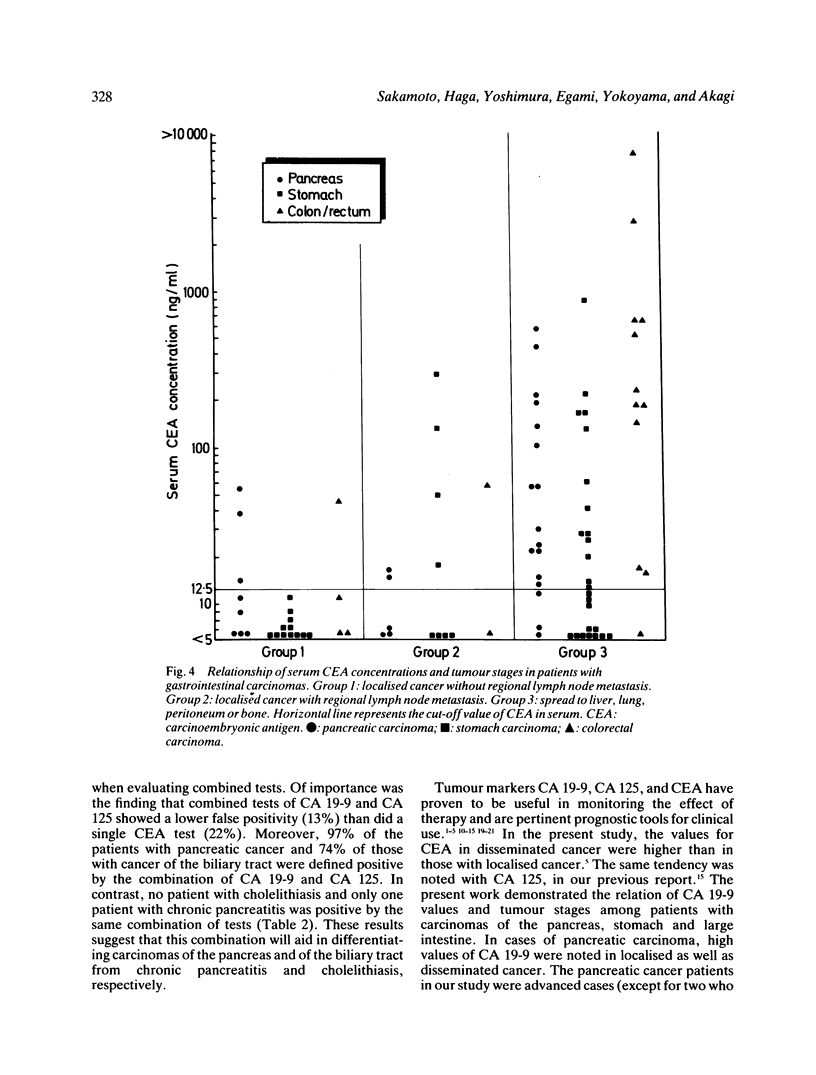
Abstract
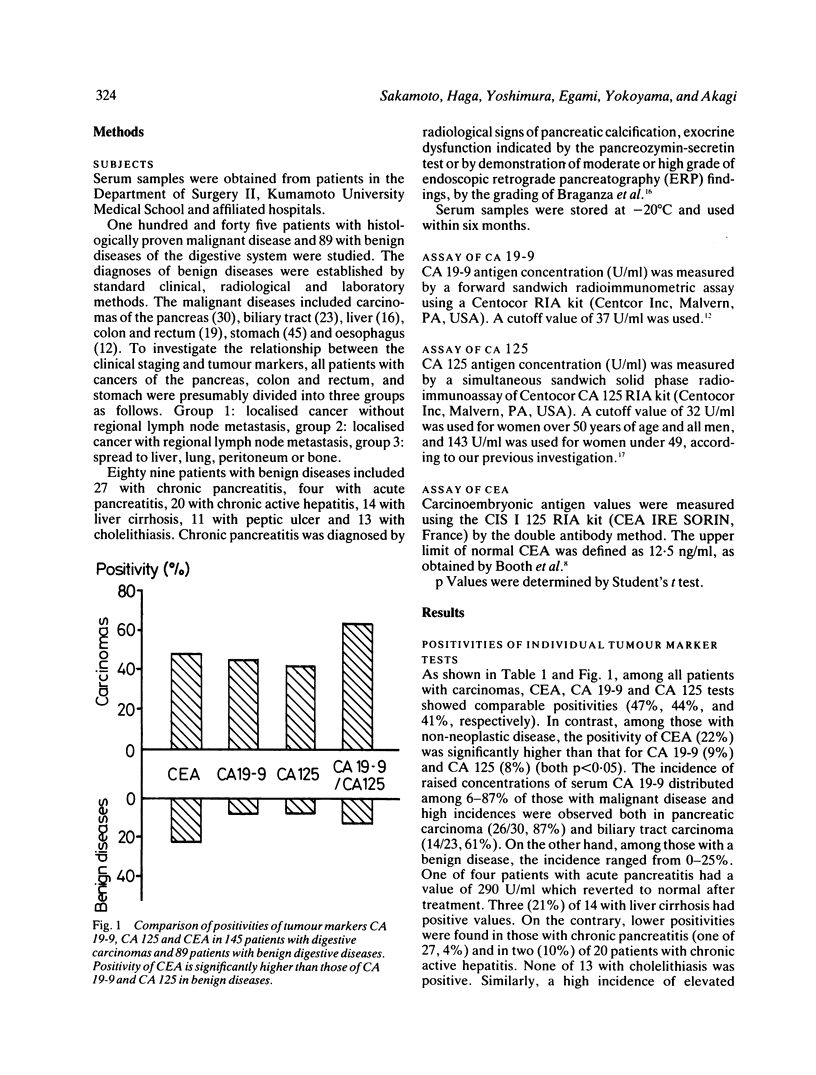
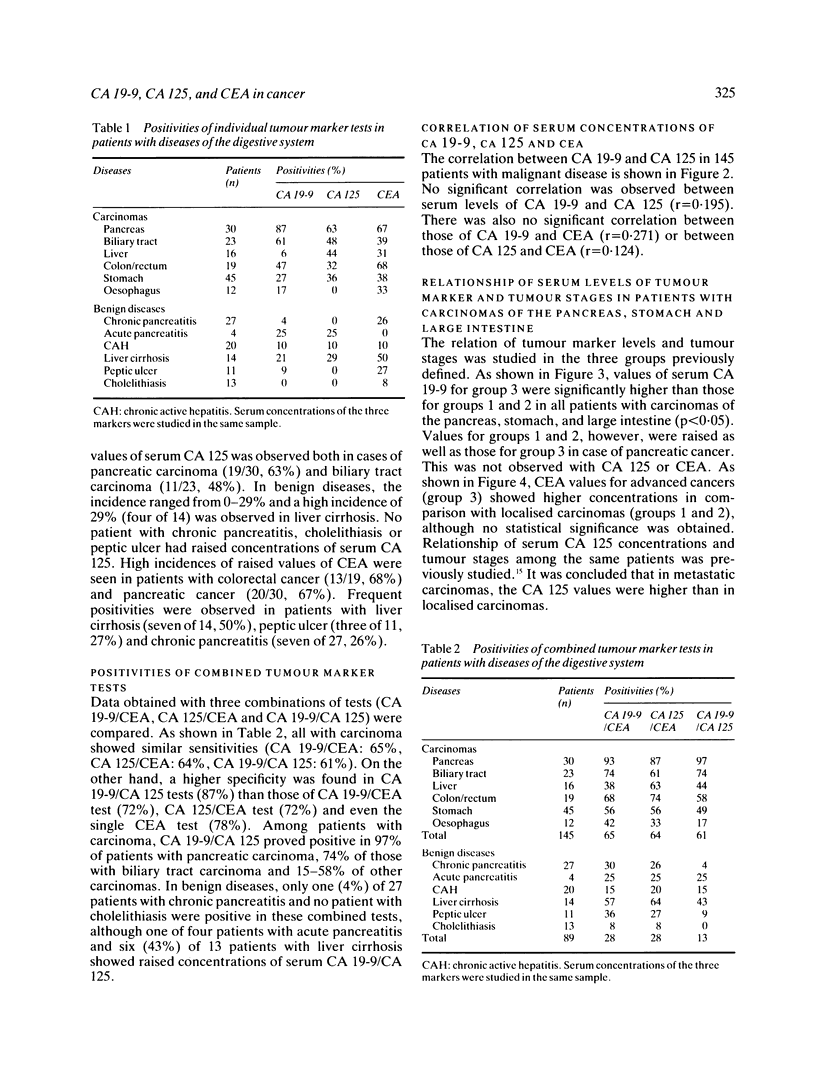
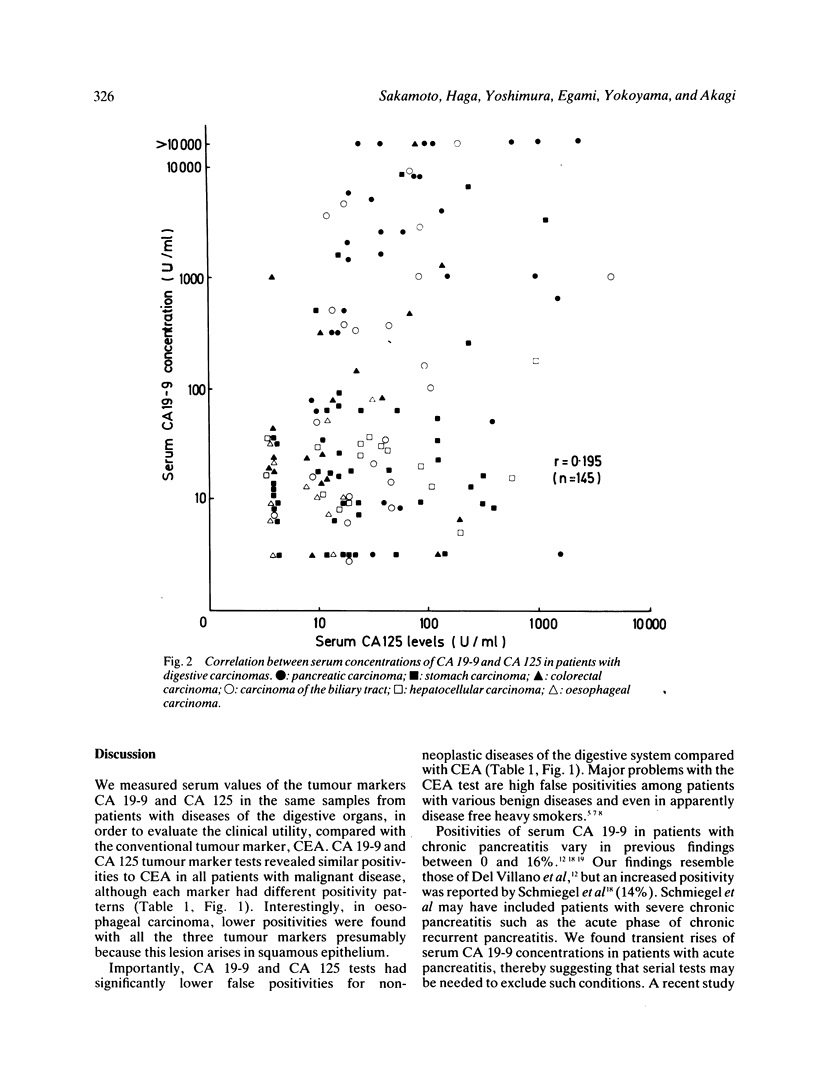
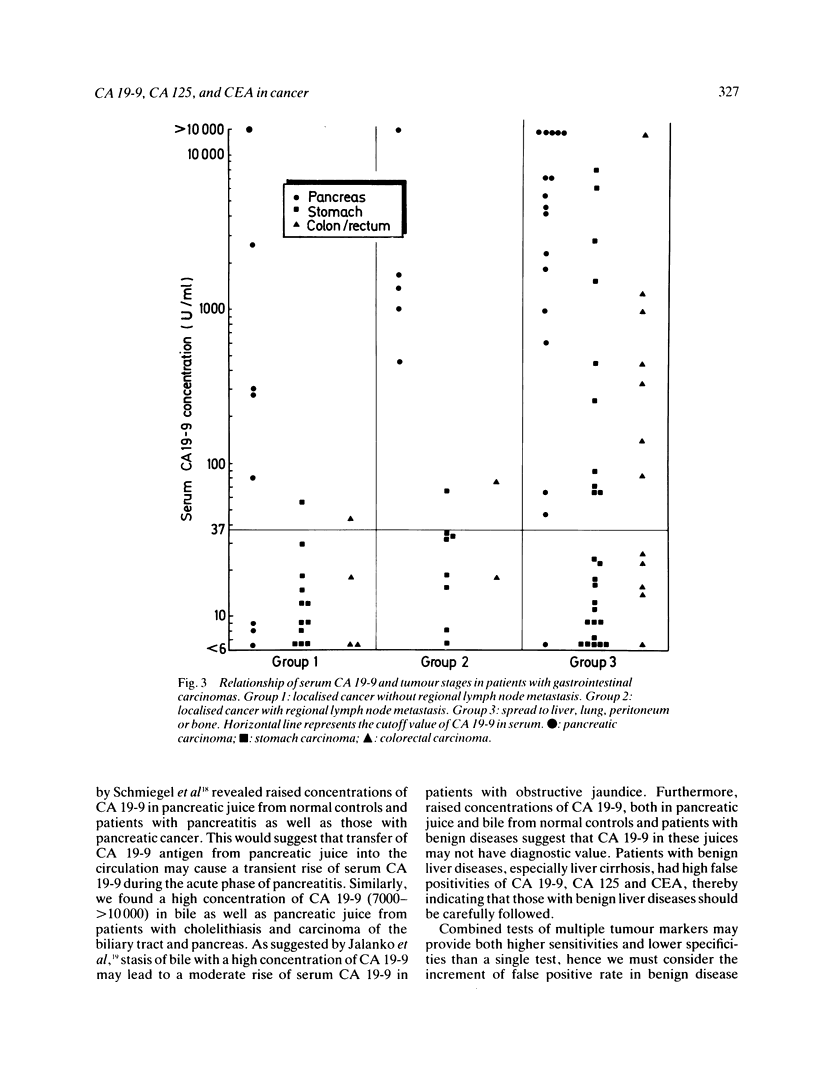
Serum concentrations of CA 19-9, CA 125 and carcinoembryonic antigen (CEA) in 145 patients with gastrointestinal carcinomas and 89 with non-neoplastic diseases were determined to compare the clinical usefulness of these tumour markers. Significantly fewer positive cases were obtained with serum CA 19-9 (9%) and CA 125 (8%) tests than the CEA test (22%) (both p less than 0.05) in patients with benign diseases, while comparable sensitivities were achieved with the CA 19-9 (44%) test, the CA 125 (41%) test and the CEA test (47%) in those with a carcinoma. High incidences of raised concentrations of serum CA 19-9 and CA 125 were observed in case of cancer of the pancreas (CA 19-9: 87%, CA 125: 67%) and biliary tract (CA 19-9: 63%, CA 125: 48%). Combined tests of CA 19-9 and CA 125 revealed increments in the sensitivity (61%) and provided a higher specificity (87%) than that of the single CEA test (78%). These combined tests were most useful for a differential diagnosis of pancreatic carcinoma (97% positive) and biliary tract carcinoma (74%) from chronic pancreatitis (4%) and cholelithiasis (0%), respectively. Studies on the relations of clinical staging and serum concentrations of CA 19-9 and CA 125 revealed significant rises in cases of disseminated carcinoma. These results clearly show that serum CA 19-9 and CA 125 tests are most pertinent for diagnosing advanced carcinomas of organs in the digestive system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R. C., Jr, Klug T. L., St John E., Jenison E., Niloff J. M., Lazarus H., Berkowitz R. S., Leavitt T., Griffiths C. T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Booth S. N., King J. P., Leonard J. C., Dykes P. W. Serum carcinoembryonic antigen in clinical disorders. Gut. 1973 Oct;14(10):794–799. doi: 10.1136/gut.14.10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braganza J. M., Hunt L. P., Warwick F. Relationship between pancreatic exocrine function and ductal morphology in chronic pancreatitis. Gastroenterology. 1982 Jun;82(6):1341–1347. [PubMed] [Google Scholar]
- Del Villano B. C., Brennan S., Brock P., Bucher C., Liu V., McClure M., Rake B., Space S., Westrick B., Schoemaker H. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983 Mar;29(3):549–552. [PubMed] [Google Scholar]
- Go V. L., Zamcheck N. The role of tumor markers in the management of colorectal cancer. Cancer. 1982 Dec 1;50(11 Suppl):2618–2623. [PubMed] [Google Scholar]
- Haga Y., Sakamoto K., Egami H., Yoshimura R., Akagi M. Evaluation of serum CA125 values in healthy individuals and pregnant women. Am J Med Sci. 1986 Jul;292(1):25–29. doi: 10.1097/00000441-198607000-00005. [DOI] [PubMed] [Google Scholar]
- Haga Y., Sakamoto K., Egami H., Yoshimura R., Mori K., Akagi M. Clinical significance of serum CA125 values in patients with cancers of the digestive system. Am J Med Sci. 1986 Jul;292(1):30–34. doi: 10.1097/00000441-198607000-00006. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Sears H. F., Steplewski Z., Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. I. Presence of antigen in sera of patients with colorectal, gastric, and pancreatic carcinoma. J Clin Immunol. 1982 Apr;2(2):135–140. doi: 10.1007/BF00916897. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Chu T. M., Holyoke E. D. Carcinoembryonic antigen (CEA) as a prognostic and monitoring test in clinically complete resection of colorectal carcinoma. Ann Surg. 1976 Jan;183(1):5–9. doi: 10.1097/00000658-197601000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanko H., Kuusela P., Roberts P., Sipponen P., Haglund C. A., Mäkelä O. Comparison of a new tumour marker, CA 19-9, with alpha-fetoprotein and carcinoembryonic antigen in patients with upper gastrointestinal diseases. J Clin Pathol. 1984 Feb;37(2):218–222. doi: 10.1136/jcp.37.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug T. L., Bast R. C., Jr, Niloff J. M., Knapp R. C., Zurawski V. R., Jr Monoclonal antibody immunoradiometric assay for an antigenic determinant (CA 125) associated with human epithelial ovarian carcinomas. Cancer Res. 1984 Mar;44(3):1048–1053. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Jalanko H., Roberts P., Sipponen P., Mecklin J. P., Pitkänen R., Mäkelä O. Comparison of CA 19-9 and carcinoembryonic antigen (CEA) levels in the serum of patients with colorectal diseases. Br J Cancer. 1984 Feb;49(2):135–139. doi: 10.1038/bjc.1984.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence D. J., Stevens U., Bettelheim R., Darcy D., Leese C., Turberville C., Alexander P., Johns E. W., Neville A. M. Role of plasma carcinoembryonic antigen in diagnosis of gastrointestinal, mammary, and bronchial carcinoma. Br Med J. 1972 Sep 9;3(5827):605–609. doi: 10.1136/bmj.3.5827.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton J. P., Martin E. W., Jr The use of serial CEA determinations to predict recurrence of colon cancer and when to do a second-look operation. Cancer. 1978 Sep;42(3 Suppl):1422–1427. doi: 10.1002/1097-0142(197809)42:3+<1422::aid-cncr2820420807>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. alpha-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- Schmiegel W. H., Kreiker C., Eberl W., Arndt R., Classen M., Greten H., Jessen K., Kalthoff H., Soehendra N., Thiele H. G. Monoclonal antibody defines CA 19-9 in pancreatic juices and sera. Gut. 1985 May;26(5):456–460. doi: 10.1136/gut.26.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears H. F., Herlyn M., Del Villano B., Steplewski Z., Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. II. A longitudinal evaluation of patients with colorectal cancer. J Clin Immunol. 1982 Apr;2(2):141–149. doi: 10.1007/BF00916898. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Rao B., Pinsky C. M., Hoffman R. G., Stearns M., Schwartz M. K., Oettgen H. F. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978 Aug 31;299(9):448–451. doi: 10.1056/NEJM197808312990904. [DOI] [PubMed] [Google Scholar]
- Wanebo J. H., Stearns M., Schwartz M. K. Use of CEA as an indicator of early recurrence and as a guide to a selected second-look procedure in patients with colorectal cancer. Ann Surg. 1978 Oct;188(4):481–493. doi: 10.1097/00000658-197810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


