sequence and the adjacent gene (RNA polymerase sigma factor which lies upstream of and is transcribed in the direction opposite that of the kat gene) were examined in N. gonorrhoeae strains FA1090 (nucleotide positions 1730489 to 1730774 in GenBank accession number AE004969), 2821 (nucleotide positions 175 to 460 in GenBank accession number U35457 [17]), and 1291 (identical to FA1090 [this study]). No obvious binding sites were observed (data not shown).
Discussion.
OxyR is a member of the LysR family of transcriptional regulators. Like the other LysR regulators, OxyR contains an N-terminal DNA-binding region and a central region associated with signaling (32). In the case of E. coli OxyR, peroxide is sensed via two cysteine residues that form an intramolecular disulfide bridge via a reaction with hydrogen peroxide. All of the available evidence indicates that, like most LysR regulators, OxyR in enteric bacteria acts as a positive regulator of gene expression. In contrast, our data suggest that in N. gonorrhoeae this transcription factor acts as a repressor. This could be due to a fundamental difference in OxyR between N. gonorrhoeae and E. coli or differences in the promoters of kat genes in these organisms. We have shown that the oxyR gene of N. gonorrhoeae has sequence features similar to those of E. coli. The helix-turn-helix DNA-binding motif in N. gonorrhoeae and E. coli are conserved (63% identity), and the oxyR gene of N. gonorrhoeae also contains the two conserved cysteine residues, C199 and C208 (Fig. 5). Furthermore, we showed that the oxyR gene of N. gonorrhoeae can complement the E. coli oxyR::kan mutant, demonstrating that OxyR of N. gonorrhoeae acts as an activator in E. coli. However, our data are consistent with OxyR in N. gonorrhoeae acting as a repressor by binding directly to the promoter of the catalase gene in its reduced form and being released upon oxidation by hydrogen peroxide. Alternately, OxyR could have an indirect role, acting via a regulatory cascade on the catalase promoter. The OxyR protein of E. coli specifically recognizes regions of DNA that share very little sequence similarity, and its functional binding sites are unusually large (>45 bp) (6, 38). Despite this, the alignment of the OxyR-regulated promoters indicates four putative OxyR-binding tetranucleotide sequences upstream of the −35 promoter elements (39). Toledano et al. (39) found that the two forms of OxyR in E. coli make different DNA contacts: oxidized OxyR recognizes four ATAG nucleotide elements in four adjacent major grooves, while reduced OxyR recognizes ATAG nucleotide elements present in two pairs of adjacent major grooves separated by one helical turn. Our examination of the catalase gene in N. gonorrhoeae strains 1291 and FA1090 revealed no obvious OxyR-binding site. This is consistent with the finding of Johnson et al. (17), who reported that unlike hktE, the gonococcal kat gene was not preceded by any sequence that exhibited significant homology with the OxyR-binding consensus sequence. In H. influenzae, an oxyR mutant was more sensitive to killing by H2O2 than the wild type (24). Again, it had a phenotype similar to those of E. coli and S. enterica serovar Typhimurium (5, 20-22) but different from that of N. gonorrhoeae (Fig. 3).
In E. coli, it is known that although expression of peroxide defense genes is activated by OxyR in the exponential phase, in the stationary phase, expression of these genes is controlled by the starvation-induced sigma factor RpoS (31). In N. gonorrhoeae, it has been established that the rpoS gene is not active (19). Thus, the regulation of peroxide defense response in this bacterium via repression by OxyR represents a much simpler peroxide defense system than that found in enteric bacteria. Analysis and comparisons of the organization of oxyR genes from several different microorganisms revealed that they are usually linked to antioxidant or associated genes. For instance, the ahpC (alkyl hydroperoxide reductase) gene in Mycobacterium leprae and M. tuberculosis (28) is upstream of the oxyR gene and is divergently transcribed from oxyR. In Pseudomonas aeruginosa, recG (DNA repair enzyme) is located downstream of oxyR (27). The oxyR gene in N. gonorrhoeae, however, is flanked by minD (septum site-determining protein) and outer membrane protein I, which do not have obvious roles in oxidative stress.
Here we report that catalase is induced by H2O2 via OxyR-mediated derepression. This unique arrangement is more interesting when we consider that although N. gonorrhoeae is known for very high levels of catalase production, the catalase activity of the oxyR::kan mutant is fourfold higher than that of the maximally induced wild-type strain. Furthermore, the oxyR::kan mutant is far more resistant to H2O2 killing than the wild-type strain, presumably due to the increased catalase activity. The reason why a regulatory system has evolved in N. gonorrhoeae that responds to H2O2 via OxyR but does not derepress the promoter to allow maximal expression, effectively limiting catalase activity, remains to be resolved.
Nucleotide sequence accession number.
The GenBank accession number for the completed N. gonorrhoeae genome is AE004969.
Acknowledgments
Hsing-Ju Tseng thanks the University of Queensland for a Postgraduate Research Scholarship. This work was supported in part by U.S. Public Health service grants AI45728, AI43924, and AI38515 from NIAID. We acknowledge the Gonococcal Genome Sequencing Project, which initially identified the oxyR gene and was supported by USPHS NIH grant AI38399.
We thank Gisela Storz (National Institutes of Health) for providing the E. coli strains.
Editor: T. R. Kozel
REFERENCES
- 1.Aebi, H. 1984. Catalase in vitro. Methods Enzymol. 105:121-126. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, H. E. 1965. The haemophilus group, p. 724-741. In R. J. Dabos and J. G. Hirsch (ed.), Bacterial and mycotic infections of man. Pitman Medical Publishing, London, England.
- 3.Archibald, F. S., and M. N. Duong. 1986. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect. Immun. 51:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bölker, M., and R. Kahmann. 1989. The Escherichia coli regulatory protein OxyR discriminates between methylated and unmethylated states of the phage Mu mom promoter. EMBO J. 8:2403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christman, M. F., R. W. Morgan, F. S. Jacobson, and B. N. Ames. 1985. Positive control of a regulon for defense against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell 41:753-762. [DOI] [PubMed] [Google Scholar]
- 6.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. USA 86:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnier, J., D. J. Osguthorpe, and B. Robson. 1978. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J. Mol. Biol. 120:97-120. [DOI] [PubMed] [Google Scholar]
- 11.González-Flecha, B., and B. Demple. 1999. Biochemistry of redox signalling in the activation of oxidative stress genes, p. 133-153. In D. L. Gilbert and C. A. Colton (ed.), Reactive oxygen species in biological systems: an interdisciplinary approach. Kluwer/Plenum, New York, N.Y.
- 12.Hassett, D. J., B. E. Britigan, T. Svendsen, G. M. Rosen, and M. S. Cohen. 1987. Bacteria form intracellular free radicals in response to paraquat and streptonigrin. Demonstration of the potency of hydroxyl radical. J. Biol. Chem. 262:13404-13408. [PubMed] [Google Scholar]
- 13.Hassett, D. J., L. Charniga, and M. S. Cohen. 1990. recA and catalase in H2O2-mediated toxicity in Neisseria gonorrhoeae. J. Bacteriol. 172:7293-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240:1302-1309. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson, D. J., and G. Storz. 1997. Transcriptional regulators of oxidative stress response, p. 91-115. In J. G. Scandalios (ed.), Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Johnson, S. R., B. M. Steiner, D. D. Cruce, G. H. Perkins, and R. J. Arko. 1993. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect. Immun. 61:1232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, S. R., B. M. Steiner, and G. H. Perkins. 1996. Cloning and characterization of the catalase gene of Neisseria gonorrhoeae: use of the gonococcus as a host organism for recombinant DNA. Infect. Immun. 64:2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J. A., and J. Mayfield. 2000. Identification of Brucella abortus OxyR and its role in control of catalase expression. J. Bacteriol. 182:5631-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskos, L., J. P. Dillard, H. S. Seifert, J. A. Fyfe, and J. K. Davies. 1998. The pathogenic neisseriae contain an inactive rpoN gene and do not utilize the pilE sigma54 promoter. Gene 208:95-102. [DOI] [PubMed] [Google Scholar]
- 20.Levin, D. E., M. Hollstein, M. Christman, M. F. Schweirs, and B. N. Ames. 1982. A new Salmonella tester strain (TA102) with A:T base pair at the site of mutation detects oxidative mutagens. Proc. Natl. Acad. Sci. USA 79:7445-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine, S. A. 1977. Isolation and characterisation of catalase-deficient mutants of Salmonella typhimurium. Mol. Gen. Genet. 150:205-209. [DOI] [PubMed] [Google Scholar]
- 22.Loewen, P. C. 1984. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J. Bacteriol. 157:622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loprasert, S., S. Atichartpongkun, W. Whangsuk, and S. Mongkolsuk. 1997. Isolation and analysis of the Xanthomonas alkyl hydroperoxide reductase gene and the peroxide sensor regulator genes ahpC and ahpF-oxyR-orfX. J. Bacteriol. 179:3944-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maciver, I., and E. J. Hansen. 1996. Lack of expression of the global regulator OxyR in Haemophilus influenzae has a profound effect on growth phenotype. Infect. Immun. 64:4618-4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan, R. W., M. F. Christman, F. S. Jacobson, G. Storz, and B. N. Ames. 1986. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc. Natl. Acad. Sci. USA 83:8059-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson, D. P., and L. A. Kiesow. 1972. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 49:474-478. [DOI] [PubMed] [Google Scholar]
- 27.Ochsner, U. A., M. L. Vasil, E. Alsabbagh, K. Parvatiyar, and D. J. Hassett. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J. Bacteriol. 182:4533-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagán-Ramos, E., J. Song, M. McFalone, M. H. Mudd, and V. Deretic. 1998. Oxidative stress response and characterization of the oxyR-ahpC and furA-katG loci in Mycobacterium marinum. J. Bacteriol. 182:4856-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha, E. R., G. Owens, Jr., and C. J. Smith. 2000. The redox-sensitive transcriptional activator OxyR regulates the peroxide response regulon in the obligate anaerobe Bacteroides fragilis. J. Bacteriol. 182:5059-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiki, R. K., D. H. Gelfand, S. Stoffel, S. J. Scharf, R. Higuchi, G. T. Horn, K. B. Mullis, and H. A. Erlich. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:4887-4891. [DOI] [PubMed] [Google Scholar]
- 31.Sak, B. D., A. Eisenstark, and D. Touati. 1989. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc. Natl. Acad. Sci. USA 86:3271-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 33.Sherman, D. R., P. J. Sabo, M. J. Hickey, T. M. Arain, G. G. Mahairas, Y. Yuan, C. E. Barry III, and C. K. Stover. 1995. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc. Natl. Acad. Sci. USA 92:6625-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 35.Stadtman, E. R., B. S. Berlett, and P. B. Chock. 1990. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 87:384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storz, G., and L. A. Tartaglia. 1992. OxyR: a regulator of antioxidant genes. J. Nutr. 122:627-630. [DOI] [PubMed] [Google Scholar]
- 37.Tao, K., K. Makino, S. Yonei, A. Nakata, and H. Shinagawa. 1989. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptive response to oxidative stress in Escherichia coli: homologies between OxyR protein and a family of bacterial activator proteins. Mol. Gen. Genet. 218:371-376. [DOI] [PubMed] [Google Scholar]
- 38.Tartaglia, L. A., G. Storz, and B. N. Ames. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 210:709-719. [DOI] [PubMed] [Google Scholar]
- 39.Toledano, M. B., I. Kullik, F. Trinh, P. T. Baird, T. D. Schneider, and G. Storz. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 40.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 42.Warne, S. R., J. M. Varley, G. J. Boulnois, and M. G. Norton. 1990. Identification and characterisation of a gene that controls colony morphology and auto-aggregation in Escherichia coli K12. J. Gen. Microbiol. 136:455-462. [DOI] [PubMed] [Google Scholar]
- 43.Wilks, K. E., K. L. Dunn, J. L. Farrant, K. M. Reddin, A. R. Gorringe, P. R. Langford, and J. S. Kroll. 1998. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 66:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng, H.-Y., D. J. Hassett, K. Bean, and M. S. Cohen. 1992. Regulation of catalase in Neisseria gonorrhoeae. Effects of oxidant stress and exposure to human neutrophils. J. Clin. Investig. 90:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng, M., F. Åslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]