Abstract
Seeligeriolysin O (LSO), one of the cholesterol-dependent cytolysins produced by Listeria seeligeri, shows 80% homology to listeriolysin O (LLO) produced by Listeria monocytogenes at the amino acid sequence level. In addition to cytolytic activity, LLO has been shown to exhibit cytokine-inducing activity. In order to determine whether LSO is also capable of exhibiting these two different activities, we constructed a recombinant full-length LSO (rLSO530) and a noncytolytic truncated derivative with a C-terminal deletion (rLSO483) and compared these molecules with recombinant LLO. The cytolytic rLSO530 molecule could induce gamma interferon (IFN-γ) production in spleen cells when the cytolytic activity was blocked by treatment with cholesterol. The noncytolytic truncated rLSO483 molecule also induced IFN-γ production. Anti-LLO polyclonal antibody inhibited not only LLO-induced IFN-γ production but also LSO-induced IFN-γ production. Both NK cells and CD11b+ cells were required for LSO-induced IFN-γ production. Among the various cytokines expressed in CD11b+ cells, interleukin-12 (IL-12) and IL-18 appeared to be essential. We concluded that LSO exhibits the same biological activity as LLO.
The cholesterol-dependent cytolysins (CDCs) are a family of structurally related cytolytic toxins produced by different species of gram-positive bacteria, including members of the genera Streptococcus, Clostridium, and Listeria (1, 5). These toxins can form membrane pores after they bind membrane cholesterol. The cytolytic activity is eliminated by cholesterol and is dependent on the presence of a highly conserved undecapeptide sequence (ECTGLAWEWWR) at the C terminus (25, 31, 38, 39).
Listeriolysin O (LLO), produced by Listeria monocytogenes, is the major virulence factor essential for escape of this bacterium from phagosomes into cytosolic space (4, 9, 32). The cytolytic activity of LLO is thought to be responsible for the high virulence of L. monocytogenes. In contrast, Listeria seeligeri, which produces seeligeriolysin O (LSO) (13, 23), cannot escape from phagosomes and hence is nonpathogenic (11, 19) in spite of the presence of the lso gene, which is highly homologous to the hly gene coding for LLO. It is well known that the hemolytic activity in culture supernatant of L. seeligeri is very weak compared with that of L. monocytogenes (12, 23, 35). The weak hemolytic activity is attributed to a low level of expression of the lso gene, since gene complementation with plcA-prfA from L. monocytogenes resulted in strong expression of the lso gene, enabling L. seeligeri to escape from phagosomes (19). In a previous study, we constructed recombinant LSO (rLSO) and recombinant LLO (rLLO) and showed that the cytolytic activity of rLSO was 25% lower than that of rLLO. Amino acid replacement analysis revealed that the difference in hemolytic activity was partially due to one amino acid change (Ala to Phe) in the undecapeptide sequence (18).
L. monocytogenes has been reported to induce various host responses, including expression of adhesion molecules, chemokines (20), and various inflammatory cytokines, such as interleukin-1 (IL-1), IL-6, IL-12, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) (15, 26, 33, 42). Among these cytokines, IFN-γ plays an important role in the protection of mice against L. monocytogenes infection (8, 10, 17, 44). We have shown that native LLO (nLLO) purified from culture supernatant of L. monocytogenes is capable of inducing IL-1 production in peritoneal macrophages (41, 45) and that nLLO and rLLO induce IFN-γ in spleen cells (22, 28, 29). In addition, we have also shown that IL-12 and IL-18 from macrophages are essential for the induction of IFN-γ (28, 29) and that the IFN-γ-inducing ability of LLO is not associated with cholesterol-binding activity based on studies in which cholesterol-treated LLO or rLLO with a C-terminal truncation was used (22). As shown in our previous studies, the IFN-γ-inducing activity of nLLO is able to serve as an adjuvant in inducing the TH1 type of cells in mice when nLLO is given after liposome encapsulation (40) or in an emulsion (43). Recently, Rose et al. reported that LLO induced IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor in human umbilical vein endothelial cells (36). Among members of the CDC family other than LLO, streptolysin O (37) and pneumolysin (PLY) (2, 7, 16) have been reported to stimulate host responses, such as cytokine production, complement activation, and nitric oxide production. The previous studies suggested that cytokine-inducing ability is also a common activity exhibited by various CDCs.
LSO from L. seeligeri is a 56-kDa protein that shows 80% homology to LLO at the amino acid sequence level (14), so it is very possible that LSO, in addition to having cytolytic activity like that of LLO, is also capable of exhibiting cytokine-inducing activity. In the present study, by using a recombinant full-length LSO (rLSO530) and a C-terminus-truncated form of LSO (rLSO483), we examined the ability of molecules to induce IFN-γ production in spleen cells, the expression of various cytokine genes of macrophages, and the effect of neutralization of the cytokines on IFN-γ production.
MATERIALS AND METHODS
Experimental animals.
Female strain C3H/HeN mice (SLC Japan, Hamamatsu, Japan) that were raised and maintained in a specific-pathogen-free environment were used for experiments when they were 7 to 10 weeks old.
Production and purification of recombinant proteins.
Full-length forms of LLO and LSO (LLO529 and LSO530) and a truncated form of LSO (LSO483) with deletion of C-terminal amino acids, including the undecapeptide, were prepared by the procedure described previously (18, 22, 24). Briefly, genomic DNAs were extracted from L. monocytogenes EGD and L. seeligeri ATCC 35967 and used as templates in PCR. The hly gene and the lso gene lacking the coding region for the predicted signal sequence were amplified by PCR. Specific primer sets containing appropriate restriction enzyme sites have been described previously (18). For construction of the truncated LSO (rLSO483), we employed the 5′ PCR primer for amplifying full-length LSO. The 3′ primer used for rLSO483 contained a HindIII site (5′ ACGCAAGCTTCTATCTTGCATAGATGTTAATATTTCT 3′), which was designed so that the region from the undecapeptide residue to the C-terminal end was truncated. The PCR fragment and the pQE31 expression vector (Qiagen, Hilden, Germany) were digested with the same restriction enzyme sets and were ligated with T4 DNA ligase (BioLabs, Hertfordshire, United Kingdom) to generate a recombinant protein with a six-His tag at the N terminus. The ligated plasmid was electroporated into Escherichia coli SG13009 by using the Gene Pulser II electroporation system (Bio-Rad Laboratories, Hercules, Calif.). The DNA sequence was confirmed with an automated sequence machine (ABI PRISM TM 310 genetic analyzer; Perkin-Elmer Applied Biosystems, Foster City, Calif.). The entire inserts were sequenced and compared with the genomic DNA to confirm that no mutation was introduced by PCR. Cultures used for preparation of His-tagged recombinant proteins were grown at 37°C in tryptic soy broth containing ampicillin (100 μg/ml) and kanamycin (25 μg/ml), and proteins were induced with 1.0 mM isopropyl-β-d-thiogalactopyranoside (Nacalai Tesque, Inc., Kyoto, Japan). Cells were harvested by centrifugation (6,000 × g for 15 min) and suspended in lysis buffer containing 1 mg of lysozyme per ml, 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 20 mM imidazole, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, 1 μg of pepstain A (Nacalai Tesque) per ml, and 1 μM phenylmethylsulfonyl fluoride (Sigma Chemical Co., St. Louis, Mo.). The suspension was incubated on ice for 30 min and vortexed with zirconia-silica beads (Bio Spec Products, Inc., Bartlesville, Okla.) for 2 min. A supernatant was obtained by centrifugation (10,000 × g for 30 min) and applied to an Ni-nitrilotriacetic acid column (Qiagen) under native conditions according to the manufacturer's instructions. Purified recombinant proteins were applied to a PD-10 column (Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, United Kingdom) for desalting and were eluted with phosphate-buffered saline. The elutant was applied to a Detoxi-Gel endotoxin-removing gel column (Pierce Chemical Company, Rockford, Ill.) in order to eliminate a possible contaminating lipopolysaccharide (LPS). The purity of recombinant proteins was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by immunostaining with anti-penta-His antibody (Qiagen). Protein concentrations were determined by a Bio-Rad protein assay. The level of LPS was determined by the Limulus color KY test (Wako Pure Chemical Industries, Osaka, Japan) and was found to be less than 10 pg/ml when a preparation was diluted in phosphate-buffered saline at a protein concentration of 10 μg/ml.
Treatment of rLLO and rLSO with cholesterol.
Full-length rLLO529 and rLSO530 at all doses were incubated with 10 μg of cholesterol per ml overnight at 4°C. Because of the absence of cytolytic activity against sheep erythrocytes, rLSO483 was not treated with cholesterol.
Preparation of cells.
A single-cell suspension was prepared from the spleens of normal mice. After treatment with 0.83% ammonium chloride in 0.17 mM Tris-HCl (pH 7.6) to lyse the erythrocytes, the cells were washed three times with Hanks' balanced salt solution and were suspended in complete medium consisting of RPMI 1640 (Gibco-BRL, Rockville, Md.) supplemented with 10% fetal calf serum and 5 μg of gentamicin (Gibco-BRL) per ml. In order to deplete NK cells, macrophages, or dendritic cells, whole spleen cells were treated with magnetic cell-sorting system (MACS) anti-NK (DX5) MicroBeads, MACS anti-CD11b MicroBeads, MACS anti-CD11c MicroBeads, and MACS anti-Ter119 MicroBeads (Miltenvi Biotec, Gladbach, Germany). The cells that were labeled magnetically with beads were enriched or depleted by passing them through a MACS column according to the manufacturer's instructions. The purity of fractionated cell populations was assessed by fluorescence-activated cell sorting.
Cytolytic activity.
Cytolytic activity was determined by measuring the level of lactate dehydrogenase (LDH) in supernatant released from the cells by using an LDH cytotoxicity detection kit (Takara Biomedical, Tokyo, Japan). Recombinant protein was added to 2.0 × 106 spleen cells in a 96-well flat-bottom tissue culture plate in complete medium. The supernatant was harvested after 6 h, and LDH activity was measured by determining the absorbance at 490 nm with an automated microplate reader. Samples treated with 1% Triton X-100 and samples treated with culture medium alone were used as positive (100% LDH release) and negative (0% LDH release) controls, respectively. The relative LDH release for each well was calculated by using the following formula: percentage of LDH released = (LDH activity in experimental group − activity in negative control)/(activity in positive control − activity in negative control) × 100.
ELISA for IFN-γ.
Cells were plated (2 × 106 cells per well) in a 96-well flat-bottom tissue culture plate and cultured with several concentrations of cholesterol-treated recombinant proteins (range, 12.5 to 200 nM) with or without 0.5 μg of polymyxin B (Nacalai Tesque) per ml. In some experiments, cells were stimulated with recombinant proteins in the presence of protein G-purified polyclonal rabbit antibody against LLO or neutralizing antibody to IL-1α (rat monoclonal antibody 40508.11; Genzyme/Techne, Minneapolis, Minn.), IL-1β (rat monoclonal antibody 30311.11; Genzyme/Techne), IL-12 p70 (goat polyclonal antibody; Genzyme/Techne), or IL-18 (rat monoclonal antibody 93-10C; MBL, Nagoya, Japan). Each culture supernatant was collected after incubation for 24 h at 37°C in the presence of 5% CO2. The IFN-γ titer was determined by a two-site sandwich enzyme-linked immunosorbent assay (ELISA) by using a combination of nonlabeled and biotin-conjugated anti-mouse IFN-γ antibodies (Endogen Inc., Woburn, Mass.) as described previously (22).
Reverse transcription (RT)-PCR.
Cells were stimulated with 50 nM cholesterol-treated rLSO530 or with non-cholesterol-treated rLSO483 for 6 h. Total cellular RNA was extracted with an RNeasy mini kit (Qiagen), and cDNA was reverse transcribed from 0.2 μg of total RNA by using a random primer as previously described (29). PCR was performed by using KOD-Plus DNA polymerase (TOYOBO, Osaka, Japan) and a specific primer set for each cytokine and β-actin. Each PCR cycle consisted of 94°C for 15 s, 60°C for 30 s, and 68°C for 60 s. Samples were amplified for 25 to 33 cycles. The most appropriate number of amplification cycles for each cytokine was determined by performing preliminary experiments. The reaction was terminated by incubation at 68°C for 7 min. The sequences of the oligonucleotide primers used are as follows: 5′-CTCTAGAGCACCATGCTACAGAC-3′ and 5′-TGGAATCCAGGGGAAACACTG-3′ for IL-1α, 5′-AAGCTCTCCACCTCAATGGACAG-3′ and 5′-CTCAAACTCCACTTTGCTCTTGA-3′ for IL-1β, 5′-CTGCATCAGCTCATCGATGG-3′ and 5′-CAGAAGCTAACCATCTCCTGGTTT-3′ for IL-12 p35, 5′-TCCGGAGTAATTTGGTGCTTCACA-3′ and 5′-ACT-GTACAACCGCAGTAATACGG-3′ for IL-12 p40, 5′-ACTGTACAACCGCAGTAATACGG-3′ and 5′-AGTGAACATTACAGATTTATCCC-3′ for IL-18, 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′ and 5′-ACATTCGAGGCTCCAGTGAATTCCA-3′ for TNF-α, 5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ and GTCACAGTTTTCAGCTGTATAGGG-3′ for IFN-γ, and 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ for β-actin. Each PCR product (5 μl) was electrophoresed on a 2% agarose gel in 0.5× Tris-acetate-EDTA buffer and stained with 0.005% ethidium bromide. The bands of the PCR product were visualized with a UV transilluminator.
Statistical analysis.
The statistical significance of the data was determined by Student's t test, and a P value of <0.05 was considered significant.
RESULTS
Purification of recombinant proteins.
The purity of the recombinant proteins obtained for this study was confirmed by SDS-PAGE and Coomassie blue staining (Fig. 1A). Anti-LLO polyclonal antibody reacted to rLLO529 and cross-reacted with rLSO530 and rLSO483 (Fig. 1B). All of these products also reacted with anti-penta-His antibody in immunoblots (data not shown).
FIG. 1.
Purity of recombinant proteins constructed and employed in this study. Each protein was analyzed by SDS-PAGE and staining with Coomassie blue (A) and immunostaining with anti-LLO polyclonal antibody after blotting (B). Lane 1, rLLO529; lane 2, rLSO530; lane 3, rLSO483.
Cytolytic activities of recombinant proteins.
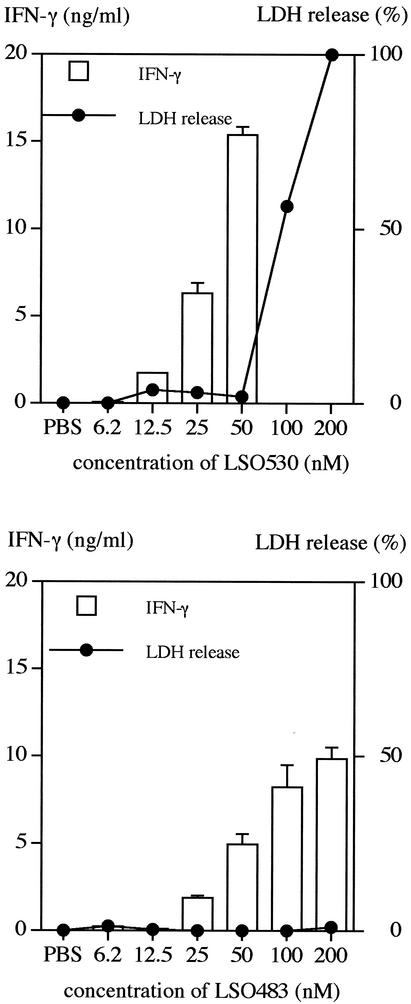
As reported previously, rLSO530 showed cholesterol-binding and hemolytic activities (18), whereas rLSO483, which was truncated at the C terminus, including the conserved undecapeptide, showed no such activities (data not shown). In order to determine cytotoxicity for spleen cells, we measured the levels of LDH in culture supernatants after stimulation with recombinant proteins. When rLSO530 was treated with 10 μg of cholesterol per ml, the cytolytic activity could be blocked at a concentration less than 50 nM, but at a higher concentration complete blocking was not observed (Fig. 2A). Without pretreatment with cholesterol, rLSO530 showed cytolytic activity at concentrations as low as 12.5 nM (data not shown), whereas rLSO483 had no activity even at a concentration of 200 nM (Fig. 2B).
FIG. 2.
IFN-γ-inducing and cytolytic activities of the recombinant proteins. Normal spleen cells were stimulated for 24 h with cholesterol-treated rLSO530 (upper graph) or non-cholesterol-treated rLSO483 (lower graph). rLSO530 at all doses was incubated with 10 μg of cholesterol per ml. The level of IFN-γ in culture supernatant was measured by ELISA. The cytolytic activity was determined by the amount of LDH released in supernatant harvested 6 h after recombinant proteins were added. Representative results are shown. Similar results were obtained in three independent experiments. The data are means ± standard errors for three determinations. PBS, phosphate-buffered saline.
IFN-γ-inducing activities of recombinant proteins.
When spleen cells were stimulated with cholesterol-treated rLSO530 or rLSO483 for 24 h, production of IFN-γ was induced in a dose-dependent manner, and rLSO530 induced strong IFN-γ production at a concentration of 50 nM. At the concentration showing cytolytic activity, rLSO530 was not able to induce IFN-γ (Fig. 2A). The absence of any IFN-γ-inducing activity of rLSO530 at higher concentrations seemed to be due to remaining cytolytic activity. The truncated, nonhemolytic rLSO483 molecule was able to induce IFN-γ production in a dose-dependent manner at concentrations up to 200 nM (Fig. 2B). The IFN-γ-inducing activity of rLSO483 was weaker than that of rLSO530. These results indicated that the C-terminal region containing the undecapeptide that is necessary for the cytotoxic activity might contribute to, but is not essential for, the cytokine-inducing activity. The level of contaminating LPS in these preparations was less than 10 pg/ml. This small amount of LPS in the preparations did not seem to contribute to IFN-γ production because the same amount of LPS did not induce IFN-γ production (data not shown) and the IFN-γ production induced by rLSO530 or rLSO483 was not blocked by addition of 0.5 μg of polymyxin B per ml, a dose that completely blocks IFN-γ production by stimulation with 1,000 pg of LPS per ml (data not shown).
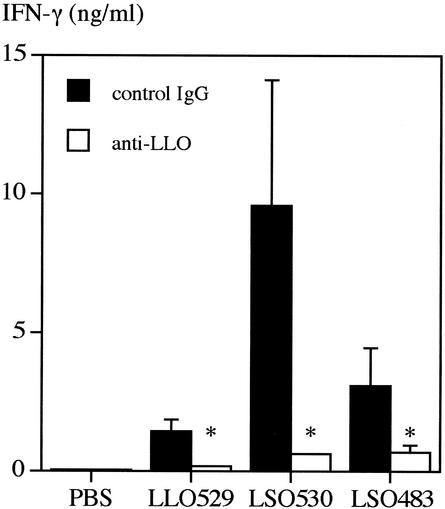
Next, in order to confirm that IFN-γ production was induced in response to rLSO530 and rLSO483, we employed anti-LLO antibody and attempted to neutralize the IFN-γ-inducing activities. The IFN-γ production by spleen cells after stimulation with 50 nM cholesterol-treated rLLO529 was significantly reduced in the presence of 5 μg of anti-LLO polyclonal antibody per ml (Fig. 3). As anti-LLO antibody recognized rLSO530 and rLSO483 (Fig. 1B) and inhibited IFN-γ production induced by rLSO530 or rLSO483, the data suggested that rLLO529, rLSO530, and rLSO483 shared the active site for IFN-γ-inducing activity. rLSO530 induced a higher level of IFN-γ than rLLO529 in the absence of anti-LLO antibody.
FIG. 3.
Effect of anti-LLO polyclonal antibody on IFN-γ production induced by rLLO529, rLSO530, or rLSO483. Normal spleen cells were stimulated with rLLO529, rLSO530, or rLSO483 (50 nM) for 24 h in the presence of anti-LLO polyclonal antibody or control rabbit immunoglobulin G (IgG) (5 μg/ml). The level of IFN-γ was measured by ELISA. Representative results are shown. Similar results were obtained in three independent experiments. The data are means ± standard errors for three determinations. An asterisk indicates that the results are significantly different from the control results (P < 0.05). PBS, phosphate-buffered saline.
Effect of depletion of a particular cell population on LSO-induced IFN-γ production.
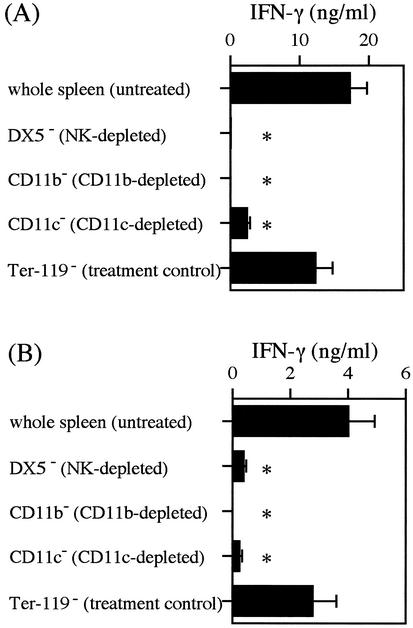
Based on our previous finding that macrophages and NK cells are involved in LLO-induced IFN-γ production (28, 29), we examined the involvement of these cell populations in LSO-induced IFN-γ production. By using MACS with MicroBeads conjugated with anti-NK (DX5), anti-CD11b (Mac-1), and anti-CD11c, spleen cells were depleted of NK cells, macrophages, and dendritic cells, respectively. Fluorescence-activated cell sorting analysis revealed that the populations of DX5+ cells (NK cells), CD11b+ cells (macrophages), and CD11c+ cells (dendritic cells) were decreased from 2.58 to 0.90%, from 10.55 to 0.41%, and from 1.02 to 0.08%, respectively. Whole spleen cells and the cells depleted for each population were stimulated with 50 nM cholesterol-treated rLSO530 (Fig. 4A) and rLSO483 (Fig. 4B) for 24 h. The levels of IFN-γ production in all groups depleted for each cell population were significantly lower than the levels of IFN-γ production in whole spleen cells. The data confirmed that the significant reductions were not artifacts caused merely by the processing of cells through the magnetic system, as there was no statistically significant reduction in the IFN-γ response in the cells processed by using MicroBeads conjugated with anti-Ter-119, an irrelevant antigen expressed on erythroid cells. As observed with LSO530 in this study, both DX5+ cells and CD11b+ cells were required for IFN-γ-inducing activity of LLO (28), suggesting that the two cytolysins induced IFN-γ production by similar mechanisms.
FIG. 4.
IFN-γ production after depletion of NK cells, macrophages, or dendritic cells. Normal spleen cells bound to anti-NK (DX5), anti-CD11b (Mac-1), anti-CD11c, or anti-Ter-119 (negative control) MicroBeads were depleted by using a MACS LS separation column. The negatively selected cells were stimulated with 50 nM rLSO530 (A) or 50 nM rLSO483 (B) for 24 h. The level of IFN-γ was measured by ELISA. Representative results are shown. Similar results were obtained in two independent experiments. The data are means ± standard errors for three determinations. An asterisk indicates that the results are significantly different from the results for the untreated group (P < 0.05).
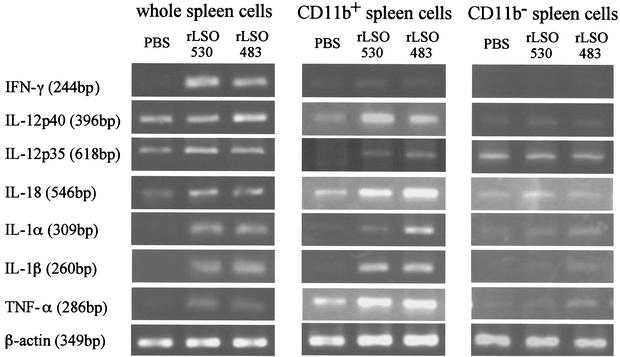
LSO-induced cytokine gene expression in spleen cells, CD11b+ spleen cells, and CD11b+ cell-depleted spleen cells.
Cell depletion experiment indicated that CD11b+ macrophages were involved in LSO-induced IFN-γ production. Accordingly, expression of the gene for the macrophage-dependent cytokine was examined by RT-PCR. We prepared whole spleen cells, spleen cells enriched for CD11b+ cells (CD11b+ spleen cells), and spleen cells depleted of CD11b+ cells (CD11b− spleen cells) by using MACS and stimulated the cells with recombinant proteins. After 6 h of stimulation, total RNA was extracted, and cytokine-specific mRNA was examined by RT-PCR (Fig. 5). In the whole spleen cells, expression of IFN-γ, IL-1α, IL-1β, and TNF-α was significantly induced. A big difference was not observed in the expression of IL-12 p35, IL-12 p40, and IL-18 between groups with stimulation and groups without stimulation. In CD11b+ cells, expression of genes for all of these cytokines except IFN-γ was observed. In contrast, no cytokine gene expression, including IFN-γ gene expression, was induced in spleen cells depleted of CD11b+ cells. These results indicated that both CD11b+ cells and CD11b− cells are required for LSO-induced IFN-γ production.
FIG. 5.
Expression of mRNAs for various cytokines after stimulation with rLSO530 or rLSO483. Whole spleen cells, spleen cells enriched for CD11b+ cells (CD11b+ spleen cells), and spleen cells depleted of CD11b+ cells (CD11b− spleen cells) were stimulated with rLSO530 or rLSO483 for 6 h. Total RNA was extracted and subjected to RT-PCR for detection of cytokine mRNAs for IFN-γ, IL-1α, IL-1β, IL-12 p35, IL-12 p40, IL-18, TNF-α, and β-actin. Representative results are shown. Similar results were obtained in two independent experiments. PBS, phosphate-buffered saline.
Effect of cytokine-specific neutralizing antibody on LSO-induced IFN-γ production.
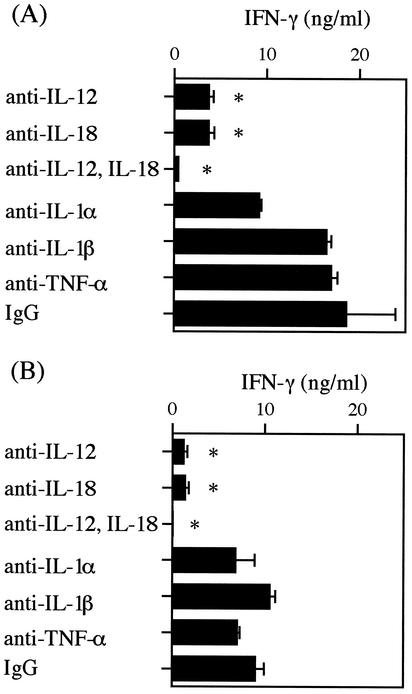
To determine the cytokine essential for LSO-induced IFN-γ production among the cytokines induced in CD11b+ cells, the effects of neutralizing antibodies on each cytokine were examined. After stimulation with 50 nM cholesterol-treated rLSO530 and rLSO483 in the presence of each cytokine-specific neutralizing antibody for 24 h, the titer of IFN-γ in the culture supernatant was determined. When anti-IL-12 p70 or anti-IL-18 antibody was added, LSO-induced IFN-γ production was significantly decreased, whereas antibodies against IL-1α, IL-1β, and TNF-α had no effect (Fig. 6).
FIG. 6.
Effect of neutralization of various cytokines on IFN-γ production induced by rLSO530 (A) or rLSO483 (B). Normal spleen cells were stimulated with rLSO530 or rLSO483 (50 nM) for 24 h in the presence of an antibody specific for IL-1α, IL-1β, IL-12 p70, IL-18, or TNF-α (5 μg/ml). The level of IFN-γ was measured by ELISA. Representative results are shown. Similar results were obtained in two independent experiments. The data are means ± standard errors for three determinations. An asterisk indicates that the results are significantly different from the control results (P < 0.05). IgG, immunoglobulin G.
DISCUSSION
In this study, we showed that both full-length rLSO530 and rLSO483 with the C terminus truncated were capable of inducing IFN-γ production in spleen cells of mice. IFN-γ production after stimulation with rLSO530 was observed only after treatment with cholesterol to eliminate the cytolytic activity of rLSO530. It is generally accepted that the C-terminal end of CDC family proteins, especially the conserved undecapeptide, is essential for cholesterol binding and cytolysis (25, 30, 38, 39). We reported previously that the lower cholesterol-binding and hemolytic activities of rLSO530 were due to the natural replacement of Ala489 with Phe (18). The C-terminus-truncated rLSO483 constructed in this study did not show any cytolytic activity without pretreatment with cholesterol, and rLSO483 could not bind immobilized cholesterol on a polyvinylidene difluoride membrane and a thin-layer chromatography plate (data not shown). Both cholesterol-treated rLSO530 and rLSO483 were capable of inducing IFN-γ production in spleen cells; therefore, the IFN-γ-inducing ability of rLSO530 appeared to be independent of cholesterol-binding activity. These findings are consistent with our previous reports on the activity of rLLO (22) and recombinant PLY (2). The possibility that contaminating LPS was responsible for IFN-γ production could be ruled out, since IFN-γ-inducing ability was not affected by treatment of the sample with polymyxin B. In addition, the level of LPS determined by the Limulus assay never exceeded 10 pg/ml, and E. coli LPS at this dose could not induce IFN-γ production in spleen cells.
In addition to our reports, there have been other reports of CDC-induced production of cytokine or nitric oxide. It has been reported that LLO induces IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor in human umbilical vein endothelial cells (36) and that PLY induces nitric oxide production from macrophages (7). Since LSO was also able to induce cytokine production, it is likely that members of the CDC family have a common feature for cytokine induction.
We reported previously that rLLO529-induced IFN-γ production was inhibited by anti-LLO polyclonal antibody (22). The rabbit anti-LLO polyclonal antibody raised by immunization with rLLO may have recognized the six-His tag at the N terminus. However, we confirmed that this antibody recognized nLLO purified from culture supernatant of L. monocytogenes and inhibited IFN-γ production induced by nLLO in spleen cells (unpublished data). The results indicated that the cross inhibition by anti-LLO polyclonal antibody of LSO-induced IFN-γ production was not associated with the binding to the six-His terminal portion. LSO exhibits approximately 80% homology with LLO at the amino acid level, and anti-LLO polyclonal antibody recognized rLSO530 and rLSO483 similarly in Western blots (Fig. 1B). The finding that rLSO530- or rLSO483-induced IFN-γ production was also reduced in the presence of anti-LLO polyclonal antibody indicated that rLLO529, rLSO530, and rLSO483 have the same active site for IFN-γ-inducing activity.
In a series of experiments, we determined the role of macrophages and NK cells in LLO-induced IFN-γ production. Of the various cytokines examined, IL-12 and IL-18 appeared to play essential roles in the induction of IFN-γ production by spleen cells after stimulation with LLO (28, 29). In the present study, we examined the role of macrophages and NK cells in LSO-induced IFN-γ production using the MACS cell depletion system. As expected, depletion of DX5+ cells (mainly NK cells), CD11b+ cells (mainly macrophages), and CD11c+ cells (mainly dendritic cells) resulted in decreases in IFN-γ production. Since NK cells are the major source of IFN-γ, the results suggested that LSO-induced IFN-γ was produced by DX5+ cells. Various cytokines from macrophages are known to induce IFN-γ production by NK cells. Depletion of CD11b+ cells resulted in the elimination of mRNA expression of various macrophage-derived cytokines, as well as the loss of IFN-γ production after stimulation with rLSO530 and rLSO483. In contrast, expression of the genes encoding these macrophage-derived cytokines was enhanced in CD11b+ cells. These cytokines appeared to be involved in IFN-γ production from NK cells. Addition of neutralizing antibodies to IL-12 p70 and IL-18 significantly affected IFN-γ production from spleen cells, and IFN-γ production was almost completely eliminated by a combination of these two antibodies (Fig. 6). Although the levels of IL-12 p70 and IL-18 production in the supernatant were not high (data not shown), these results indicated that small amounts of LSO-induced IL-12 p70 and IL-18 produced from macrophages stimulated NK cells for IFN-γ production.
It is known that IL-12 p40 and IL-12 p35 are expressed even after depletion of macrophages from spleen cells (3), and they do not always indicate production of functional IL-12 (IL-12 p70). This may explain why the levels of mRNA for IL-12 p40 and IL-12 p35 remained almost the same in the whole spleen cells (Fig. 5). In contrast, LSO was able to induce the mRNAs for both IL-12 p40 and IL-12 p35 in the CD11b+ population, so it is possible that functionally active IL-12 p70 was produced in the CD11b+ population.
IL-18 induces IFN-γ production from NK cells and TH1 cells in concert with IL-12 (30). There is a report that neutralization of IL-18 inhibited IFN-γ, TNF-α, and nitric oxide production in L. monocytogenes infection (27). IL-18 is constitutively expressed in macrophages and is processed by caspase-1 (34). LPS and oligodeoxynucleotide CpG motifs induce the expression of IL-18 (6, 21). A low but definite amount of IL-18 was produced from thioglycolate-induced peritoneal macrophages after stimulation with rLSO (data not shown). Therefore, rLSO itself may enhance IL-18 gene expression via caspase activation, resulting in strong induction of IFN-γ production.
In conclusion, we found that LSO, a cytolysin homologous to LLO, could induce IFN-γ production in spleen cells by a mechanism different from cytolytic activity. In LSO-induced IFN-γ production, NK cells and CD11b+ cells played an essential role and IL-12 and IL-18 were important among the various cytokines produced by CD11b+ cells. We concluded that some members of the CDC family have the same biological activity (cytokine-inducing activity with similar mechanisms). The minimum region required for the cytokine-inducing activity of the N-terminal portion of CDCs remains to be determined.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research on Priority Areas 13226042 from The Ministry of Education, Culture, Sports, Science and Technology of Japan and by Research for the Future Program grant 97L00706 and Grants-in-Aid for Scientific Research B/14370092 and C/13670270 from The Japan Society for the Promotion of Science.
Editor: J. D. Clements
REFERENCES
- 1.Alouf, J. E. 1999. Introduction to the family of the structurally related cholesterol-binding cytolysins (′sulfhydryl-activated' toxins), p. 443-456. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 2.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2002. Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity. Infect. Immun. 70:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bette, M., S. C. Jin, T. Germann, M. K. Schafer, E. Weihe, E. Rude, and B. Fleischer. 1994. Differential expression of mRNA encoding interleukin-12 p35 and p40 subunits in situ. Eur. J. Immunol. 24:2435-2440. [DOI] [PubMed] [Google Scholar]
- 4.Bielecki, J., P. Youngman, P. Connelly, and D. A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175-176. [DOI] [PubMed] [Google Scholar]
- 5.Billington, S. J., B. H. Jost, and J. G. Songer. 2000. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol. Lett. 182:197-205. [DOI] [PubMed] [Google Scholar]
- 6.Bohle, B., B. Jahn-Schmid, D. Maurer, D. Kraft, and C. Ebner. 1999. Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-γ production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur. J. Immunol. 29:2344-2353. [DOI] [PubMed] [Google Scholar]
- 7.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier, N. A., and R. D. Schreiber. 1985. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 82:7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossart, P., M. F. Vicente. J. Mengaud, F. Baquero, J. C. Perez-Diaz, and P. Berche. 1989. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, P. L., and R. J. North. 1991. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect. Immun. 59:2892-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1989. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J. Gen. Microbiol. 135:481-487. [DOI] [PubMed] [Google Scholar]
- 13.Gouin, E., J. Mengaud, and P. Cossart. 1994. The virulence gene cluster of Listeria monocytogenes is also present in Listeria ivanovii, an animal pathogen, and Listeria seeligeri, a nonpathogenic species. Infect. Immun. 62:3550-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas, A., M. Dumbsky, and J. Kreft. 1992. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim. Biophys. Acta 1130:81-84. [DOI] [PubMed] [Google Scholar]
- 15.Havell, E. A., L. L. Moldawer, D. Helfgott, P. L. Kilian, and P. B. Sehgal. 1992. Type I IL-1 receptor blockade exacerbates murine listeriosis. J. Immunol. 148:1486-1492. [PubMed] [Google Scholar]
- 16.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-γ receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 18.Ito, Y., I. Kawamura, C. Kohda, H. Baba, T. Kimoto, I. Watanabe, T. Nomura, and M. Mitsuyama. 2001. Difference in cholesterol-binding and cytolytic activities between listeriolysin O and seeligeriolysin O: a possible role of alanine residue in tryptophan-rich undecapeptide. FEMS Microbiol. Lett. 203:185-189. [DOI] [PubMed] [Google Scholar]
- 19.Karunasagar, I., R. Lampidis, W. Goebel, and J. Kreft. 1997. Complementation of Listeria seeligeri with the plcA-prfA genes from L. monocytogenes activates transcription of seeligerolysin and leads to bacterial escape from the phagosome of infected mammalian cells. FEMS Microbiol. Lett. 146:303-310. [DOI] [PubMed] [Google Scholar]
- 20.Kayal, S., A. Lilienbaum, C. Poyart, S. Memet, A. Israel, and P. Berche. 1999. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-κB and upregulation of adhesion molecules and chemokines. Mol. Microbiol. 31:1709-1722. [DOI] [PubMed] [Google Scholar]
- 21.Kim, Y. M., J. Y. Im, S. H. Han, H. S. Kang, and I. Choi. 2000. IFN-γ up-regulates IL-18 gene expression via IFN consensus sequence-binding protein and activator protein-1 elements in macrophages. J. Immunol. 165:3198-3205. [DOI] [PubMed] [Google Scholar]
- 22.Kohda, C., I. Kawamura, H. Baba, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect. Immun. 70:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leimeister-Wächter, M., and T. Chakraborty. 1989. Detection of listeriolysin, the thiol-dependent hemolysin in Listeria monocytogenes, Listeria ivanovii, and Listeria seeligeri. Infect. Immun. 57:2350-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mengaud, J., M. F. Vicente, J. Chenevert, J. M. Pereira, C. Geoffroy, B. Gicquel-Sanzey, F. Baquero, J. C. Perez-Diaz, and P. Cossart. 1988. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect. Immun. 56:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 26.Nakane, A., A. Numata, and T. Minagawa. 1992. Endogenous tumor necrosis factor, inerleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect. Immun. 60:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon γ production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishibori, T., H. Xiong, I. Kawamura, M. Arakawa, and M. Mitsuyama. 1996. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect. Immun. 64:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomura, T., I. Kawamura, K. Tsuchiya, C. Kohda, H. Baba, Y. Ito, T. Kimoto, I. Watanabe, and M. Mitsuyama. 2002. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect. Immun. 70:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamura, H., S. I. Kashiwamura, H. Tsutsui, T. Yoshimoto, and K. Nakanishi. 1998. Regulation of interferon-γ production by IL-12 and IL-18. Curr. Opin. Immunol. 10:259-264. [DOI] [PubMed] [Google Scholar]
- 31.Owen, R. H. G., G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1994. A role in cell-binding for the C-terminus of pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae. FEMS Microbiol. Lett. 121:217-222. [DOI] [PubMed] [Google Scholar]
- 32.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poston, R. M., and R. J. Kurlander. 1992. Cytokine expression in vivo during murine listeriosis. J. Immunol. 149:3040-3044. [PubMed] [Google Scholar]
- 34.Puren, A. J., G. Fantuzzi, and C. A. Dinarello. 1999. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 96:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez, L. D., J. A. V. Boland, J. F. F. Garayzabal, P. E. Tranchant, E. Gomez-Lucia, E. F. R. Ferri, and G. S. Fernandez. 1986. Microplate technique to determine hemolytic activity for routine typing of Listeria strains. J. Clin. Microbiol. 24:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose, F., S. A. Zeller, T. Chakraborty, E. Domann, T. Machleidt, M. Knonke, W. Seeger, F. Grimminger, and U. Sibelius. 2001. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect. Immun 69:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27:337-346. [DOI] [PubMed] [Google Scholar]
- 38.Sekino-Suzuki, N., M. Nakamura, K. I. Mitsui, and Y. Ohno-Iwashita. 1996. Contribution of individual tryptophan residues to the structure and activity of θ-toxin (perfringolysin O), a cholesterol-binding cytolysin. Eur. J. Biochem. 241:941-947. [DOI] [PubMed] [Google Scholar]
- 39.Shimada, Y., M. Nakamura, Y. Naito, K. Nomura, and Y. Ohno-Iwashita. 1999. C-terminal amino acid residues are required for the folding and cholesterol binding property of perfringolysin O, a pore-forming cytolysin. J. Biol. Chem. 274:18536-18542. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe, Y., H. Xiong, T. Nomura, M. Arakawa, and M. Mitsuyama. 1999. Induction of protective T cells against Listeria monocytogenes in mice by immunization with a listeriolysin O-negative avirulent strain of bacteria and liposome-encapsulated listeriolysin O. Infect. Immun. 67:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukada, H., I. Kawamura, T. Fujimura, K. Igarashi, M. Arakawa, and M. Mitsuyama. 1992. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell. Immunol. 140:21-30. [DOI] [PubMed] [Google Scholar]
- 42.Xiong, H., S. Ohya, Y. Tanabe, and M. Mitsuyama. 1997. Persistent production of interferon-gamma (IFN-γ) and IL-12 is essential for the generation of protective immunity against Listeria monocytogenes. Clin. Exp. Immunol. 108:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong, H., Y. Tanabe, S. Ohya, and M. Mitsuyama. 1998. Administration of killed bacteria together with listeriolysin O induces protective immunity against Listeria monocytogenes in mice. Immunology 94:14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, J., I. Kawamura, and M. Mitsuyama. 1997. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect. Immun. 65:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshikawa, H., I. Kawamura, M. Fujita, H. Tsukada, M. Arakawa, and M. Mitsuyama. 1993. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect. Immun. 61:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]