Abstract
Penicillium marneffei is an intracellular opportunistic fungus causing invasive mycosis in AIDS patients. T cells and macrophages are important for protection in vivo. However, the role of T-cell cytokines in the immune response against P. marneffei is still unknown. We studied by semiquantitative reverse transcription-PCR and biological assays the patterns of expression of Th1 and Th2 cytokines in the organs of wild-type (wt) and gamma interferon (IFN-γ) knockout (GKO) mice infected intravenously with P. marneffei conidia. At 3 × 105 conidia/mouse, a self-limiting infection developed in wt BALB/c mice, whereas all GKO mice died at day 18 postinoculation. Splenic and hepatic granulomas were present in wt mice, whereas disorganized masses of macrophages and yeast cells were detected in GKO mice. The infection resolved faster in the spleens than in the livers of wt mice and was associated with the local expression of type 1 cytokines (high levels of interleukin-12 [IL-12] and IFN-γ) but not type 2 cytokines (low levels of IL-4 and IL-10). Conversely, both type 1 and type 2 cytokines were detected in the livers of wt animals. Disregulation of the cytokine profile was seen in the spleens but not in the livers of GKO mice. The inducible nitric oxide synthase mRNA level was low and the TNF-α level was high in both spleens and livers of GKO mice compared to wt mice. These data suggest that the polarization of a protective type 1 immune response against P. marneffei is regulated at the level of individual organs and that the absence of IFN-γ is crucial for the activation of fungicidal macrophages and the development of granulomas.
Penicillium marneffei is a dimorphic pathogen that causes disseminated mycoses in the lungs, spleens, and livers of infected subjects, especially human immunodeficiency virus (HIV)-positive patients in Southeast Asia (8, 13). In vivo (21, 29) and in vitro (6, 9), P. marneffei undergoes phase transition and multiplies as yeast cells inside macrophages. The histopathological characteristic of P. marneffei infection in humans is granuloma formation, with suppurative and anergic reactions and necrosis (9, 13, 29). In mice, disseminated granulomas are also present, with epithelioid cells containing growing yeast cells. Hepatosplenomegaly is common in both mice and humans (8).
The immune response against P. marneffei infection is mediated mainly by T cells and macrophages: in nude mice or T-cell-depleted animals, the mycosis is fatal, whereas in healthy hosts, the fungus is cleared in 2 to 3 weeks, depending on the size of the inoculum (22, 32). In vitro, both human and mouse macrophages are able to control P. marneffei growth and to kill intracellular yeast cells when activated by T-cell-derived cytokines, such as gamma interferon (IFN-γ) (6, 23, 30). It has been shown that fungus death is due mostly to the release of nitric oxide (NO) by activated macrophages (6, 23, 30).
Little is known about the role of inflammatory cytokines in vivo and whether resistance to P. marneffei infection is mediated by a Th1 or a Th2 immune response or both. Granuloma formation can be associated with either a predominantly Th1 response, with high levels of tumor necrosis factor alpha (TNF-α), IFN-γ, and interleukin-12 (IL-12), as in mycobacterial infections (7, 15), or a Th2 response, with high levels of IL-10 and IL-4, as in the reaction to Schistosoma eggs (34). As reported for other fungi, such as Candida spp., Aspergillus fumigatus, or Histoplasma capsulatum (4, 20, 28, 35), the cytokine microenvironment dictates whether a protective or a deleterious immune response is generated. IFN-γ is the hallmark cytokine for the Th1 immune response, and its production is essential in the control of intracellular pathogens, such as Mycobacterium tuberculosis (7). In mice with a disrupted IFN-γ gene (IFN-γ knockout [GKO] mice) or in patients with defects in IFN-γ signaling, susceptibility to mycobacterial infections has been reported (14, 18, 19, 26).
Dissecting the mechanism(s) of P. marneffei infection in immunocompetent hosts may help to understand the pathogenicity of penicilliosis marneffei in immunocompromised patients. This goal is particularly relevant for AIDS patients living in Southeast Asia, where this infection ranks as the third most important opportunistic infection (8) and where the vast majority of HIV-infected patients cannot yet afford treatment with highly active antiretroviral therapy.
The purpose of this study was to characterize the cytokine microenvironment leading to the formation of granulomas and to the activation of a protective immune response in healthy or GKO BALB/c mice sublethally infected with P. marneffei conidia. We also investigated the histopathological changes occurring in the organs of wild-type and GKO mice. Although the natural infection likely is acquired via the respiratory tract, in this set of experiments and in agreement with previous reports (9, 22), we preferred the intravenous (i.v.) route of infection to better control the size of the inoculum and, consequently, the extent of colonization of the different organs.
MATERIALS AND METHODS
Mice.
Pathogen-free female BALB/c mice weighing 20 to 24 g were obtained from Charles River Italia (Calco, Como, Italy) and used at the age of 6 to 8 weeks. GKO mice (11) in a BALB/c background (BALB/c-Ifngtm1Ts) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Homozygous mice were identified by PCR of tail-derived DNA with primers flanking the neo gene (sense, 5′-CAAGTGGCATAGATGTGGAAG-3′; antisense, 5′-GGCAATACTCATGAATGCATCC-3′); the wild-type gene gave an amplified fragment of 340 bp, whereas the disrupted gene gave a 2,400-bp fragment. Homozygous mice were bred and maintained at the Istituto Nazionale Tumori, Milan, Italy, and were kindly provided to us by M. P. Colombo, Istituto Nazionale Tumori. All mice were housed, fed, and handled under standard conditions according to institutional guidelines.
Microorganism, infections, and in vivo analysis.
The strain of P. marneffei used in this study, IUM885346, was kindly provided by M. A. Viviani, Milan, Italy. It was isolated from the blood of an Italian HIV-positive i.v. drug user infected in Thailand (33). The fungus was cultured on yeast morphology agar (YMA) (Biolife, Milan, Italy) at 25°C for 10 to 14 days. P. marneffei conidia were collected by flooding the culture surface with saline, centrifuged at 1,800 × g for 20 min, and filtered to remove mixed mycelial debris. The conidia were washed twice with saline, counted with a hemocytometer, and then suspended at the desired concentration.
Mice were injected i.v. via the tail vein with a sublethal suspension of 3 × 105 conidia/mouse and sacrificed after specified time intervals. Control mice of the same sex and age were injected with sterile saline. To check the inoculum viability, the suspension was diluted and plated in triplicate on YMA, and the number of CFU was determined after 72 h at 25°C. Viability was always 60 to 80%.
To evaluate in vivo the growth of the fungus, weighed organ samples were mechanically homogenized and centrifuged at 800 × g. The pellet was suspended in 0.1% Tween 20 in phosphate-buffered saline (Sigma, Milan, Italy). The suspension was plated on YMA as described above, and the results were expressed as CFU per gram of tissue. The data are the cumulative results for all available specimens from five mice per group in four experiments.
For histological analysis, tissues samples were excised and immediately fixed in 10% formalin in 0.1 M phosphate buffer at pH 7.4. Serial 3-μm sections of paraffin-embedded tissues were stained with hematoxylin-eosin (HE) stain and periodic acid-Schiff (PAS) stain.
In vitro macrophage stimulation.
Murine macrophage cell line J774 of BALB/c origin was maintained in minimal essential medium (Gibco BRL, Milan, Italy) supplemented with 10% heat-inactivated fetal calf serum (Euroclone, Ltd., Cellbio Sre, Milan, Italy), 1% glutamine, 2% HEPES buffer, 1% penicillin-streptomycin solution (Biological Ind., Kibbutz, Israel), and 1% nonessential amino acids (Gibco BRL) (complete medium [CM]) in 5% CO2 at 37°C in petri dishes. J774 macrophages in CM were seeded in 24-well plates (no. 3524; Costar, Milan, Italy) at 105 cells/well and incubated at 37°C in 5% CO2 for 24 h; confluent monolayers were treated with P. marneffei conidia (2 × 105/well) as described previously (30). Phagocytosis of conidia was allowed for 2 h in the absence of opsonins. J774 monolayers were then washed with warm phosphate-buffered saline to remove nonphagocytized conidia and incubated for different times in CM. Control wells were treated with CM only or activated with 10 U of recombinant murine IFN-γ (Genzyme)/ml plus 1 μg of lipopolysaccharide (LPS) (Sigma)/ml. At the end of the incubation, supernatants were collected and assayed for the presence of nitrite or cytokines. For the evaluation of cytokine mRNA expression, cells were lysed with 1 ml of Trizol reagent (Invitrogen, Milan, Italy) 24 h after stimulation with recombinant murine IFN-γ plus LPS or 4, 24, and 48 h after treatment with P. marneffei conidia.
RT-PCR detection of cytokine mRNA.
For reverse transcriptase PCR (RT-PCR) analysis, organ tissues from healthy or P. marneffei-infected mice were kept at −20°C in RNase-free solution (RNA-later; Celbio, Milan, Italy). The medium was removed, and the tissues were homogenized in 1 ml of Trizol reagent by using an ULTRA-TURRAX homogenizer (IKA-WERK, Staufen, Germany). Total RNA was isolated according to the manufacturer's instructions. RT-PCR was performed to determine the relative quantities of mRNAs for IL-10, IL-12, IL-4, IFN-γ, TNF-α, and inducible nitric oxide synthase (iNOS) and two housekeeping genes, those for β-actin and glyceraldehyde-3-phosphate dehydrogenase.
The sequences for the PCR primers used are shown in Table 1. All of the PCRs were performed with a TECHNE thermal cycler by using a final volume of 50 μl containing reaction buffer (1 mM Tris-HCl, 5 mM KCl, 0.1% Triton X-100 [Promega]), 2 mM MgCl2, 200 μM deoxynucleoside triphosphates, 0.2 μM each primer, 1.25 U of Taq thermostable polymerase (Promega), and template cDNA.
TABLE 1.
Oligonucleotide primer sequences for PCR
| Primer target | Sequencea | Product size, (bp) |
|---|---|---|
| IL-10 | 5′-CGG GAA GAC AAT AAC TG-3′ + | 186 |
| 5′-CAT TTC CGA TAA GGC TTG G-3′ − | ||
| IL-12 | 5′-CGT GCT CAT GGC TGG TGC AAA G-3′ + | 220 |
| 5′-CTT CAT CTG CAA GTT CTT GGG C-3′ − | ||
| IFN-γ | 5′-AAC GCT ACA CAC TGC ATC TTG G-3′ + | 236 |
| 5′-GAC TTC AAA GAG TCT GAG G-3′ − | ||
| TNF-α | 5′-ATG AGC ACA GAA AGC ATG ATC-3′ + | 276 |
| 5′-TAC AGG CTT GTC ACT CGA ATT-3′ − | ||
| iNOS | 5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′ + | 496 |
| 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′ − | ||
| IL-4 | 5′-TCG GCA TTT TGA ACG AGG TC-3′ + | 216 |
| 5′-GAA AAG CCC GAA AGA GTC TC-3′ − | ||
| β-Actin | 5′-GTG GGA ATT CGT CAG AAG GAC TCC TAT GTG-3′ + | 537 |
| 5′-GAA GTC TAG AGC AAC ATA GCA CAG CTT CTC-3′ − | ||
| G3PDHb | 5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′ + | 983 |
| 5′-CAT GTA GGC CAT GAG GTC CAC CAC-3′ − |
+, sense strand; −, antisense strand.
G3PDH, glyceraldehyde-3-phosphate dehydrogenase.
After an initial incubation at 95°C for 2 min, temperature cycling was begun. The cycles consisted of 20 s of denaturation at 94°C, 30 s of primer annealing at 55°C, and 30 s of primer extension at 72°C for 30 cycles. PCR conditions were strictly defined for each cytokine primer pair such that a linear relationship between the input RNA and the final PCR product was obtained. As a control for calibration, an equivalent amount of input cDNA for β-actin was amplified, and all samples were normalized (if necessary) for β-actin cDNA content prior to analysis of cytokine cDNA. A negative control was included in each assay to confirm that only cDNA PCR products were detected and that none of the reagents was contaminated with cDNA of previous PCR products.
Dilution analysis of template cDNA was used to optimize the concentration of cDNA required in each PCR. Optimal amounts of template cDNA ranged from 0.5 to 4 μl.
All PCR products were analyzed by electrophoresis on 1.5% agarose gels and visualized by ethidium bromide staining. The amplifications were repeated at least twice with two separately prepared cDNA samples for each mouse. The reported data are representative of at least three different experiments.
Cytokine bioassay and nitrite determination.
TNF-α activity in the supernatants of P. marneffei-stimulated J774 macrophages was assayed by using the Wehi 164 clone 13 cell line in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma catalog no. M2128) cytotoxicity assay as described previously (31). The results are expressed as units of TNF-α per milliliter calculated from a standard curve with recombinant TNF-α (Boehringer Mannheim, Milan, Italy) used as an internal standard for each assay.
The accumulation of nitrite was measured by mixing 100 μl of culture supernatants with an equal volume of Griess reagent (1% sulfanilamide- 0.1% naphthylethylenediamine dihydrochloride in 5% concentrated H3PO4). Plates were incubated at room temperature for 10 min, and the A550 was measured with a microtiter plate reader (Molecular Devices) (30). Nitrite concentrations were determined by a least-squares linear regression analysis of a sodium nitrite standard curve (from 5 to 100 μM) generated for each experiment.
Statistical analyses.
All assays were performed at least three times in triplicate, and the results were analyzed by a one-way analysis of variance.
RESULTS
In vitro production of cytokines by J774 macrophages stimulated with P. marneffei conidia.
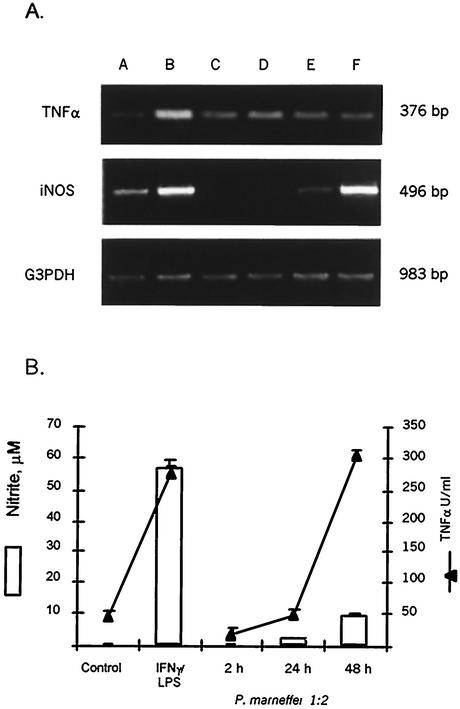
Using a recently developed in vitro coculture system (30), we evaluated the production of inflammatory cytokines by J774 macrophages stimulated with P. marneffei conidia. As shown in Fig. 1A, mRNA levels, measured by semiquantitative RT-PCR, for TNF-α and iNOS were augmented in P. marneffei-treated cells compared to control cells. mRNA for TNF-α was evident after only 2 h, and its level increased by 24 h, whereas the iNOS mRNA level increased only after 48 h. In parallel, NO and TNF-α were found in cell supernatants, and their levels increased with time in cultures. The amount of TNF-α found in macrophage-fungus cocultures was similar to that obtained after IFN-γ- LPS stimulation of J774 macrophages (Fig. 1B).
FIG. 1.
In vitro production of cytokines by P. marneffei-stimulated macrophages. (A) RT-PCR analysis of TNF-α and iNOS mRNAs in J774 macrophages treated with P. marneffei conidia (1:2 ratio) for different times. Samples were processed as described in Materials and Methods. Lanes: A, mRNA from control, unstimulated J774 cells; B, mRNA from J774 cells stimulated with IFN-γ plus LPS for 24 h; C to F, mRNA from J774 cells stimulated with live P. marneffei conidia for 2 h (C), 4 h (D), 24 h (E), or 48 h (F). G3PDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Kinetics of TNF-α and NO production by J774 macrophages stimulated with P. marneffei conidia (1:2 ratio). Supernatants were collected after different times and assayed for the presence of cytokines as described in Materials and Methods. Error bars indicate standard deviations.
Course of P. marneffei infection in wild-type BALB/c mice.
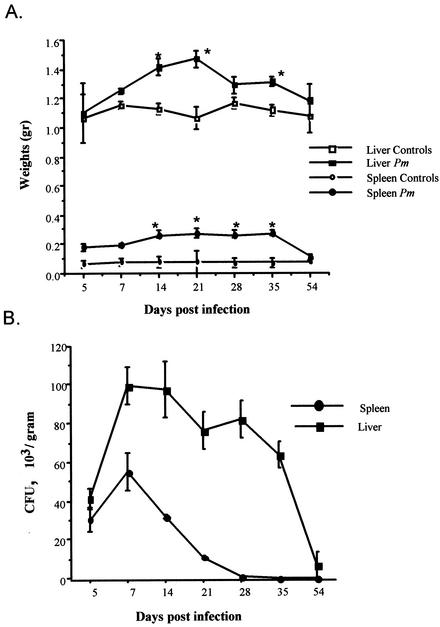
To establish an experimental model of disseminated penicilliosis marneffei, immunocompetent wild-type BALB/c mice were infected i.v. with a sublethal dose (3 × 105/mouse) of P. marneffei conidia. A higher dose (2 × 106) was lethal in 18 to 20 days. Marked hepatosplenomegaly (Fig. 2A) occurred at an early stage of the infection and reached a maximum between 14 and 20 days after inoculation. Thereafter, the weights of the spleens and livers gradually decreased, with a return to normal after 50 days.
FIG. 2.
Course of P. marneffei infection in BALB/c mice. Mice were injected i.v. with 3 × 105 conidia. At the indicated time points, spleens and livers were excised, weighed, and processed for CFU evaluation. (A) Spleen and liver weights in control and P. marneffei-infected (Pm) mice. (B) Number of CFU recovered on YMA after 72 h from the organs of P. marneffei-infected mice. Data represent the means and standard deviations of three different experiments with four or five mice for each time point. Asterisks indicate a P value of <0.01 for comparisons between control mice and P. marneffei-infected mice.
In parallel, there was a rapid increase in the numbers of live fungi recovered from the spleens and livers of infected animals by day 5 postinfection (Fig. 2B). Fungal growth reached a maximum of approximately 105 CFU/g of tissue at day 7 in both the livers and the spleens. Subsequently, a rapid clearance of the microorganisms occurred in the spleens, with complete elimination of fungi by day 21. The livers showed a slower decline in the number of fungus colonies, with complete disappearance only after 54 days postinfection. The infected mice were still alive 70 days after challenge.
Cytokine pattern in the spleens and livers of infected wild-type BALB/c mice.
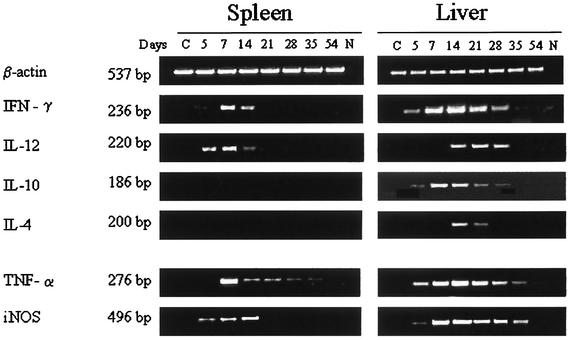
The production of cytokines in the organs of infected mice was evaluated by RT-PCR with the primers shown in Table 1. By day 5 postinfection, the up-regulation of type 1 cytokines, IFN-γ and IL-12, was observed in the spleens of infected mice but not in those of healthy control mice (Fig. 3). The highest levels of IFN-γ and IL-12 expression were noted between 7 and 14 days after inoculation and subsequently declined in parallel with the number of colonies. Type 2 cytokines, IL-10 and IL-4, were not detected in the spleens.
FIG. 3.
RT-PCR analysis of cytokine mRNA expression in BALB/c mice infected with P. marneffei. Mice were injected i.v. with 3 × 105 conidia. At the indicated times, spleens and livers were excised and processed for evaluation of cytokine gene expression by RT-PCR. Lanes C, control mRNA from three uninfected wild-type mice; lanes N, no DNA was added to the amplification mixture during PCR.
A clear dichotomy between type 1 and type 2 cytokines was not found in the livers. Starting from day 7, significant levels of IL-4 and IL-10 as well as IFN-γ and IL-12 were detected in the livers of infected but not control mice. IL-10 appeared and its levels declined earlier than IL-12 and IL-4. iNOS and TNF-α mRNAs were produced in both organs at an early stage of the infection and persisted at high levels until the infection was completely resolved.
Role of IFN-γ in immunity against P. marneffei.
To verify the role of IFN-γ in resistance to P. marneffei infection, GKO BALB/c mice were injected with a sublethal dose of P. marneffei conidia. All GKO mice succumbed to systemic penicilliosis 18 days postinfection, whereas all wild-type mice were still alive by day 60, when the experiment was terminated.
On monitoring the number of CFU per gram of tissue in infected GKO mice at days 7 and 14 postinfection, we found massive fungus overgrowth in the organs of infected GKO mice. Compared to wild-type mice, approximately 40- and 7-fold increases in CFU were seen in the spleens and livers, respectively, 14 days after inoculation (Table 2).
TABLE 2.
Course of P. marneffei infection in wild-type and GKO BALB/c micea
| Days postinfection | Mean CFU (103)/g ± SD in the following organs in the indicated mice:
|
|||
|---|---|---|---|---|
| Spleens
|
Livers
|
|||
| Wild type | GKOb | Wild type | GKO | |
| 7 | 30.2 ± 7.9 | 66.9 ± 8 | 59.8 ± 6 | 73.1 ± 4 |
| 14 | 34.5 ± 10 | 1,392 ± 57 | 41.1 ± 9 | 292 ± 47 |
Mice were injected i.v. with 4 × 105 conidia. At the indicated time postinfection, the organs were excised and processed. Fungus growth was evaluated by counting CFU on YMA in triplicate after 72 h. There were three mice per group. Statistical analyses were performed by analysis of variance by comparing each experimental group from GKO mice to the corresponding wild-type control group at different times postinfection. P values for spleens at days 7 and 14 were <0.05 and <0.0001, respectively. P values for livers at days 7 and 14 were <0.04 and <0.002, respectively.
All GKO mice died of generalized mycosis on day 18 postinfection.
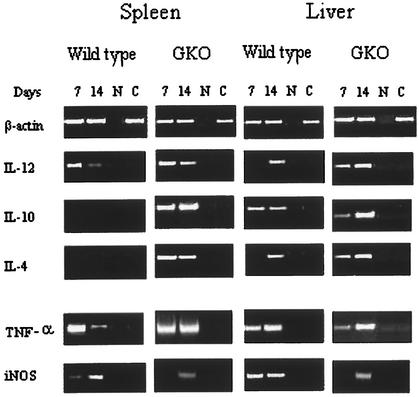
A disruption of the characteristic cytokine pattern of wild-type mice was observed in GKO mice (Fig. 4). mRNA for IL-12 was present by day 7 postinfection in both the spleens and the livers of infected GKO mice. In contrast, mRNAs for IL-10 and IL-4, type 2 cytokines, which were absent in the spleens of wild-type mice, were strongly up-regulated in the spleens of GKO mice. In the livers of GKO mice, mRNA for IL-4 appeared at 7 days postinfection, 1 week earlier than in wild-type mice. The expression of mRNA for IL-10 was increased at day 14 in GKO mice compared to wild-type mice. iNOS mRNA levels were extremely low in GKO mice compared to wild-type mice, whereas TNF-α mRNA levels appeared similar in both strains of mice.
FIG. 4.
RT-PCR analysis of cytokine mRNA in the spleens and livers of wild-type and GKO BALB/c mice infected with P. marneffei. Mice were injected i.v. with 3 × 105 conidia and sacrificed at 7 and 14 days after infection. Spleens and livers were recovered and processed for evaluation of cytokine gene expression by RT-PCR. Lanes C, control mRNA from uninfected wild-type mice; lanes N, no DNA was added to the amplification mixture during PCR.
Histological analysis of infected wild-type BALB/c mice.
Spleen sections of wild-type mice at day 7 postinfection revealed an expansion of red pulp and white pulp associated with scattered single macrophages that contained yeast cells within their cytoplasm. No granulomas were observed. By day 14, the expansion of spleen red pulp and white pulp was associated with occasional small granulomas either embedded within the follicles or localized at the edge of the follicles and the red pulp (Fig. 5A). They consisted of a few macrophages with cytoplasmic yeast cells, surrounded by a few fibroblast-like cells (Fig. 5B). Tissue necrosis was never observed. By day 21, hyperplasia of both the red pulp and the white pulp was seen, granulomas were not detected, and the sparse macrophages were negative for yeast cells.
FIG. 5.
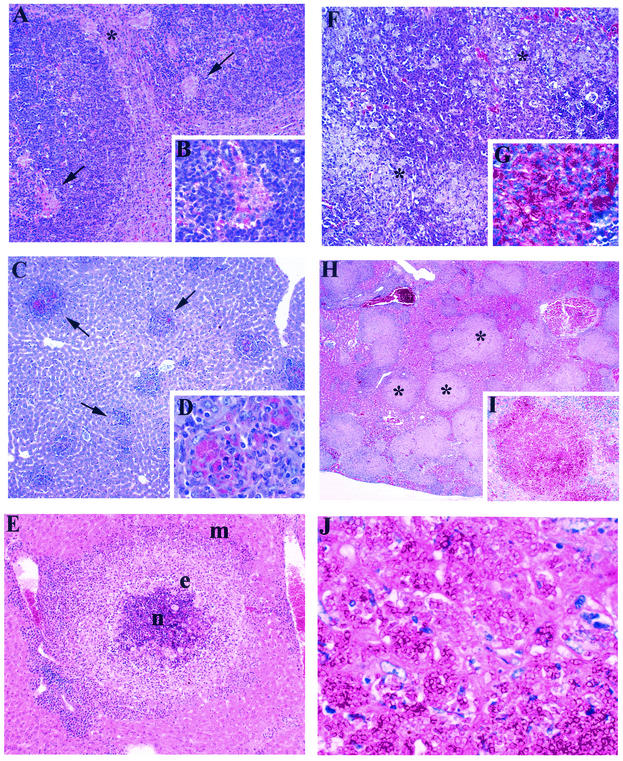
Composite illustration of the main histological findings in the livers and spleens of wild-type and GKO BALB/c mice infected with P. marneffei. Mice were injected i.v. with 3 × 105 conidia and sacrificed at different times postinfection for histological analysis. (A) Wild-type BALB/c mouse spleen section at day 14 postinfection. Isolated single small granulomas were seen within the white pulp of the spleen. The asterisk indicates a granuloma at the edge; the arrows indicate granulomas within follicles. HE stain. Magnification, ×10. (B) P. marneffei yeast cells within macrophages. HE stain. Magnification, ×100. (C) Wild-type BALB/c mouse liver section at 7 days postinfection. Numerous small granulomas (arrows) are present. PAS stain. Magnification, ×4. (D) P. marneffei yeast cells within the macrophage cytoplasm. PAS stain. Magnification, ×100. (E) BALB/c mouse liver section showing a large granuloma with a target appearance; the central necrotic area (n) is circled by epithelioid histiocytes (e), macrophages (m), and granulation tissue. HE stain. Magnification, ×40. (F) GKO BALB/c mouse spleen section. The spleen structure is subverted by masses of macrophages loaded with yeast cells (asterisks). HE stain. Magnification, ×10. (G) Macrophages from GKO spleen cells filled with yeast cells. PAS stain. Magnification, ×100. (H) GKO BALB/c mouse liver section. Round masses of macrophages (asterisks) largely replace the liver parenchyma. HE stain. Magnification, ×4. (I) Macrophages from GKO liver cells filled with yeast cells. PAS stain. Magnification, ×100. (J) Macrophage cytoplasm occupied by a large number of yeast cells. PAS stain. Magnification, ×100.
Liver sections at day 7 showed a significant number of small granulomas in the parenchyma and numerous yeast cells within the macrophages (Fig. 5C and D). The portal tracts contained a moderate lymphocytic infiltrate but no granulomas. By day 14, liver sections were characterized by large granulomas with central areas of necrosis, and the macrophages contained a large number of yeast cells (Fig. 5E). By day 21, there were fewer large granulomas, the necrotic debris had disappeared, and some yeast cells could still be seen within the macrophages. A rim of granulation tissue surrounded the granulomas. By day 28, round areas of granulation tissue had replaced the liver granulomas.
Spleen and liver sections were within normal limits at 35 and 54 days, respectively.
Histological analysis of infected BALB/c GKO mice.
Tissues of GKO mice revealed extensive morphological differences with respect to granuloma formation and fungus load. Enlarged masses of macrophages containing a large number of yeast cells in the parenchyma replaced the red pulp and the white pulp of the spleen. The spleen had also lost the follicular or nodular structure and showed diffuse hyperplasia of lymphoplasmocytic cells (Fig. 5F and G).
The liver parenchyma was largely replaced by nodular masses of aggregated macrophages filled with yeast cells (Fig. 5H and I). These macrophage aggregates lacked the typical granuloma structure, and the individual macrophages did not feature an epithelioid morphology. Necrosis was not observed, and lymphocytes were also absent. At a higher magnification, an overgrowth of intracellular yeast cells was seen within the macrophages, in the absence of hyphae. Residual hepatocytes showed occasional microvesicular degenerative changes and were surrounded by small foci of macrophage microaggregates filled with yeast cells (Fig. 5J). The sinusoids were dilated with fibrin-like material in their lumina or intermingled with the macrophages.
DISCUSSION
Immunocompetent wild-type BALB/c mice challenged with an i.v. sublethal dose of P. marneffei develop hepatosplenomegaly associated with fungal growth and an immune response. The liver appears to be the major target organ affected, with extensive distribution of granulomas and an abundant lymphomonocytic infiltrate. Macrophages contain large amounts of yeast cells, resulting in a high level of CFU per gram of liver tissue. In contrast, the spleen has only a few granulomas, with occasional yeast cell-containing macrophages and a lower level of CFU per gram. The kinetics of recovery from the infection also differ between the two organs, with rapid fungal clearance from the spleen and slower elimination of P. marneffei from the liver.
As local immune factors may be responsible for the differential fungal growth and elimination observed in the spleen and the liver, Th1- and Th2-like responses in both organs were studied. In the spleen, a Th1-biased pattern of cytokines (IFN-γ and IL-12) was observed, starting early after infection. In contrast, both Th1 (IFN-γ and IL-12) and Th2 (IL-4 and IL-10) cytokine messages were detected in the liver during the first 2 weeks, followed by a dominant Th1 cytokine pattern. These results, together with the early resolution of the fungus burden in the spleen, in contrast to the later clearance in the liver, suggest that the development of protective immunity and the inhibition of P. marneffei growth are associated with the induction of a Th1 response that, in the liver, ultimately dominates Th2 reactivity. They also expand previous observations that T cells and in particular CD4+ T cells are necessary for clearing P. marneffei infection in mice (22, 32).
Similar findings have been reported with other fungal disease models. A Th1 response (high levels of IFN-γ and TNF-α and low levels of IL-4) plays a protective role in mice with systemic candidiasis, pulmonary histoplasmosis, or invasive aspergillosis, whereas a Th2 response is associated with disease progression (1, 4, 12, 25, 28, 35). However, as in the livers of P. marneffei-infected animals, both Th1 and Th2 cytokines have been detected in mice with pulmonary Cryptococcus neoformans infection or with gastric candidiasis (2, 17). The cytokine pattern seems to vary depending on the type of pathogen, the affected organ, or the infection route. Our data on P. marneffei infection are consistent with the general concept that a Th1 response plays a crucial role in host resistance to intracellular pathogens (27) and support the evidence that T-cell responses can be compartmentalized at the tissue level to favor the development of distinct protective immunity in a given tissue (24).
Our results show for the first time that IFN-γ is essential to prevent the unrestrained growth of P. marneffei in different organs in vivo. Indeed, all GKO mice died of systemic mycosis with massive fungal overgrowth within 18 days, while all wild-type mice survived. IFN-γ is crucial for the structural organization of the cellular response (7, 11, 26). In the livers of GKO mice, apparently not enough T cells could be recruited to the infected area to form granulomas, as in wild-type mice. In GKO mice, huge aggregates of phagocytes were seen, although they failed to restrict fungal growth, suggesting that macrophage chemotaxis was unimpaired. Individual macrophages were filled with growing yeast cells and did not feature an epithelioid morphology, and no necrosis was observed.
These results confirm the notion that the central deficit of GKO mice is the inability to activate the microbicidal activity of macrophages (7). It is likely that the recruitment of macrophages during P. marneffei infection is induced by other cytokines or chemokines, whose production and/or activity are not influenced by IFN-γ. Such an early migration of inflammatory cells but a delay or lack of granuloma formation has also been described for GKO mice with tuberculosis (26) or with hypersensitivity pneumonitis (16). The chemokine pattern during P. marneffei infection is currently under investigation.
The failure of macrophages from GKO mice to exert fungicidal activity against P. marneffei may be related to their impaired production of NO, due to a major reduction in iNOS activity (11). Indeed, both IFN-γ and TNF-α are required for the expression of iNOS in macrophages (10), and GKO mice suffer from deficient iNOS expression (26), which could result in an overwhelming and fatal infection. It has been demonstrated that NO contributes to the intracellular killing of P. marneffei yeast cells by both murine and human macrophages in vitro (23, 30); in addition, mouse macrophages produce NO when stimulated with P. marneffei conidia (this study). The elevated iNOS mRNA levels found in the organs of P. marneffei-infected mice during the week preceding the resolution of the infection further support the hypothesis that NO plays a crucial role in protection against P. marneffei.
The progression of the disease and the onset of a nonprotective immune response leading to the death of GKO mice are associated with a shift in the cytokine balance from Th1 to Th2. High levels of mRNAs for IL-4 and IL-10 are present in GKO mouse spleen cells but not in wild-type mouse spleen cells. In the liver, where the cytokine pattern at day 14 after infection was much less Th1 oriented than in the spleen, IL-4 and IL-10 mRNAs appeared earlier in GKO mice than in wild-type mice and their levels remained elevated for a longer period. Moreover, TNF-α and IL-12 mRNA levels did not change in GKO mice relative to wild-type mice. With regard to TNF-α production, these results were not completely unexpected, since the pathways of IFN-γ and TNF-α in protective immunity to other pathogens have been shown to be independent; i.e., the absence of one cytokine (IFN-γ) does not diminish the production of the other (1, 28).
These data also suggest that TNF-α alone is not sufficient to control P. marneffei infection in GKO mice. Being a potent chemoattractant, TNF-α may contribute in our model to the recruitment of macrophages into inflamed tissues by up-regulating the expression of adhesion molecules or the production of chemokines by the endothelium. However, this activity seems not to be sufficient for the structural organization of the granuloma.
The lack of a Th1 response and of protective immunity in GKO mice occurs in the presence of a high level of IL-12 message production. IL-12 is considered one of the most potent inducers of IFN-γ; in turn, IFN-γ can act on macrophages to augment IL-12 secretion via a direct effect on the IL-12 p40 promoter (28). The fact that in GKO mice IL-12 up-regulation is observed in the absence of IFN-γ suggests that factors other than IFN-γ contribute to IL-12 production. In mice with systemic candidiasis, the presence of IL-12 in the absence of IFN-γ correlates with a loss of responsiveness of CD4+ Th1 cells to IL-12, due to the failure of up-regulation of the β2 chain of the IL-12 receptor (3). We do not know at present whether the same situation may also apply to P. marneffei infection. Clearly, both IL-12 production and responsiveness appear to be required for an optimal Th1-type response in the mice.
In conclusion, the in vivo analysis of a sublethal P. marneffei infection in BALB/c mice indicates that protective immunity follows a Th1 response that involves T cells and macrophages and that IFN-γ is an essential mediator. NO is largely responsible for intracellular fungus death, as iNOS reduction in vivo leads to unrestrained fungal growth.
These data may help explain the high prevalence of P. marneffei infections in AIDS patients in countries in which such infections are endemic. The progression toward AIDS is associated with a loss of CD4+ T cells and an altered cytokine profile with diminished IFN-γ levels, which favor the spread of opportunistic fungi. It will be interesting to determine whether the immune recovery observed with highly active antiretroviral therapy (5) is associated with the restoration of IFN-γ production in response not only to HIV but also to other opportunistic pathogens, including P. marneffei.
Acknowledgments
This work was supported by the I.S.S., National Program for Research on AIDS, Rome, Italy (contracts 50B.037 and 50C.030).
We acknowledge L. Riviera-Uzielli, recently deceased, for valuable suggestions and support during the course of these studies. We also thank M. P. Colombo for providing GKO mice.
Editor: T. R. Kozel
REFERENCES
- 1.Allendoerfer, R., and G. Deepe, Jr. 1997. Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect. Immun. 65:2564-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cenci, E., A. Mencacci, R. Spaccapelo, L. Tonnetti, P. Mosci, K. Enssle, P. Puccetti, L. Romani, and F. Bistoni. 1995. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 171:1279-1288. [DOI] [PubMed] [Google Scholar]
- 3.Cenci, E., A. Mencacci, G. Del Sero, C. Fe'd'Ostiani, P. Mosci, A. Bacci, C. Montagnoli, M. Kopf, and L. Romani. 1998. IFN-γ is required for IL-12 responsiveness in mice with Candida albicans infection. J. Immunol. 161:3543-3550. [PubMed] [Google Scholar]
- 4.Cenci, E., S. Perito, K. Enssle, P. Mosci, J. P. Latgé, L. Romani, and F. Bistoni. 1997. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect. Immun. 65:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici, M., E. Seminari, F. Suter, F. Castelli, A. Pan, M. Biasin, F. Colombo, D. Trabattoni, F. Maggiolo, G. Carosi, R. Maserati, and the Master Group. 2000. Different immunologic profiles characterize HIV infection in highly active antiretroviral therapy-treated and antiretroviral-naive patients with undetectable viraemia. AIDS 14:109-116. [DOI] [PubMed] [Google Scholar]
- 6.Cogliati, M., A. Roverselli, J. R. Boelaert, D. Taramelli, L. Lombardi, and A. M. Viviani. 1997. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect. Immun. 65:279-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A., D. Dalton, T. Stewart, J. Griffin, D. Russel, and I. Orme. 1993. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, C. R. 1998. From bamboo rats to humans: odyssey of Penicillium marneffei. ASM News 64:390-397 [Google Scholar]
- 9.Cui, J., R. Tanaka, H. Taguchi, A. Sano, E. Ito, K. Fukushima, K. Takeo, S. Yoshida, K. Nishimura, and M. Miyaji. 1997. Histopathological and electron microscopical studies on experimental Penicillium marneffei infection in mice. J. Med. Vet. Mycol. 35:347-353. [PubMed] [Google Scholar]
- 10.Cunha, F. Q., J. Assreuy, D. W. Moss, D. Rees, L. M. Leal, S. Moncada, M. Carrier, C. A. O'Donnell, and F. Y. Liew. 1994. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology 81:211-215. [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton, D., S. Pitts-Meek, S. Keshav, I. Figari, A. Bradley, and T. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 12.Deepe, G., Jr., L. Romani, V. Calich, G. Huffnagles, C. Arruda, E. Molinari-Madlum, and J. Perfect. 2000. Knockout mice as experimental models of virulence. Med. Mycol. 38(Suppl. 1):87-98. [PubMed] [Google Scholar]
- 13.Duong, T. A. 1996. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin. Infect. Dis. 23:125-130. [DOI] [PubMed] [Google Scholar]
- 14.Erb, K., J. Kirman, B. Delahunt, and G. LeGros. 1999. Infection of mice with Mycobacterium bovis BCG induces both Th1 and Th2 immune responses in the absence of interferon-γ signalling. Eur. Cytokine Netw. 10:147. [PubMed] [Google Scholar]
- 15.Fenhalls, G., A. Wong, J. Bezuidenhout, P. van Helden, P. Bardin, and P. T. Lukey. 2000. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect. Immun. 68:2827-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudmundsson, G., and G. W. Huninghake. 1997. Interferon-gamma is necessary for the expression of hypersensitivity pneumonitis. J. Clin. Investig. 99:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huffnagle, G. B., and M. F. Lipscomb. 1998. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 149:387-396. [DOI] [PubMed] [Google Scholar]
- 18.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. Casanova. 1999. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo, R., J. Le, D. Shapiro, E. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaposzta, R., P. Tree, L. Marodi, and S. Gordon. 1998. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 66:1708-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudeken, N., K. Kawakami, and A. Saito. 1997. CD4+ T cell-mediated fatal hyperinflammatory reactions in mice infected with Penicillium marneffei. Clin. Exp. Immunol. 107:468-473. [DOI] [PubMed] [Google Scholar]
- 22.Kudeken, N., K. Kawakami, N. Kusano, and A. Saito. 1996. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J. Med. Vet. Mycol. 34:371-378. [DOI] [PubMed] [Google Scholar]
- 23.Kudeken, N., K. Kawakami, and A. Saito. 1998. Different susceptibilities of yeasts and conidia of Penicillium marneffei to nitric oxide (NO)-mediated fungicidal activity of murine macrophages. Clin. Exp. Immunol. 112:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzo, A. L., V. Vezys, K. Williams, D. F. Tough, and L. Lefrancois. 2002. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J. Immunol. 168:4504-4510. [DOI] [PubMed] [Google Scholar]
- 25.Nagai, H., J. Guo, H. Choi, and V. Kurup. 1995. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J. Infect. Dis. 172:1554-1560. [DOI] [PubMed] [Google Scholar]
- 26.Pearl, J., B. Saunders, S. Ehlers, I. Orme, and A. Cooper. 2001. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-γ-deficient mouse. Cell. Immunol. 211:43-50. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani, S. 1997. The Th1/Th2 paradigm. Immunol. Today 18:263-266. [DOI] [PubMed] [Google Scholar]
- 28.Romani, L. 1999. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 2:363-367. [DOI] [PubMed] [Google Scholar]
- 29.Supparatpinyo, K., C. Khamwan, V. Baosoung, K. Nelson, and T. Sirisanthana. 1994. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet 344:110-113. [DOI] [PubMed] [Google Scholar]
- 30.Taramelli, D., S. Brambilla, G. Sala, A. Bruccoleri, C. Tognazioli, L. L. Riviera-Uzielli, and J. R. Boelaert. 2000. Effect of iron on the extracellular and intracellular growth of Penicillium marneffei. Infect. Immun. 68:1724-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taramelli, D., M. Malabarba, G. Sala, N. Basilico, and G. Cocuzza. 1996. Production of cytokines by alveolar and peritoneal macrophages stimulated by Aspergillus fumigatus conidia or hyphae. J. Med. Vet. Mycol. 34:49-56. [PubMed] [Google Scholar]
- 32.Viviani, M. A., J. O. Hill, and D. M. Dixon. 1993. Penicillium marneffei: dimorphism and treatment, p. 413-422. In H. van den Bossche, F. Odds, and D. Kerridge (ed.), Dimorphic fungi in biology and medicine. Plenum Press, New York, N.Y.
- 33.Viviani, M. A., A. M. Tortorano, G. Rizzardini, T. Quirino, L. Kaufman, A. A. Padhye, and L. Ajello. 1993. Treatment and serological studies of an Italian case of penicillinosis marneffei contracted in Thailand by a drug addict infected with the human immunodeficiency virus. Eur. J. Epidemiol. 9:79-85. [DOI] [PubMed] [Google Scholar]
- 34.Wynn, A., I. Eltoum, A. Cheever, F. Lewis, W. Gause, and A. Sher. 1993. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J. Immunol. 151:1430-1440. [PubMed] [Google Scholar]
- 35.Zhou, P., G. Miller, and R. Seder. 1998. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of interferon-γ. J. Immunol. 160:1359-1368. [PubMed] [Google Scholar]