Abstract
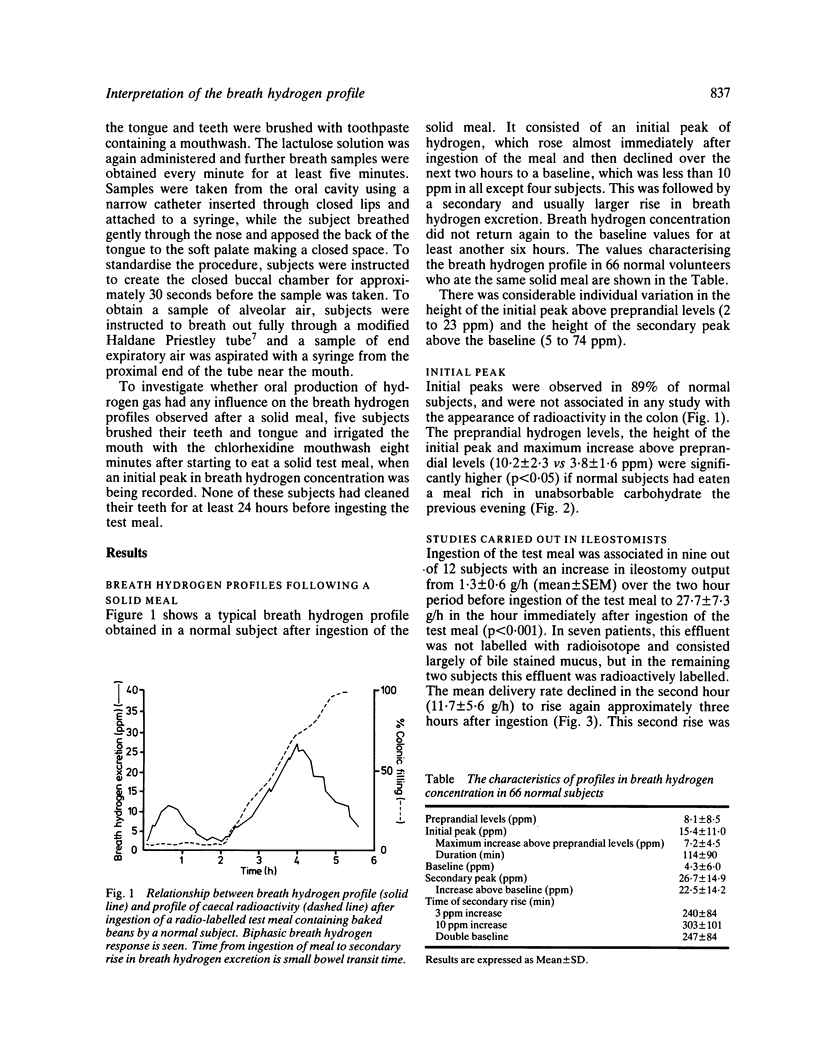
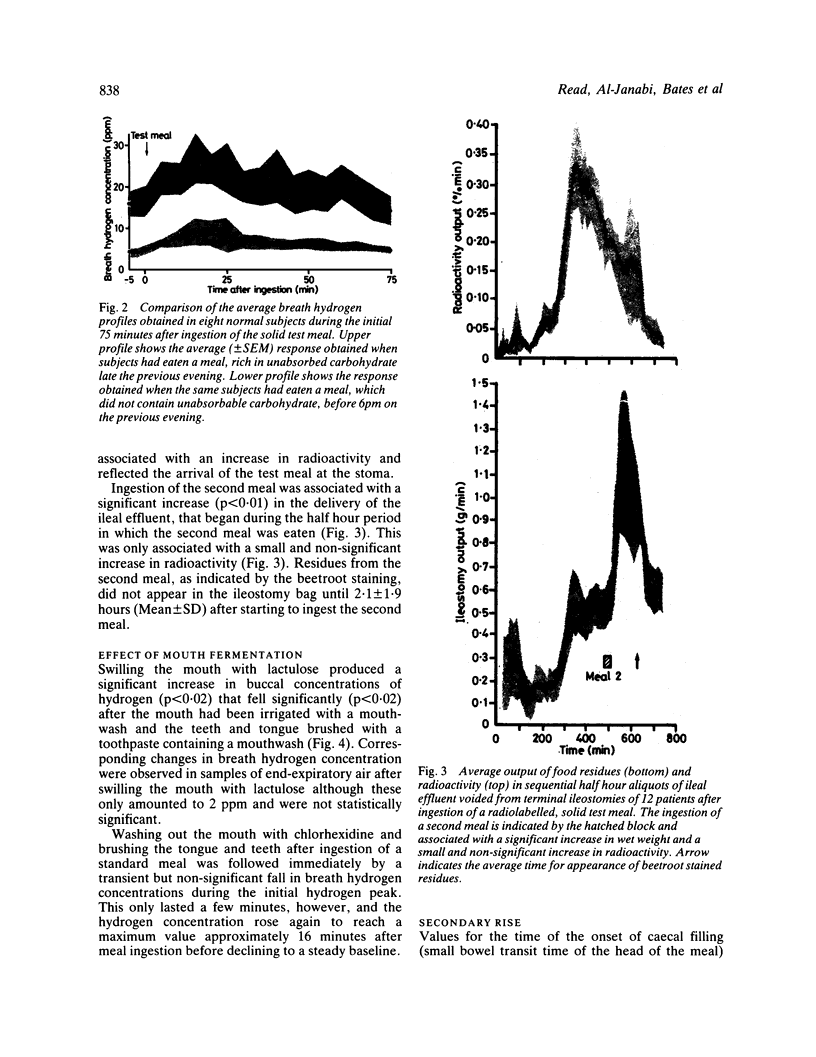
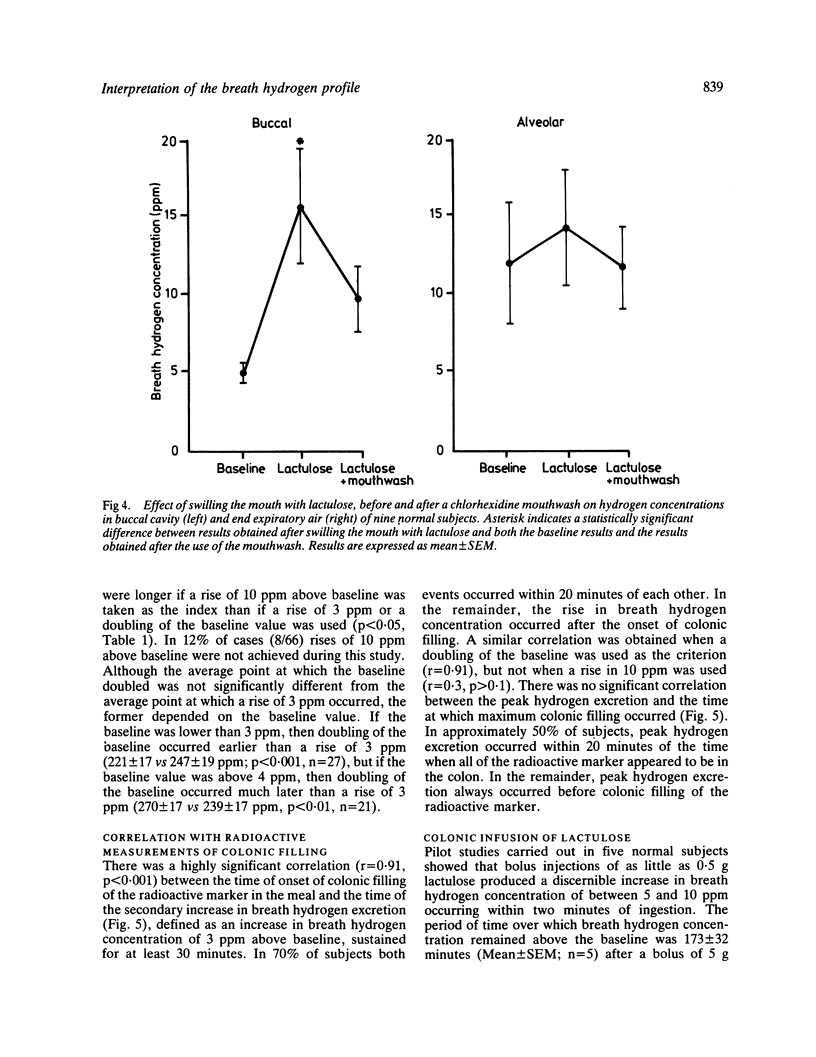
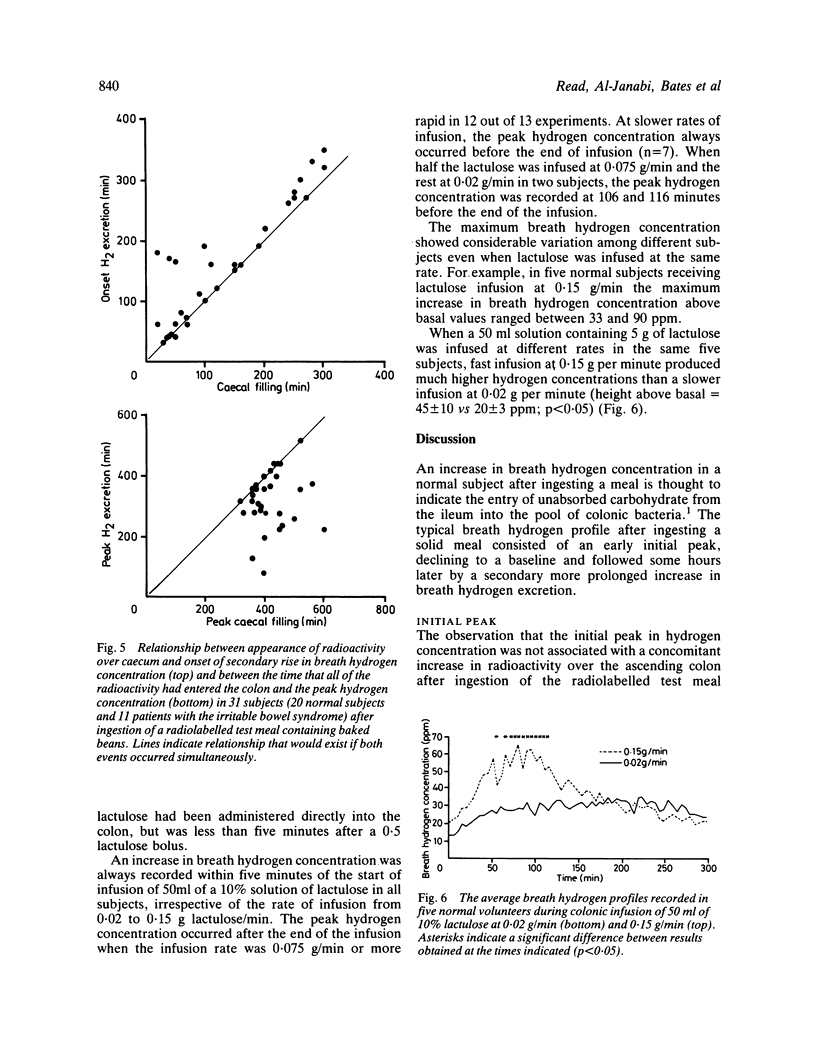
The extent to which monitoring breath hydrogen excretion provides information concerning the entry of the residues of a solid test meal into the colon was investigated in 89 normal subjects, and 11 patients with the irritable bowel syndrome. The profile of breath hydrogen concentration showed an early peak, that occurred soon after ingesting the test meal in 89% subjects. This was followed by a later more prolonged rise in breath hydrogen concentration. The early peak occurred well before a radioactive marker, incorporated in the test meal, reached the caecum and the data suggest it was predominantly caused by the emptying of the remnants of the previous meal from the ileum into the colon. This hypothesis was supported by direct measurements of the rate of delivery of ileostomy effluent in 12 subjects with terminal ileostomies. Fermentation of carbohydrate in the mouth may, however, contribute to the initial peak, but this contribution may be avoided by collecting gas samples from the nares. The secondary rise in breath hydrogen excretion was closely correlated with the arrival of the radioactive marker in the caecum (r = 0.91), p less than 0.001), though the time, at which the secondary peak of breath hydrogen excretion occurred was poorly correlated with the time that all the radioactive test meal had entered the colon. When lactulose was infused directly into the colon, as little as 0.5 g produced a discernible hydrogen response, which occurred within two minutes of the infusion. Increasing the rate of colonic infusion of a 50 ml solution of 10% lactulose from 0.02 to 0.15 g/min in five subjects significantly increased the breath hydrogen concentration. At infusion rates below 0.075 g lactulose/minute, the peak breath hydrogen response preceded the end ot the infusion, while at higher rates of infusion, the peak hydrogen response occurred after the end of the infusion. Although these results confirmed that monitoring breath hydrogen concentration usefully signalled the time taken for a meal containing unabsorbed carbohydrate to reach the colon, it did not reliably indicate the time when all of the meal had entered the colon. Finally, the use of the maximum increase in breath hydrogen concentration as an index of the degree of carbohydrate malabsorption assumes uniform rates of entry into the colon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. H., Jr, Levitt M. D., Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975 Apr;85(4):546–555. [PubMed] [Google Scholar]
- Bond J. H., Jr, Levitt M. D. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972 May;51(5):1219–1225. doi: 10.1172/JCI106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. H., Levitt M. D. Quantitative measurement of lactose absorption. Gastroenterology. 1976 Jun;70(6):1058–1062. [PubMed] [Google Scholar]
- Cann P. A., Read N. W., Brown C., Hobson N., Holdsworth C. D. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983 May;24(5):405–411. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett C. L., Thomas S., Read N. W., Hobson N., Bergman I., Holdsworth C. D. Electrochemical detector for breath hydrogen determination: measurement of small bowel transit time in normal subjects and patients with the irritable bowel syndrome. Gut. 1981 Oct;22(10):836–840. doi: 10.1136/gut.22.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz A. F. The ileo-caecal sphincter. J Physiol. 1913 Oct 17;47(1-2):54–56. doi: 10.1113/jphysiol.1913.sp001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate A. M., Read N. W. Relationship between small bowel transit time and absorption of a solid meal. Influence of metoclopramide, magnesium sulfate, and lactulose. Dig Dis Sci. 1983 Sep;28(9):812–819. doi: 10.1007/BF01296904. [DOI] [PubMed] [Google Scholar]
- Metz G., Gassull M. A., Leeds A. R., Blendis L. M., Jenkins D. J. A simple method of measuring breath hydrogen in carbohydrate malabsorption by end-expiratory sampling. Clin Sci Mol Med. 1976 Mar;50(3):237–240. doi: 10.1042/cs0500237. [DOI] [PubMed] [Google Scholar]
- Perman J. A., Modler S. Glycoproteins as substrates for production of hydrogen and methane by colonic bacterial flora. Gastroenterology. 1982 Aug;83(2):388–393. [PubMed] [Google Scholar]
- Read N. W., Haynes W. G., Bartolo D. C., Hall J., Read M. G., Donnelly T. C., Johnson A. G. Use of anorectal manometry during rectal infusion of saline to investigate sphincter function in incontinent patients. Gastroenterology. 1983 Jul;85(1):105–113. [PubMed] [Google Scholar]
- Read N. W., Miles C. A., Fisher D., Holgate A. M., Kime N. D., Mitchell M. A., Reeve A. M., Roche T. B., Walker M. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology. 1980 Dec;79(6):1276–1282. [PubMed] [Google Scholar]


