Abstract
Tuberculosis remains one of humankind's greatest killers, and new therapeutic strategies are needed to combat the causative agent, Mycobacterium tuberculosis, which is rapidly developing resistance to conventional antibiotics. Using the highly demanding guinea pig model of pulmonary tuberculosis, we have investigated the feasibility of inhibiting M. tuberculosis glutamine synthetase (GS), an enzyme that plays a key role in both nitrogen metabolism and cell wall biosynthesis, as a novel antibiotic strategy. In guinea pigs challenged by aerosol with the highly virulent Erdman strain of M. tuberculosis, the GS inhibitor l-methionine-SR-sulfoximine (MSO) protected the animals against weight loss, a hallmark of tuberculosis, and against the growth of M. tuberculosis in the lungs and spleen; MSO reduced the CFU of M. tuberculosis at 10 weeks after challenge by ∼0.7 log unit compared with that in control animals. MSO acted synergistically with isoniazid in protecting animals against weight loss and bacterial growth, reducing the CFU in the lungs and spleen by ∼1.5 log units below the level seen with isoniazid alone. In the presence of ascorbate, which allows treatment with a higher dose, MSO was highly efficacious, reducing the CFU in the lungs and spleen by 2.5 log units compared with that in control animals. This study demonstrates that inhibition of M. tuberculosis GS is a feasible therapeutic strategy against this pathogen and supports the concept that M. tuberculosis enzymes involved in cell wall biosynthesis, including major secretory proteins, have potential as antibiotic targets.
Tuberculosis remains one of the world's major health problems and the leading cause of death from a single infectious agent. Compounding the problem, strains of Mycobacterium tuberculosis, the primary causative agent of tuberculosis, that are resistant to the major drugs used to treat tuberculosis are rapidly emerging worldwide (3, 14). The World Health Organization has declared tuberculosis a global emergency, the first disease so designated.
New therapeutic strategies to combat M. tuberculosis are urgently needed. In previous studies in this laboratory, the enzyme glutamine synthetase (GS) (E.C. 6.3.1.2) was identified as a potential antibiotic target (6, 7). In addition to its well-characterized role in nitrogen metabolism, in pathogenic mycobacteria GS appears to play an important role in cell wall biosynthesis, specifically, the synthesis of a poly-l-glutamate-glutamine cell wall component found exclusively in pathogenic mycobacteria. Treatment of M. tuberculosis with the GS inhibitor l-methionine-SR-sulfoximine (MSO) or with antisense oligodeoxyribonucleotides specific to M. tuberculosis GS mRNA inhibits the formation of the poly-l-glutamate-glutamine cell wall structure (6, 9). Paralleling this effect, these agents also inhibit bacterial growth, indicating that the enzyme plays an important role in bacterial homeostasis (6, 9). MSO selectively blocks the growth in broth cultures of pathogenic mycobacteria, including M. tuberculosis, M. bovis, and M. avium, but has no effect on nonpathogenic mycobacteria or nonmycobacterial microorganisms (6). The inhibitor also blocks the growth of M. tuberculosis and M. avium within human mononuclear phagocytes, the primary host cells of these pathogens, and at concentrations that are completely nontoxic to the mammalian cells, likely reflecting the 100-fold greater sensitivity to MSO of bacterial GS than of mammalian GS (6).
In the present study, we explore the feasibility of inhibiting M. tuberculosis GS as a therapeutic strategy against this pathogen in vivo. Using the highly demanding guinea pig model of pulmonary tuberculosis, we demonstrate that MSO is an effective antibiotic in vivo and that it acts synergistically with isoniazid (INH), one of the major antibiotics used to treat tuberculosis.
MATERIALS AND METHODS
Bacterial cultures.
M. tuberculosis strain Erdman (ATCC 35801) was maintained in Middlebrook 7H9 medium (Difco Laboratories) supplemented with 2% glucose at 37°C in a 5% CO2- 95% air atmosphere as unshaken broth cultures.
Determination of the MIC of MSO for M. tuberculosis in broth cultures and human macrophages.
The MIC in broth cultures was determined by setting up triplicate cultures of M. tuberculosis (1 × 105 to 5 × 105 cells ml−1) in 7H9 broth medium in tissue culture flasks (Fisher) and maintaining the cultures for 6 weeks. MSO (Sigma Chemical Co.) was added to cultures at final concentrations ranging from 0.036 to 36 μg ml−1 (0.2 to 200 μM). The growth of the cultures was monitored by gently sonicating the cultures to break up bacterial clumps, removing small aliquots, plating serial dilutions of washed bacteria on Middlebrook 7H11 agar (Difco Laboratories), and enumerating CFU after incubation for 2 weeks. The MIC was defined as the lowest dose that restricted growth to less than twofold the starting inoculum.
The MIC of MSO for M. tuberculosis growing in human macrophages was determined by studying the same range of drug concentrations in infected THP-1 cells, a human acute monocytic leukemia cell line (ATCC TIB 202). At the highest dose studied (36 μg ml−1, or 200 μM), MSO had no inhibitory effect on the growth of uninfected undifferentiated THP-1 cells in cultures. THP-1 cells were prepared by seeding 107 cells ml−1 in a total volume of 10 ml of RPMI 1640 medium (Irvine Scientific) and differentiating the cells with 100 nM phorbol-12-myristate-13-acetate (Sigma). THP-1 cells were then infected with M. tuberculosis that had been grown for 7 to 8 days on 7H11 agar plates, washed, sonicated to obtain a single-cell suspension, and enumerated. The bacteria were added to the THP-1 cells at a multiplicity of infection of 1:1 in the presence of human serum (Irvine Scientific), and the mixture was allowed to stand for 90 min; 6 to 11% of the monocytes became infected, based on bacterial counts determined 3 h after infection. THP-1 cells were washed extensively and incubated at 37°C in a 5% CO2- 95% air atmosphere for up to 5 days in the presence of various concentrations of MSO. At various times, cultures were lysed by the addition of 0.1% sodium dodecyl sulfate and vortexing, and CFU in the lysates were enumerated by serial dilution on 7H11 agar. The MIC was defined as the lowest dose that restricted growth to less than twofold the starting inoculum.
Determination of the maximum tolerated dose of MSO in guinea pigs.
Specific-pathogen-free outbred male Hartley strain guinea pigs of ∼500 g were purchased from Charles River Laboratories and housed in groups of three in stainless steel cages with unlimited access to food and water. After a quarantine period of 7 days, animals in groups of two or three per drug concentration were administered MSO daily intraperitoneally (i.p.) in doses ranging in twofold increments from 1.6 to 100 mg kg of body weight−1, with or without ascorbate (3 mmol kg−1 day−1 i.p. in two equally divided doses). Animals were weighed every day and examined for adverse effects of the drugs. For animals that did not show severe side effects, the treatment was continued for 21 days, at which time all surviving animals were euthanized and necropsied to assess any pathological effects of the drugs.
Efficacy of MSO in guinea pigs challenged by aerosol with M. tuberculosis.
After a 7-day quarantine period, guinea pigs were challenged with an aerosol generated from a 10-ml suspension of 5 × 104 guinea pig lung-passaged M. tuberculosis strain Erdman cells, a dose which delivers ∼40 primary lesions to the lungs of each animal. Afterward, the animals were housed individually in stainless steel cages contained within a laminar flow biohazard safety enclosure for the 10-week treatment period and allowed free access to food and water. Animals were left untreated (control animals) or were administered a specific drug regimen adjusted to their weights each day and monitored for signs of illness.
In experiment 1, animals were treated with MSO at a dose of 0.75 or 1.5 mg kg−1 day−1 i.p. starting immediately or 7 days after challenge with M. tuberculosis. In experiment 2, animals were treated with MSO at 1.5 mg kg−1 day−1 i.p. and/or INH (Sigma) at 1.0 or 4.0 mg kg−1 day−1 orally starting immediately or 14 days after challenge with M. tuberculosis (see Fig. 2 and 3). By day 14 after challenge, M. tuberculosis infection is well established in the lungs. In experiment 3, animals were not treated with MSO or were treated with MSO at a dose of 1.5, 3.13, or 6.25 mg kg−1 day−1 i.p. and with ascorbate at a dose of 3 mmol kg−1 day−1 i.p. in two equally divided doses.
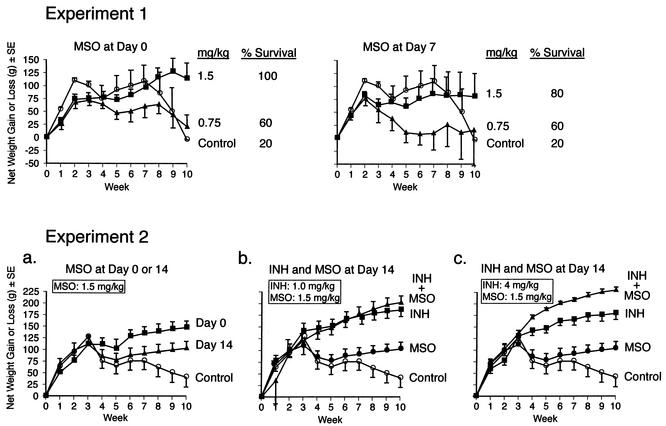
FIG. 2.
Weight loss and death after M. tuberculosis challenge. Animals in groups of five were infected with M. tuberculosis and treated with MSO and/or INH beginning 0, 7, or 14 days after challenge or not treated (control animals). All animals were weighed weekly for 10 weeks after challenge and monitored for survival. Weight data are the mean net weight gain or loss and standard error (SE) for each group of animals compared with their weights immediately before challenge. The survival data in experiment 1 are the percentages of animals surviving to the end of the 10-week observation period. In experiment 2, all animals survived to the end of the observation period.
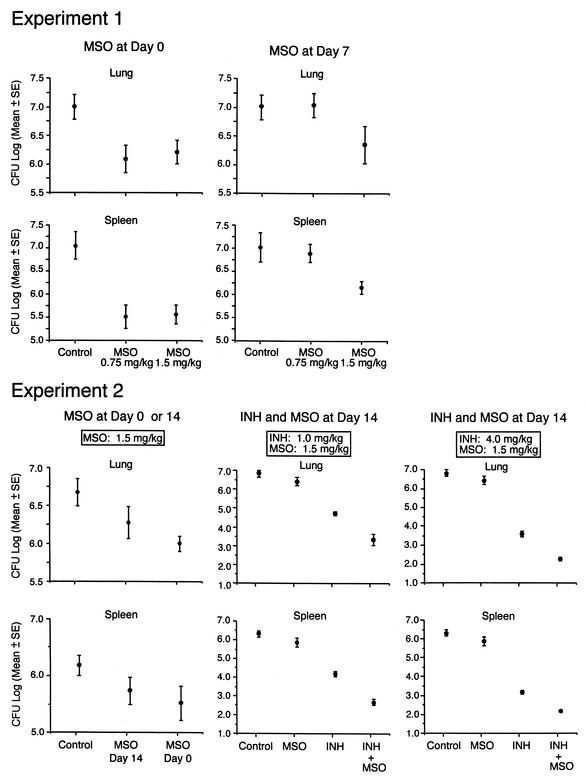
FIG. 3.
Growth of M. tuberculosis in the lungs and spleens of guinea pigs after M. tuberculosis challenge. At the end of the observation period, the animals described in the legend to Fig. 2 were euthanized, and the numbers of CFU of M. tuberculosis in the right lung and spleen were assayed. Samples from the few animals that died before the end of the observation period were cultured immediately after death. Data are the mean and standard error (SE) for all animals in a group (Table 3). The lower limit of detection was 2.0 log units per organ (1 CFU on a plate seeded with an undiluted 1% sample of an organ, i.e., 100 μl of a total sample volume of 10 ml). In experiment 2, two lung cultures and four spleen cultures from the group treated with INH (4.0 mg kg−1 day−1) plus MSO (1.5 mg kg−1 day−1) had 0 CFU on plates seeded with undiluted samples. For statistical purposes, these organs were scored as containing 2.0 log units.
After 10 weeks, the animals were euthanized, and the lungs, spleen, and liver of each animal were aseptically removed and inspected for pathological effects. The right lung and spleen were cultured for CFU of M. tuberculosis on Middlebrook 7H11 agar for 2 weeks at 37°C in a 5% CO2- 95% air atmosphere. The left lung and liver were fixed in 10% formaldehyde-phosphate-buffered saline and subsequently photographed by using Kodak Ectochrome 200 slide film and a Mini Flo lighting system (Kino Flo, Inc.). Slides were converted to digitized photo files by using a Nikon Photoshot 3000 scanner. The digitized images were further processed by using the Adobe Photoshop 5.5 software program, merged into one composite, and printed on a Fuji Pictrography 3000 photo developer. All animal studies were approved by the University of California, Los Angeles, Animal Research Committee.
Statistical analysis.
Analyses of variance (ANOVA) were used to compare mean weight gain or loss during the observation period and to compare mean log CFU in animal organs across treatment groups. Post hoc comparisons were judged statistically significant by using the Fisher-Tukey least-significant-difference criterion.
RESULTS
MIC of MSO for M. tuberculosis in vitro and maximum tolerated dose of MSO in vivo.
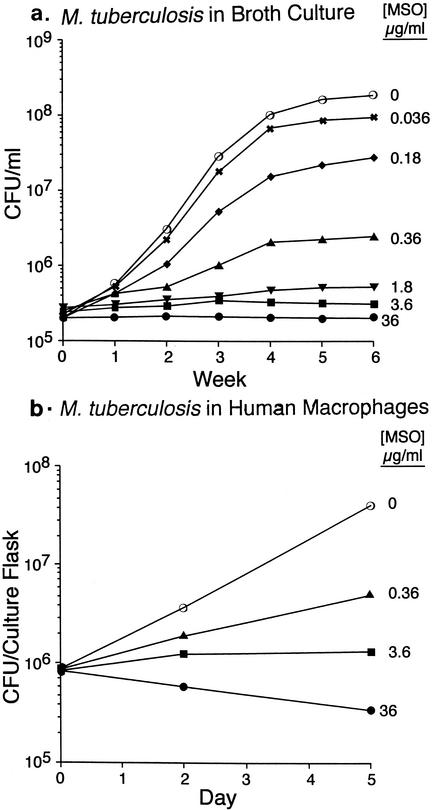
To test the efficacy of MSO in treating tuberculosis, we selected the guinea pig model of pulmonary tuberculosis. Guinea pigs are highly susceptible to tuberculosis, and they develop disease that closely resembles human disease clinically, immunologically, and pathologically. As an initial guide to dosing in guinea pigs, in preliminary in vitro experiments, we determined the MIC of MSO for M. tuberculosis growing in broth cultures and in human macrophages. The MICs were ∼1.8 μg ml−1 (10 μM) for M. tuberculosis in broth cultures and ∼3.6 μg ml−1 (20 μM) for M. tuberculosis in human macrophages (Fig. 1). These data are consistent with those of a previous study of the efficacy of MSO in vitro (6).
FIG. 1.
Susceptibility of M. tuberculosis growing in broth cultures and in human macrophages to MSO. (a) M. tuberculosis growing in broth cultures was incubated in the presence of various concentrations of MSO, and the numbers of CFU in the cultures were determined weekly for 6 weeks. Data are mean CFU per milliliter for triplicate cultures; the standard error (SE) was always ≤3%, except for one value of 6.1% (no MSO at 6 weeks). (b) Differentiated THP-1 cells, a human macrophage cell line, were infected with M. tuberculosis and incubated in the presence of various concentrations of MSO. The numbers of CFU in the cultures were determined on day 0 (3 h after infection), day 2, and day 5. Data are mean CFU per culture flask for triplicate cultures; all SEs were ≤2.4%.
As no information was available on doses of MSO tolerated by guinea pigs with long-term administration, we next determined the maximum tolerated dose of MSO delivered to the animals daily by i.p. injection. We administered MSO for 21 days in doses ranging in twofold increments from 1.6 to 100 mg kg−1 day−1 to animals in groups of two to five and observed the animals for weight loss and other adverse effects. Doses of ≥12.5 mg kg−1 day−1 were 100% lethal; a dose of 6.25 mg kg−1 day−1 was 33% lethal and otherwise poorly tolerated, inducing lethargy and anorexia; and doses of ≤3 mg kg−1 day−1 were nonlethal (Table 1). In a subsequent experiment, we determined that a dose of 3.0 mg kg−1 day−1 was well tolerated by uninfected guinea pigs but not by guinea pigs infected with M. tuberculosis, which exhibited early weight loss. It is possible that the known negative impact of MSO on glutathione synthesis in the absence of ascorbate (see below) reduced the capacity of the animals to counter the stress of infection. In infected animals, 1.5 mg kg−1 day−1 was well tolerated; hence, this dose was judged to be the maximum tolerated dose for guinea pigs infected with M. tuberculosis. In subsequent experiments, in which infected animals were treated with MSO at 1.5 mg kg−1 day−1 for 10 weeks, no side effects of MSO were observed.
TABLE 1.
Maximum tolerated dose of MSO in guinea pigs
| MSO concn (mg kg−1 day−1) | Ascorbate (3 mmol kg−1 day−1)a | No. of animals tested | % of animals dying | Median days to death |
|---|---|---|---|---|
| 100 | − | 3 | 100 | 1 |
| 50 | − | 3 | 100 | 2 |
| 25 | − | 5 | 100 | 2.5 |
| 12.5 | − | 2 | 100 | 4 |
| 6.25 | − | 3 | 33 | 5 |
| 3.13 | − | 3 | 0 | |
| 1.56 | − | 3 | 0 | |
| 25 | + | 3 | 100 | 6 |
| 12.5 | + | 3 | 0 | |
| 6.25 | + | 3 | 0 | |
| 3.13 | + | 3 | 0 | |
| 1.56 | + | 3 | 0 |
−, absent; +, present.
MSO protects guinea pigs infected with M. tuberculosis from death and disease.
To test the in vivo efficacy of MSO, we infected guinea pigs in groups of five with the highly virulent Erdman strain of M. tuberculosis, administered MSO to the animals at a dose of 1.5 or 0.75 mg kg−1 day−1 for 10 weeks beginning immediately or 7 days after challenge, and monitored the subsequent course of infection. Control animals were untreated. Death is not an end point in the majority of our studies because untreated guinea pigs usually do not succumb to tuberculosis until after 10 weeks postchallenge, the point at which we terminate the studies. However, deaths do occasionally occur earlier than 10 weeks, and this was the case in the present study. Whereas almost all of the animals treated with MSO at 1.5 mg kg−1 day−1 (n = 10) survived the 10-week observation period, whether treatment was begun on day 0 (100% survival) or day 7 (80% survival) after challenge, only 20% of the control animals (n = 5) survived (Fig. 2, experiment 1). Animals treated with MSO at 0.75 mg kg−1 day−1 (n = 10) were partially protected against death; 60% of these animals survived whether treatment was initiated on day 0 or 7 after challenge. Differences in survival were statistically significant between untreated control animals and (i) animals treated with MSO at 1.5 mg kg−1 day−1 beginning on day 0 (P < 0.05; chi-square statistic), (ii) all animals treated with MSO at 1.5 mg kg−1 day−1 (i.e., regardless of starting time) (P < 0.02), and (iii) all animals treated with MSO (i.e., regardless of dose or starting time) (P < 0.05).
As an objective indicator of illness, we assessed weight loss, a major physical sign of tuberculosis in humans and a hallmark of the disease in the guinea pig model of this chronic infectious disease. Compared with untreated control animals, animals treated with MSO at 1.5 mg kg−1 day−1 were protected from weight loss in the final weeks of the observation period, by which time the disease in control animals was far advanced. Differences in net weight gain between MSO-treated and control animals were statistically significant when treatment was begun immediately after challenge (P = 0.02) (Table 2, experiment 1). Animals treated with MSO at 0.75 mg kg−1 day−1 had less weight gain than control animals in the first 9 weeks after challenge but were protected from a precipitous decline in weight loss in the final week of the observation period (Fig. 2, experiment 1). Animals treated with MSO at 1.5 or 0.75 mg kg−1 day−1 beginning immediately after challenge gained slightly more weight than animals treated with the same dose beginning 7 days after challenge, but the survival rates were comparable. Similarly, in a second experiment, animals treated beginning immediately after challenge gained more weight than animals in which treatment was begun 14 days after challenge (Fig. 2, experiment 2, panel a). Differences in net weight gain between MSO-treated and control animals were statistically significant whether MSO treatment was started immediately (P = 0.003) (Table 2, experiment 2a) or 14 days after challenge (P = 0.04).
TABLE 2.
Key statistical analyses of net weight gain or loss
| Expt | Comparison (no. of animals/group) | P valuea |
|---|---|---|
| 1 | Control (5) vs MSO at 1.5 mg kg−1 (5) at day 0 | 0.02 |
| Control (5) vs MSO at 0.75 mg kg−1 (5) at day 0 | NS | |
| MSO at 1.5 mg kg−1 (5) vs MSO at 0.75 mg kg−1 (5) at day 0 | <0.05 | |
| Control (5) vs MSO at 1.5 mg kg−1 (5) at day 7 | NS | |
| Control (5) vs MSO at 0.75 mg kg−1 (5) at day 7 | NS | |
| MSO at 1.5 mg kg−1 (5) vs MSO at 0.75 mg kg−1 (5) at day 7 | NS | |
| 2a | Control (5) vs MSO (5) at day 0 | 0.003 |
| Control (5) vs MSO (5) at day 14 | 0.04 | |
| MSO (5) at day 0 vs MSO (5) at day 14 | 0.07 | |
| 2b | Control (5) vs INH at 1 mg kg−1 (5) | 0.001 |
| Control (5) vs INH at 1 mg kg−1 + MSO (5) | 0.001 | |
| MSO (5) vs INH at 1 mg kg−1 (5) | 0.004 | |
| MSO (5) vs INH at 1 mg kg−1 + MSO (5) | 0.02 | |
| INH at 1 mg kg−1 (5) vs INH at 1 mg kg−1 + MSO (5) | NS | |
| 2c | Control (5) vs INH at 4 mg kg−1 (5) | 0.001 |
| Control (5) vs INH at 4 mg kg−1 + MSO (5) | <0.0001 | |
| MSO (5) vs INH at 4 mg kg−1 (5) | 0.005 | |
| MSO (5) vs INH at 4 mg kg−1 + MSO (5) | <0.0001 | |
| INH at 4 mg kg−1 (5) vs INH at 4 mg kg−1 + MSO (5) | 0.007 | |
| 3 | Control (4) vs MSO at 1.5 mg kg−1 (5) | 0.001 |
| Control (4) vs MSO at 3.13 mg kg−1 (5) | <0.0001 | |
| Control (4) vs MSO at 6.25 mg kg−1 (5) | <0.0001 |
As determined by ANOVA. NS, not significant.
To assess the capacity of MSO treatment to restrict the growth of M. tuberculosis in tissues of challenged guinea pigs, we assayed the numbers of bacteria in the lungs, the primary site of infection, and the spleen, a major site of bacterial dissemination, at the end of the 10-week observation period. Animals treated with MSO at either 1.5 mg kg−1 day−1 beginning on day 0 or 7 after challenge or 0.75 mg kg−1 day−1 beginning on day 0 after challenge had approximately 1 log unit fewer CFU of M. tuberculosis in their organs than control animals (Fig. 3, experiment 1); these differences were statistically significant and highly so in the spleen (Table 3, experiment 1). When treatment was begun on day 0, doses of MSO of 0.75 and 1.5 mg kg−1 day−1 yielded comparable reductions in CFU. However, when treatment was delayed until day 7 after challenge, only the higher dose of MSO was effective in reducing bacterial counts in the lungs and spleen. In a second experiment, MSO at 1.5 mg kg−1 day−1 was effective at reducing bacterial counts whether treatment was initiated at day 0 or 14 after challenge, although early initiation was somewhat more efficacious (Fig. 3, experiment 2, left panels) and the differences from control animals were statistically significant (Table 3, experiment 2).
TABLE 3.
Key statistical analyses of CFU in lungs and spleens
| Expt | Comparison (no. of animals/group) |
P valuea for:
|
|
|---|---|---|---|
| Lungs | Spleens | ||
| 1 | Control (5) vs MSO at 0.75 mg kg−1 (5) at day 0 | 0.01 | 0.0001 |
| Control (5) vs MSO at 1.5 mg kg−1 (5) at day 0 | 0.04 | 0.0002 | |
| Control (5) vs MSO at 0.75 mg kg−1 (5) at day 7 | NS | NS | |
| Control (5) vs MSO at 1.5 mg kg−1 (5) at day 7 | 0.08 | 0.01 | |
| 2 | Control (5) vs MSO at 1.5 mg kg−1 (5) at day 14 | 0.1 | 0.1 |
| Control (5) vs MSO at 1.5 mg kg−1 (5) at day 0 | 0.015 | 0.02 | |
| Control (5) vs INH at 1 mg kg−1 (5) | <0.0001 | <0.0001 | |
| Control (5) vs INH at 4 mg kg−1 (4) | <0.0001 | <0.0001 | |
| INH at 1 mg kg−1 (5) vs INH + MSO (5) | <0.0001 | <0.0001 | |
| INH at 4 mg kg−1 (4) vs INH + MSO (5) | <0.0001 | <0.0001 | |
| 3 | Control (4) vs MSO at 1.5 mg kg−1 (5) | 0.001 | NS |
| Control (4) vs MSO at 3.13 mg kg−1 (5) | <0.0001 | <0.0001 | |
| Control (4) vs MSO at 6.25 mg kg−1 (5) | <0.0001 | <0.0001 | |
| MSO at 1.5 mg kg−1 (5) vs MSO at 3.13 mg kg−1 (5) | 0.001 | 0.02 | |
| MSO at 3.13 mg kg−1 (5) vs MSO at 6.25 mg kg−1 (5) | 0.004 | 0.001 | |
As determined by ANOVA. NS, not significant.
MSO acts synergistically with INH to protect guinea pigs infected with M. tuberculosis.
Tuberculosis in humans is generally treated with a combination of antibiotics to gain better control over the infection and to prevent the emergence of resistant organisms. To determine the efficacy of MSO in combination with another antituberculosis drug, we studied the efficacy of MSO in combination with the major antituberculosis drug INH, which was previously found to act synergistically with MSO against M. tuberculosis in broth cultures (6). We studied orally administered INH at the maximum effective dose in guinea pigs of 4 mg kg−1 day−1 and at the slightly less effective dose of 1 mg kg−1 day−1. The drugs were administered beginning 14 days after challenge, a point at which animals begin to exhibit signs of disease.
Administered alone, both MSO and INH protected animals from weight loss; INH was more effective at both doses studied (Fig. 2, experiment 2, middle and right panels). INH in combination with MSO yielded even greater protection against weight loss than INH alone, especially at the higher dose of INH, where the difference was statistically significant (Table 2, experiment 2c). Weight gain in animals treated with the two drugs in combination approximated that in untreated animals of similar ages in previous experiments (11). Paralleling these results, both MSO and INH reduced the CFU of M. tuberculosis in the lungs and spleen. INH was much more effective than MSO. At a dose of 1 mg kg−1 day−1, INH reduced the CFU in the lungs and spleen by ∼1.5 log units below the level obtained with MSO, and at a dose of 4 mg kg−1 day−1, INH reduced the CFU by ∼2.5 log units below the level obtained with MSO (Fig. 3, experiment 2, middle and right panels). However, when the two drugs were administered in combination, they reduced the CFU in the lungs and spleen even further, by ∼1.5 log units below the level obtained with INH alone; these differences were statistically highly significant in both organs at both doses of INH (P < 0.0001) (Table 3, experiment 2). Indeed, in animals treated with MSO plus the higher dose of INH (4.0 mg kg−1 day−1), the reduction in CFU was even greater than that indicated in Fig. 2; CFU were actually undetectable in the lungs of two animals and the spleens of four animals out of the five animals in this group but were scored as 2.0 log units, the lower limit of detection.
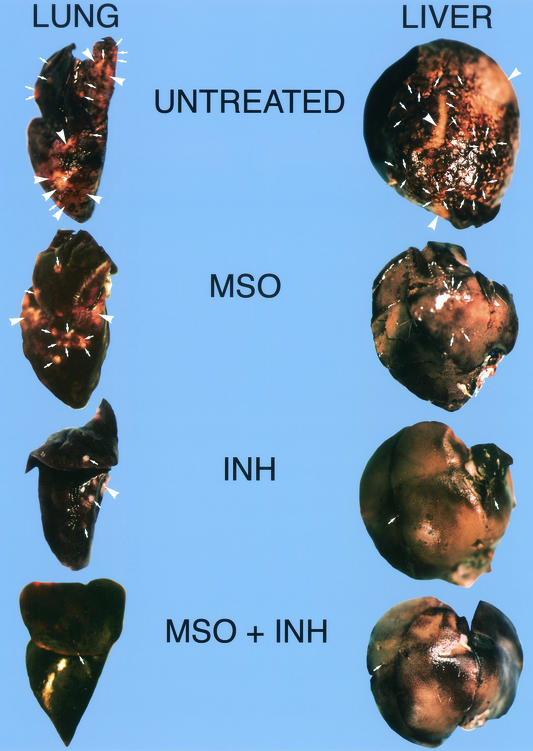
The findings at necropsy mirrored these results. On visual inspection, the lungs and livers of animals treated with MSO, INH, and the combination of MSO and INH had progressively fewer lesions than those of untreated control animals (Fig. 4). Lesions were particularly scarce in the lungs and livers of animals treated with the combination of MSO and INH.
FIG. 4.
Gross pathology of lungs and livers from untreated animals or animals treated with MSO and/or INH in experiment 2. Representative organs from untreated control animals or from animals treated with MSO (1.5 mg kg−1 day−1) alone starting on day 0, INH (4.0 mg kg−1 day−1) alone starting on day 14, or INH (4.0 mg kg−1 day−1) plus MSO (1.5 mg kg−1 day−1) starting on day 14 are shown. The organs were fixed in 10% formaldehyde-phosphate-buffered saline and photographed. Arrows indicate single primary tubercles, and arrowheads indicates areas where many tubercles have coalesced into a large lesion.
Thus, the combination of MSO and INH was more efficacious than either drug alone in protecting animals from disease, as reflected by weight loss, and the two drugs acted synergistically to suppress the growth of M. tuberculosis in the lungs and spleen.
MSO is highly efficacious as a single agent at higher doses tolerated in the presence of ascorbate administered concomitantly.
In addition to inhibiting GS, MSO also inhibits γ-glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme in glutathione synthesis. The glutathione deficiency that results contributes to the toxicity of MSO. Ascorbate (vitamin C) can offset this toxic effect of MSO and preserve glutathione levels (5, 12).
To determine the maximum tolerated dose of MSO in guinea pigs in the presence of ascorbate, we administered to animals MSO at various doses in the presence of ascorbate at 3 mmol kg−1 day−1 i.p. in two equally divided doses. A preliminary study determined that a total ascorbate dose of 3 mmol kg−1 day−1 i.p. but not 9 mmol kg−1 day−1 was well tolerated by guinea pigs. In the presence of ascorbate, the maximum tolerated dose of MSO was 12.5 mg kg−1 day−1, fourfold higher than that in the absence of ascorbate (Table 1).
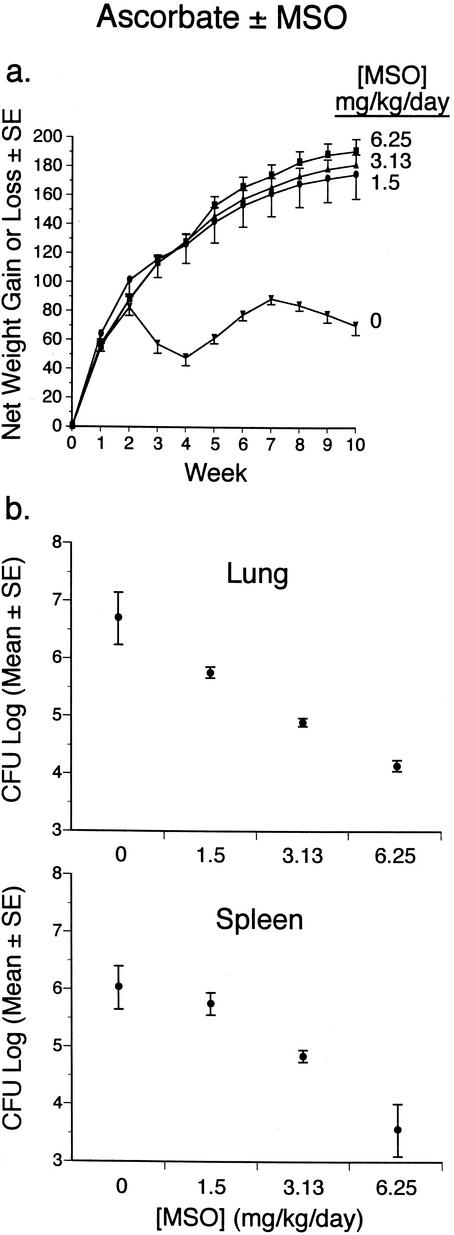
To determine the efficacy of the higher doses of MSO tolerated in the presence of ascorbate, we infected guinea pigs with M. tuberculosis and then left them untreated or treated them with MSO at concentrations of 1.5, 3.13, and 6.25 mg kg−1 day−1 i.p. in the presence of ascorbate at 3 mmol kg−1 day−1 i.p. starting immediately after challenge. As in previous experiments, we observed the animals for 10 weeks and then euthanized them to determine the CFU in the lungs and spleen. All animals survived the observation period, except for one untreated control animal (receiving no MSO) that died 3.5 weeks after challenge. Animals administered MSO at each of the three doses gained significantly more weight than untreated control animals (Fig. 5 and Table 2). MSO-treated animals also had significantly fewer CFU in the lungs and spleen (Fig. 5 and Table 3). The effect of MSO on CFU was strongly dose dependent, with 6.25 > 3.13 > 1.5 mg kg−1 day−1. At the highest dose, 6.25 mg kg−1 day−1, CFU were reduced by 2.5 log units in the lungs and spleens compared with those in untreated control animals; these differences were highly statistically significant in both organs (P < 0.0001) (Table 3).
FIG. 5.
Efficacy of MSO in the presence of ascorbate. Animals in groups of five were infected with M. tuberculosis by aerosol and then treated with MSO at the concentrations indicated in the presence of ascorbate for 10 weeks. (a) Weight data. All animals were weighed weekly for 10 weeks. Data are the mean net weight gain or loss and standard error (SE) for each group of animals compared with their weights immediately before challenge. (b) Numbers of CFU in the lungs and spleens. Data are the mean and SE for all animals in a group (Table 3).
DISCUSSION
This study demonstrates that M. tuberculosis GS is a promising target for new antibiotic drug development. MSO, an inhibitor of M. tuberculosis GS, protected guinea pigs challenged by aerosol with a highly virulent strain of M. tuberculosis from (i) death; (ii) disease, as manifested by protection against weight loss; and (iii) growth and dissemination of M. tuberculosis in animal organs, as manifested by decreased CFU in the lungs and spleen. MSO acted synergistically with the major antituberculosis drug INH, reducing CFU in guinea pig organs by ∼1.5 log units below the level attained with INH alone. MSO was effective in guinea pigs despite the need to use a low dose in this relatively sensitive species (see below). In the presence of ascorbate, which allowed treatment with a fourfold-higher dose, MSO was highly efficacious as a single agent, reducing CFU in guinea pig organs by 2.5 log units.
We have postulated that one reason why inhibiting M. tuberculosis GS is an effective therapeutic strategy is that the enzyme is involved in the formation of a poly-l-glutamate- glutamine structure in the cell walls of M. tuberculosis and other pathogenic mycobacteria (6, 9). The formation of this cell wall structure is inhibited by the GS inhibitor MSO and by antisense oligodeoxyribonucleotides specific to M. tuberculosis GS mRNA (6, 9). These same agents inhibit bacterial growth, suggesting that disruption of the poly-l-glutamate-glutamine structure interferes with bacterial homeostasis. If GS is involved in the formation of the poly-l-glutamate-glutamine cell wall structure, inhibiting GS may have an additional advantage. By interfering with the integrity of the bacterial cell wall, drugs directed against GS may amplify their uptake through the cell wall, which, in M. tuberculosis, is normally relatively impermeable to foreign molecules.
By analogy, other proteins of M. tuberculosis involved in cell wall biogenesis also may be promising targets for new antituberculosis drugs; in particular, secretory proteins, which function at the exterior of the cell wall, may be more accessible to drugs (1, 8). Such proteins include the three members of the 30- and 32-kDa (antigen 85) complex of mycolyl transferases (2), already shown to be potent immunoprotective antigens in vaccines (10, 11). Consistent with this idea, it has been found that antisense oligodeoxyribonucleotides complementary to the mRNAs of the three mycolyl transferases are unusually potent inhibitors of M. tuberculosis growth in broth cultures (9b). Moreover, proteins that have no homologs in mammals, such as the mycolyl transferases, may be superior targets, since drugs targeted against them may exhibit more favorable toxicity/therapeutic effect ratios than drugs targeted against more conserved proteins.
MSO is a known epileptogenic agent, a property first observed in dogs fed biscuits contaminated with MSO (15). The biscuits were contaminated because they were made from flour bleached with nitrogen trichloride or agene, a common practice in the first half of the 20th century. However, the sensitivities of various animal species to this effect of MSO vary enormously. Dogs are unusually sensitive. In one study, an injection of 3 mg of racemic d,l-methionine-SR-sulfoximine kg−1, about one-half the potency of the MSO isomer used here, was lethal to dogs, whereas an injection of 100 to 150 mg kg−1 produced no ill effects in monkeys and rats (4). Previous studies of the susceptibility of animals to agenized (nitrogen trichloride-treated) food suggested that guinea pigs were less sensitive to MSO than dogs (15). However, in our study, guinea pigs appeared highly sensitive to repeatedly administered MSO, as the maximum tolerated daily doses were only 3 mg kg−1 in uninfected animals and 1.5 mg kg−1 in infected animals. This finding may have had less to do with the capacity of MSO to inhibit GS than with its capacity to inhibit γ-GCS. In animals such as guinea pigs and newborn rats, which cannot synthesize ascorbate, the inhibition of γ-GCS results in glutathione deficiency and mitochondrial damage (12). Ascorbate protects guinea pigs from toxicity induced by buthionine sulfoximine, a specific inhibitor of γ-GCS (5). Similarly, in our study, we found that the addition of ascorbate to MSO increased the maximum tolerated dose of MSO in guinea pigs by fourfold.
Humans, like monkeys and rats, may be relatively insensitive to MSO. In contrast to dogs, humans fed large amounts of agenized food exhibited no significant clinical, electroencephalographic, or biochemical abnormalities (13, 15). If humans can tolerate higher doses of MSO than guinea pigs, greater efficacy against tuberculosis may be achievable in humans than in guinea pigs with this agent. It may also be possible to improve the toxicity/therapeutic effect ratio of this treatment approach by the use of MSO analogs that are specific to GS, i.e., noninhibitory for γ-GCS (5), and that are poorly transported into the brain; an example is α-ethyl-MSO, which induces convulsions in only a minority of mice at doses 16-fold higher than the dose of MSO that produces convulsions in 100% of animals (5). Along these lines, it has been found that α-ethyl-MSO inhibits broth-grown M. tuberculosis as effectively as MSO (G. Harth, O. W. Griffith, and M. A. Horwitz, unpublished data). In addition, it may be possible to develop MSO analogs that are even more specific to M. tuberculosis GS relative to mammalian GS.
In conclusion, this study demonstrates that inhibiting M. tuberculosis GS is a feasible therapeutic strategy against this pathogen and supports the concept that M. tuberculosis proteins involved in cell wall biogenesis, including major secretory proteins, have potential as antibiotic targets. This study justifies further evaluation of MSO or its analogs as chemotherapeutic agents against tuberculosis.
Acknowledgments
We thank Barbara Jane Dillon, Chalermchai Chaloyphian, and Saša Masleša-Galic for assistance with animal necropsies and Jeffrey Gornbein for statistical evaluation of data.
This work was supported by NIH grant AI42925.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Anderson, D. H., G. Harth, M. A. Horwitz, and D. Eisenberg. 2001. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kDa major secretory protein (antigen 85B), a mycolyl transferase. J. Mol. Biol. 307:671-681. [DOI] [PubMed] [Google Scholar]
- 2.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 3.Cohn, D. L., F. Bustreo, M. C. Raviglione, and the International Union Against Tuberculosis and Lung Disease. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. Clin. Infect. Dis. 24(Suppl. 1):S121-S130. [DOI] [PubMed] [Google Scholar]
- 4.Gershoff, S. N., and C. A. Elvehjem. 1951. The relative effect of methionine sulfoximine on different animal species. J. Nutr. 45:451-458. [DOI] [PubMed] [Google Scholar]
- 5.Griffith, O. W., and A. Meister. 1978. Differential inhibition of glutamine and γ-glutamylcysteine synthetases by α-alkyl analogs of methionine sulfoximine that induce convulsions. J. Biol. Chem. 253:2333-2338. [PubMed] [Google Scholar]
- 6.Harth, G., and M. A. Horwitz. 1999. An inhibitor of exported Mycobacterium tuberculosis glutamine synthetase selectively blocks the growth of pathogenic mycobacteria in axenic culture and in human monocytes: extracellular proteins as potential novel drug targets. J. Exp. Med. 189:1425-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harth, G., D. L. Clemens, and M. A. Horwitz. 1994. Glutamine synthetase of Mycobacterium tuberculosis: extracellular release and characterization of its enzymatic activity. Proc. Natl. Acad. Sci. USA 91:9342-9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harth, G., B.-Y. Lee, and M. A. Horwitz. 1997. High-level heterologous expression and secretion in rapidly growing nonpathogenic mycobacteria of four major Mycobacterium tuberculosis extracellular proteins considered to be leading vaccine candidates and drug targets. Infect. Immun. 65:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harth, G., P. C. Zamecnik, J.-Y. Tang, D. Tabatadze, and M. A. Horwitz. 2000. Treatment of Mycobacterium tuberculosis with antisense oligonucleotides to glutamine synthetase mRNA inhibits glutamine synthetase activity, formation of the poly-l-glutamine/glutamate cell wall structure, and bacterial replication. Proc. Natl. Acad. Sci. USA 97:418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b.Harth, G., M. A. Horwitz, D. Tabatedze, and P. C. Zamecnik. 2002. Targeting the Mycobacterium tuberculosis 30/32-kDa mycolyl transferase complex as a therapeutic strategy against tuberculosis: proof of principle by using antisense technology. Proc. Natl. Acad. Sci. USA 99:15614-15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz, M. A., B.-W. E Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Masleša-Galic. 2000. Recombinant BCG vaccines expressing the Mycobacterium tuberculosis 30 kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meister, A. 1995. Mitochondrial changes associated with glutathione deficiency. Biochim. Biophys. Acta 1271:35-42. [DOI] [PubMed] [Google Scholar]
- 13.Newall, G. W., T. C. Erickson, W. E. Gilson, S. N. Gershoff, and C. A. Elvehjem. 1949. Studies on human subjects receiving highly agenized food materials. J. Clin. Lab. Investig. 34:239-245. [PubMed] [Google Scholar]
- 14.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. B. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, P. Nunn, and the World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. 1998. Global surveillance for antituberculosis-drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. [DOI] [PubMed] [Google Scholar]
- 15.Proler, M., and P. Kellaway. 1962. The methionine sulfoximine syndrome in the cat. Epilepsia 3:117-130. [DOI] [PubMed] [Google Scholar]