Abstract
In addition to their effects on alveolar surface tension, some components of lung surfactant also have immunological functions. We found recently that the hydrophobic lung surfactant protein SP-C specifically binds to the lipid A region of lipopolysaccharide (LPS). In this study, we show that SP-C also interacts with CD14. Four observations showed cross talk between the three molecules SP-C, LPS, and CD14. (i) Like LBP, SP-C allows the binding of a fluorescent LPS to cells expressing CD14 (the other surfactant components were ineffective). (ii) Recombinant radiolabeled CD14 and SP-C (or a synthetic analog of SP-C) interact in a dose-dependent manner. (iii) LPS blocks the binding of radiolabeled CD14 to SP-C-coated wells. (iv) SP-C enhances the binding of radiolabeled CD14 to LPS-coated wells. These results, obtained with native murine SP-C and with three synthetic analogs, suggest that LPS and CD14 interact with the same region of SP-C and that binding of SP-C modifies the conformation of CD14 or the accessibility of its LPS-binding site, allowing it to bind LPS. This ability of SP-C to interact with the pattern recognition molecule CD14 extends the possible immunological targets of SP-C to a large panel of microorganisms that can enter the airways.
The lipopolysaccharide (LPS) of gram-negative bacteria is a potent stimulator of the immune system (27, 34). When minute amounts of LPS reach the blood circulation system, a rapid innate response of the host aims at containing and destroying bacteria. However, in mammals, an excessive response to LPS often leads to major clinical problems mediated by LPS-induced bioactive products of monocytes/macrophages, such as tumor necrosis factor alpha, interleukins, reactive oxygen intermediates, and nitric oxide (7, 28).
When LPS enters the host via the airways, it interacts with alveolar macrophages in a fluid environment which is markedly different from that of the blood circulation system. Pulmonary surfactant is a major constituent of the alveolar surface fluid. The surfactant acts to prevent alveoli from collapsing during expiration (22). Pulmonary surfactant is a complex mixture of lipids and proteins secreted by alveolar type II epithelial cells (15), the main component being dipalmitoylphosphatidylcholine. Surfactant contains the two hydrophilic proteins SP-A and SP-D, which belong to the C-type (collagen-like) mammalian lectin family referred to as collectins (9), and the two hydrophobic proteins SP-B and SP-C (8). On a weight basis, SP-A constitutes about 75% of the surfactant-associated proteins, while SP-B, SP-C, and SP-D make up 10, 6, and 8%, respectively. However, on a molar basis, SP-C is the major surfactant protein (65%), whereas SP-A, SP-B, and SP-D, the three other surfactant-associated proteins, represent only 5, 30, and 0.5%, respectively (18). In addition to its surface tension-lowering activity, pulmonary surfactant also displays host defense capacities (32). SP-A and SP-D have been shown to interact with LPSs of various phenotypes (20, 25), and we have shown recently that SP-C, but not SP-B, can also do so (3, 4).
Because LPS induces a variety of responses in alveolar macrophages (31) and because the responses of macrophages to physiological amounts of LPS depend in part on membrane-bound CD14 (40), it appeared important to examine the influence of the surfactant proteins SP-A, SP-D, and SP-C on CD14. Concerning lung collectins, Sano et al. (35) demonstrated in 1999 that SP-A binds to CD14 and modifies its interaction with LPS. SP-D can also bind CD14, but unlike SP-A, which recognizes a peptide region of CD14, SP-D recognizes a carbohydrate moiety of CD14 (36). The consequence of these interactions is that the binding of a rough-type LPS to CD14 is enhanced by SP-A and is decreased by SP-D. The purpose of this study was to determine if SP-C interacts with CD14, and if so, how such an interaction influences the binding of LPS to CD14.
MATERIALS AND METHODS
Animals.
Swiss mice were provided by R. Janvier (Le Genest Saint-Isle, France). C3H/HeOU mice were bred at the Pasteur Institute (Paris, France). Eight- to ten-week-old mice were used in all experiments.
Media and reagents.
Culture medium (CM) was RPMI 1640 (GIBCO, Grand Island, N.Y.) containing 2 mM l-glutamine, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml and supplemented with 10% heat-inactivated (56°C, 30 min) fetal calf serum (FCS). The tripalmitoyl pentapeptide was from Bachem (Bubendorf, Switzerland). Recombinant soluble murine CD14 (rmCD14) was from Biometec (Greifswald, Germany). 1,3,4,6-Tetrachloro-3α,6β-diphenylglycouril (Iodogen) was from Sigma Chemical Co. (St. Louis, Mo). Na125I (0.78 MBq/μl) was from ICN Biomedical Inc. (Irvine, Calif.), and tritium-labeled sodium borohydride (481 GBq/mmol) was from Amersham-Pharmacia Biotech (Buckinghamshire, England). The liquid scintillation reagent Aqualyte was from Baker (Deventer, The Netherlands).
LPS.
The LPS from Salmonella enterica serovar Minnesota (rough mutant Re595) was from Sigma Chemical Co. The LPS from Salmonella enterica serovar Choleraesuis (serotype 62,7,14) was prepared as described previously (12).
A fluorescein isothiocyanate (FITC)-labeled suspension of LPS from S. enterica serovar Choleraesuis (FITC-LPS) (12) was used. Briefly, FITC (250 μl; 1 mg/ml in dimethyl sulfoxide) was incubated (150 min, 20°C) with a suspension of lysine-LPS (0.9 ml in 0.1 M NaHCO3; pH 9) obtained by incubation of CNBr-activated LPS (5.2 mg; 700 μl; pH 10) with lysine chloride (200 μl; 5 mg/ml in 1 M NaHCO3). After dialysis against phosphate-buffered saline, FITC-LPS (3.7 mg/ml) was stored in the dark and at −20°C until used.
Tritium-labeled LPS (3H-LPS) was obtained by a modification of the procedure of Watson and Riblet (38). A sample (2 mg) of LPS from S. enterica serovar Minnesota Re595 was oxidized (150 min, 20°C) with sodium periodate (3 × 10−2 M). After destruction of the oxidant with 1 M ethylene glycol, aldehyde groups were reduced (18 h at 4°C) with an ice-cold solution of NaB3H4 (0.46 GBq, 481 GBq/mmol) in 200 μl of ice-cold borate buffer (0.05 M, pH 9.5). Excess sodium borohydride was destroyed with 5 μl of acetic acid. After the LPS was washed (centrifugation at 100,000 × g for 15 min) twice in 400 μl of an ice-cold water-ethanol mixture (1:1 [vol]), the radiolabeled 3H-LPS (9 × 105 cpm/μg; 2 × 103 cpm/pmol) was stored at −20°C until use. Nitric oxide production induced by 2 and 5 ng/ml of this radiolabeled material in mouse macrophages was not significantly different from that induced by the same concentrations of unlabeled LPS, indicating that the bioactivity of the LPS was not modified by the radiolabeling procedure.
Radiolabeled mouse CD14.
The Iodogen method of Greenwood et al. (13) was used. A freshly prepared solution (60 μl) of 1,3,4,6-tetrachloro-3α,6β-diphenylglycouril (Iodogen; 1 mg/ml in chloroform) was evaporated under vacuum in a glass tube. NaCl (0.15 M; 30 μl), rmCD14 (1 mg/ml in 0.15 M NaCl; 5 μl), and Na125I (3.9 MBq; 5 μl) were sequentially added to the Iodogen-coated tube. After incubation for 10 min at room temperature, the solution was transferred to a polypropylene tube containing dithiothreitol (2 mg/ml in 0.15 M NaCl; 7.5 μl). Radioiodinated rmCD14 was separated from the unreacted Na125I by chromatography on a Sephadex G-100 column (0.8 by 18 cm) preequilibrated with 0.15 M NaCl containing 60 μg of bovine serum albumin (BSA) per ml and eluted with the same solution. Fractions (0.5 ml) were collected and assessed for 125I by counting on an automatic gamma counter (1275 Minigamma; LKB). The specific activity of 125I-labeled CD14 was 5.9 × 106 cpm/μg, and its purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
Mouse surfactant.
Crude surfactant was isolated from the bronchoalveolar lavage specimens of 5- to 10-week-old Swiss mice; surfactant was isolated on an NaCl/NaBr density gradient by the method of Katyal et al. (21). The different surfactant components were prepared by a modification of the method of Beers (6). A suspension of crude surfactant (2.5 mg) was extracted (1 h, 4°C) in 2.5 ml of a mixture containing chloroform, methanol, and 1 M HCl (60:40:0.1 [vol]). After centrifugation (10 min, 12,000 × g), the hydrophilic components SP-A and SP-D in the pellet were purified by chromatography on a Biogel P-60 column (20 ml) eluted with apyrogenic water. The organic solvents of the supernatant were evaporated. After sonication of the dried residue in a solution of 5 mM Tris-HCl and 75 mM NaCl (pH 7.4) and removal of phospholipids by extraction with a mixture of diisopropylether-1-butanol (3:2 [vol]), the hydrophobic surfactant components at the interphase were isolated. Solvents were evaporated, and the dried residue was extracted with 2.5 ml of an ethanol-diethyl ether mixture (1:3 [vol]). SP-B was recovered in the insoluble material isolated after centrifugation (15 min, 12,000 × g) and reextraction with the same solvent. SP-C was purified from the supernatant as described previously (3). The purity of the different surfactant components was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. For experiments of incubation with bone marrow cells (BMC), dried mixtures (sterilized under UV light) of purified surfactant components (2 μg) and dipalmitoylphosphatidylcholine (18 μg) were sonicated in 1 ml of CM, and 100-μl samples of these suspensions were added to the cells in a final volume of 250 μl.
FACS analysis of the binding of LPS to cellular CD14.
Bone marrow cells (BMC) collected from femurs of C3H/HeOU mice (5 × 105 cells in 125 μl of CM) were induced to express CD14 by incubation (18 to 24 h, 37°C) with LPS from S. enterica serovar Choleraesuis (10 ng/ml in 125 μl of CM). The cultures were then maintained for 1 h at 4°C and reincubated (18 h, 4°C) with FITC-LPS (0.2 μg/ml in 250 μl of CM), alone, or in the presence of serum or surfactant components. The cells were then layered on a 50% FCS solution and centrifuged, and the cell pellet was resuspended in 0.5 ml of staining buffer (phosphate-buffered saline, 5% FCS, 0.02% sodium azide) containing propidium iodide (0.2 μg/ml) to stain dead cells. Viable (propidium iodide-negative) cells with high numbers of LPS-binding sites (LpsR+ cells) were detected by analysis (5,000 cells per sample) on a fluorescence-activated cell sorter (FACS) flow cytometer (FACScan; Becton-Dickinson Electronic Laboratories, Mountain View, Calif.) using Cell Quest Software. Cells with a fluorescence intensity higher than the maximal level of autofluorescence (channel 222 with the gain setting used) were scored as LpsR+ cells.
RESULTS
Pulmonary mouse surfactant enhances the binding of LPS to BMC expressing CD14.
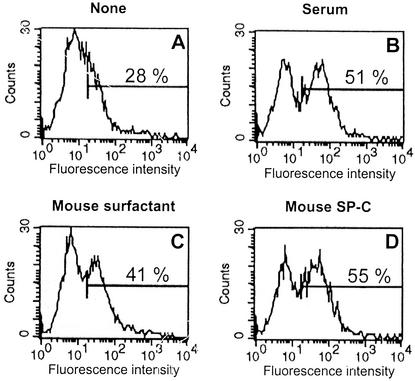
Our previous observations showing an interaction between LPS and the lung surfactant component SP-C lead us to try to evaluate the biological importance of this interaction. Because CD14 is the first cellular target of LPS, it was important to determine if SP-C can influence the binding of LPS to cells expressing CD14. We used a system consisting of fluorescent LPS (FITC-LPS) and BMC. We have shown previously that BMC do not constitutively express membrane CD14, and thus, unstimulated BMC do not bind FITC-LPS. However, after stimulation with different agents, including low concentrations of LPS, the expression of CD14 is induced on the membranes of bone marrow granulocytes (BMG). We have also shown previously that in BMG, FITC-LPS binds exclusively to an inducible glycosylphosphatidylinositol-anchored molecule (29) that we identified further as CD14 (30). This binding to CD14 occurs exclusively in the presence of serum or serum component LBP (12, 30). Therefore, we prepared LPS-stimulated BMC containing the granulocyte population expressing the LPS receptor CD14 by the method described previously (30), and we used these cells to analyze the influence of mouse surfactant and its protein components on the binding of FITC-LPS. The results in Fig. 1 confirm that FCS enhances the binding of FITC-LPS to the cells compared to cells incubated in the absence of serum (51% versus 28% of fluorescent cells) (Fig. 1A and B). The mean fluorescence and the number of fluorescent cells were both increased. Enhanced binding of FITC-LPS to BMC was also found when complete mouse surfactant (protein content, 800 ng/ml) was used instead of serum. An increase of 13% of the number of fluorescent cells was observed (Fig. 1C). When purified mouse SP-C was used at the same protein concentration (800 ng/ml) and in the presence of the same amount of lipids as in complete surfactant, the effect was even higher (27% increase in the number of fluorescent cells) (Fig. 1D). In the presence of void vesicles of dipalmitoylphosphatidylcholine (without SP-C), the binding of FITC-LPS to the cells was not modified (data not shown). These results indicate that SP-C, like LBP, allows an interaction between FITC-LPS and BMG expressing CD14.
FIG. 1.
Influence of serum and mouse surfactant on the binding of FITC-LPS to BMC. BMC (5 × 105 cells) from C3H/HeOU mice, pretreated (18 h, 37°C) with LPS from S. enterica serovar Choleraesuis (10 ng/ml) in serum-free CM, were reincubated (18 h, 4°C) with FITC-LPS (0.2 μg/ml) in medium alone (A) or in medium containing 8% FCS (B), mouse surfactant (800 ng of proteins per ml) (C), or mouse SP-C (800 ng of proteins per ml) (D). Histograms represent fluorescence analyzed by FACS on the gated granulocyte population of viable (propidium iodide-negative) cells. The mean fluorescence of the fluorescent population is indicated in each panel. Data are from one representative experiment.
The serum-like activity of mouse surfactant is mainly due to its SP-C component.
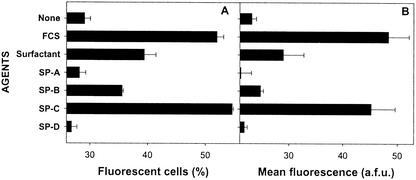
In a second experiment, we compared the influence of SP-C to those of the three other protein components of mouse surfactant (SP-A, SP-B, and SP-D). We found that SP-C is clearly the most efficient surfactant component for this effect (Fig. 2). A moderate activity was also observed with SP-B, whereas SP-A and SP-D were inactive.
FIG. 2.
Comparison of the influence of different mouse surfactant proteins on the binding of FITC-LPS to BMC. BMC (5 × 105 cells) from C3H/HeOU mice, pretreated (18 h, 37°C) with LPS from S. enterica serovar Choleraesuis (10 ng/ml) in serum-free CM, were reincubated (18 h, 4°C) with FITC-LPS (0.2 μg/ml) in the presence of different protein components (800 ng of proteins per ml) isolated from mouse surfactant. Crude mouse surfactant (800 ng of proteins per ml) and FCS (8%) were also used for comparison. Histograms represent the percentage of fluorescent cells (A) and the mean fluorescence (B) determined by FACS on the gated granulocyte population of viable (propidium iodide-negative) cells. Data are the arithmetic mean of duplicates, in one representative experiment. a.f.u., arbitrary fluorescence units.
Interaction of mouse SP-C with recombinant CD14 and inhibition by LPS.
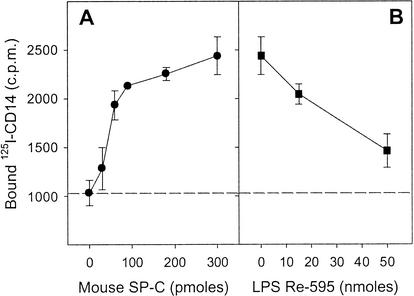
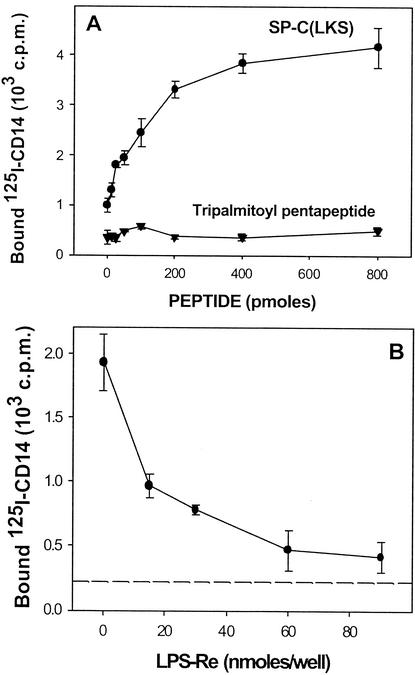
The results mentioned above suggested that SP-C, which binds LPS, can carry or transfer this LPS to cellular CD14. There must be an interaction between SP-C and CD14 for SP-C to carry LPS to CD14. To evaluate this, we used radiolabeled rmCD14, and we analyzed its interaction with mouse SP-C in an acellular system. Polypropylene wells, resistant to organic solvents, were coated with various amounts of mouse SP-C by evaporation of a chloroform-methanol solution of the protein, and the coated plate was incubated for 2 h at 20°C with 125I-labeled CD14. The results in Fig. 3A show binding of 125I-labeled CD14, which is dependent on the amount of SP-C coating the wells. This indicates that mouse SP-C binds directly to CD14. Because mouse SP-C also binds to LPS (3), it was interesting to examine the influence of LPS on the interaction between SP-C and CD14. Polypropylene wells coated with a constant (300 pmol) amount of mouse SP-C were thus preincubated (2 h, 20°C) with various concentrations of the S. enterica serovar Minnesota Re595 LPS. After the wells were washed, the binding of 125I-CD14 was analyzed as described above. We found (Fig. 3B) that the preincubation with LPS induced a dose-dependent inhibition of the binding of 125I-CD14 to mouse SP-C. This result suggests that LPS and CD14 interact with a common binding site of SP-C.
FIG. 3.
Interaction of 125I-labeled CD14 and LPS with mouse SP-C. Polypropylene plates were coated with various amounts (0 to 300 pmol) (A) or with a fixed amount (300 pmol) (B) of mouse SP-C by evaporation of solutions of the peptide in chloroform-methanol (1:1 [vol]). A solution (100 μl) of 125I-CD14 (30,000 cpm) in 0.15 M NaCl containing BSA (600 μg/ml) alone (A) or preincubated (2 h, 20°C) with various concentrations of S. enterica serovar Minnesota Re595 LPS (B) was added to the SP-C-coated wells. After the wells were incubated for 2 h at 20°C and washed three times, the bound radioactivity was measured. The broken line represents the nonspecific binding of 125I-CD14 to the surface of uncoated wells. The results are the means ± standard deviations of three wells from one representative experiment.
The synthetic SP-C analog SP-C(LKS) behaves like mouse SP-C.
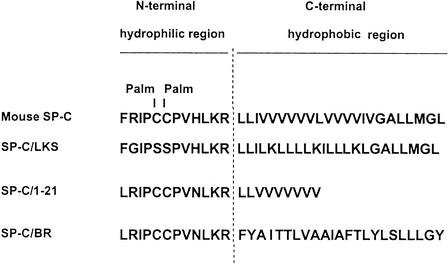
To ensure that in the experiments described above, the binding of 125I-labeled CD14 is not due to some undetectable contaminant of mouse SP-C, we used the synthetic peptide SP-C(LKS), which is a nonpalmitoylated analog of mouse SP-C. The sequences of SP-C and SP-C(LKS) are shown in Fig. 4. The molecular masses of SP-C and SP-C(LKS) are 4,255 and 3,832 kDa, respectively. We have shown recently (4) that SP-C(LKS) interacts with LPS as efficiently as native mouse SP-C. Using the binding technique described above, with polypropylene wells coated with SP-C(LKS), we found that 125I-CD14 also binds to this synthetic peptide (Fig. 5A). In contrast, the labeled ligand did not bind to an unrelated palmitoylated peptide (tripalmitoyl pentapeptide), thus indicating that binding to murine SP-C and to the synthetic SP-C(LKS) is specific. Again, this binding was markedly inhibited by the presence of the S. enterica serovar Minnesota Re595 LPS.
FIG. 4.
Sequences of natural and synthetic analogs of SP-C used. Palmitoyl residues (Palm) are indicated.
FIG. 5.
Interaction of 125I-labeled CD14 and LPS with the synthetic analog SP-C(LKS). Polypropylene plates were coated with various amounts (0 to 300 pmol) (A) of SP-C(LKS) or tripalmitoyl pentapeptide or with a fixed amount (300 pmol) (B) of SP-C(LKS) by evaporation of solutions of the peptides in chloroform-methanol (1:1 [vol]). The plates were preincubated (2 h, 20°C) with 90 μl of BSA (667 μg/ml in 0.15 M NaCl) in the absence (A) or presence (B) of various concentrations of S. enterica serovar Minnesota Re595 LPS. 125I-CD14 (30,000 cpm; 10 μl in 0.15 M NaCl) was then added, and the plates were reincubated for 2 h at 20°C. After the wells were washed five times with saline, bound radioactivity was measured. The broken line represents the nonspecific binding of 125I-CD14 to the surface of uncoated wells. The results are the means ± standard deviations of three wells from one representative experiment.
Comparison of different synthetic analogs of SP-C.
We then compared the capacity of three synthetic analogs of SP-C to bind 125I-labeled CD14 and the ability of LPS to block this interaction. We used SP-C(LKS), SP-C(1-21), and SP-C/BR (Fig. 4). Using the binding and inhibition techniques mentioned above, we found that both SP-C(LKS) and SP-C/BR have a common binding site for CD14 and LPS (Fig. 6). In contrast, SP-C(1-21) did not bind CD14. This ability of the synthetic SP-C analogs to bind 125I-CD14 correlates with their previously reported capacity to bind LPS (3): in that report, the LPS-binding capacities of 400 pmol of SP-C(LKS), SP-C(1-21), and SP-C/BR were 77, 5, and 75%, respectively. This correlation confirms that CD14 and LPS interact with the same region of SP-C.
FIG. 6.
Interaction of 125I-labeled CD14 and LPS with different synthetic analogs of SP-C. Solutions of 125I-CD14 (30,000 cpm in 100 μl of 0.15 M NaCl containing 60 μg of BSA) were preincubated in glass tubes (2 h, 20°C) in the absence or presence of S. enterica serovar Minnesota Re595 LPS (50 nmol). The mixtures were then added to polypropylene wells coated with 300 pmol of different SP-C analogs, and the plates were reincubated for 2 h at 20°C. After the wells were washed five times with saline, bound radioactivity was measured. The results are the means ± standard deviations of three wells from one representative experiment.
SP-C(LKS) enhances the specific interaction between LPS and recombinant CD14.
The inhibition of the interaction between SP-C and CD14 induced by LPS seemed to contradict the result of Fig. 1D showing that SP-C triggers an interaction between LPS and cell surface CD14. Therefore, we reexamined the results of Fig. 1D in an acellular system and with a synthetic analog of SP-C. We used polystyrene wells coated with the S. enterica serovar Minnesota Re595 LPS and saturated with BSA. A solution of 125I-labeled CD14 preincubated (2 h, 20°C) with various concentrations of SP-C(LKS) was added to uncoated or LPS-coated wells, and the plate was incubated for 2 h at 20°C. The specific binding of 125I-CD14 to LPS was calculated as the difference of radioactive material bound to LPS-coated and uncoated wells. The results in Fig. 7 show a binding of 125I-CD14 to LPS which is dependent on the amount of SP-C(LKS). This result confirms the observations of Fig. 1D and Fig. 2 showing that the interaction between CD14 and LPS requires an additional molecule and that SP-C(LKS) can play this role.
FIG. 7.
Influence of SP-C(LKS) on the interaction between 125I-labeled CD14 and LPS. Polystyrene plates were coated with LPS by incubation (18 h, 37°C) with suspensions (150 μl) of the S. enterica serovar Minnesota Re595 LPS (20 μg/ml in Tris buffer [pH 9.6]). The plastic surface was then saturated with BSA (1 mg/ml, 1 h, 37°C) and washed. A solution (100 μl) of 125I-CD14 (30,000 cpm in 0.15 M NaCl containing 600 μg of BSA per ml), alone or preincubated (2 h, 20°C) with various concentrations of SP-C(LKS), was added to uncoated or LPS-coated wells. After the wells were incubated for 2 h at 20°C and washed five times, the bound radioactivity was measured. Specific binding of 125I-CD14 was the calculated difference of radioactive material bound to LPS-coated and uncoated wells. The results are the mean ± standard deviations of three wells from one representative experiment.
DISCUSSION
A growing body of evidence suggests for most epithelial cells a role as sensors for microorganisms (19) and a capacity to produce molecules with antimicrobial properties (26). The main cells lining the lung alveolus are the thin epithelial type I cells and the bulky epithelial type II cells. The type II cells are key cells of the alveolus because they secrete the components of pulmonary surfactant and, after alveolar injury, will divide to form more type I cells. They can also play an immunological role by producing proteins that cause neutrophil chemotaxis and surfactant proteins that bind to alveolar macrophages, type II alveolar cells, and microorganisms that have entered the alveolus. Of the different bacterial components, LPS is one of the most active in the host. Recent work in this laboratory (3, 4) has demonstrated a new feature of the surfactant component SP-C, its ability to interact with LPS. This opens new perspectives for a possible role of SP-C in innate immunity, since by binding to LPS, SP-C may affect the interaction of this molecule with cellular receptors and thus influence the cell signaling reactions and the ensuing pathophysiological cascade.
Because CD14, which has been identified as a cell surface binding site for LPS (40), enhances the LPS signal mediated by the TLR4-MD2 complex (1), we analyzed how SP-C, CD14, and LPS interact together. Our data indicate that mouse SP-C and the two synthetic analogs SP-C(LKS) and SP-C/BR interact with rmCD14. Converging evidence show that CD14 and LPS interact with the same region of the SP-C molecule. First, LPS inhibits the binding of CD14 to SP-C. Second, the palmitoyl chains of SP-C, shown previously to be unnecessary for LPS binding (4), are also not required for CD14 binding, since the two synthetic peptides which bind to CD14 are not palmitoylated. Third, the synthetic peptide SP-C(1-21) which previously exhibited a reduced capacity to bind LPS (4) is also almost unable to bind CD14 (Fig. 6).
Once it was established that LPS binds to SP-C and that CD14 binds to SP-C, it may be expected that CD14 should bind to the LPS/SP-C complex by interacting with its two components, and it may appear paradoxical that the presence of LPS inhibits the binding of CD14. To understand this, we should note that in the absence of a “presenting” molecule (LBP or SP-C), CD14 alone does not bind efficiently to LPS. This is clearly shown in cellular (Fig. 1A) and acellular (Fig. 7) experiments. Only LBP/CD14 or SP-C/CD14 complexes can recognize LPS. For example, in the absence of SP-C, there was no detectable specific binding of CD14 to LPS-coated wells (Fig. 7). This explains why LPS, when attached to SP-C on the wells, is not recognized by CD14. Actually, this attachment even blocks the interaction between CD14 and SP-C (Fig. 3B and 5B). One explanation is that on SP-C, the LPS-binding site and the CD14-binding site are very close to each other, so that the binding of LPS causes a steric hindrance of the other adjacent site. However, this is unlikely because the hydrophilic site of SP-C, which binds LPS according to our previous study (4), is rather short (12 amino acids) and can hardly contain two distinct binding sites (one for SP-C and another for CD14). Another explanation is that the two binding sites are actually identical. Actually, these two possibilities can be grouped in a more general interpretation stating that LPS and CD14 interact with the same “region” of SP-C.
The competition between LPS and CD14 for binding to SP-C cannot be explained by a structural analogy between these molecules: indeed, our previous study (4) demonstrated that SP-C essentially reacts with the α-1-phosphate group of lipid A and that an ester-linked fatty acid of this ligand may also play a role in the recognition. In contrast, the rmCD14 used lacks phosphate and fatty acids, as the glycosylphosphatidylinositol moiety which carries such residues is not present in the recombinant soluble form of CD14 used in our study. In view of this apparent absence of structural analogy between LPS and CD14, our hypothesis is that CD14 does not represent the ligand of SP-C but its receptor. Actually, this is not very surprising inasmuch as CD14 is now considered a pattern recognition receptor (24, 33) which, in addition to LPS, is able to recognize a number of ligands such as peptidoglycan (14), polyuronic acids of Pseudomonas (17), rhamnose-glucose polymers of Streptococcus mutans (37), lipoteichoic acid of Staphylococcus aureus (16), lipoarabinomannan from mycobacteria (42), and an outer membrane lipoprotein of Borrelia burgdorferi (39). Therefore, it is conceivable that the pattern recognition receptor region of CD14, possibly its leucine-rich repeat region (11), which in general is a versatile binding motif (23), interacts with the LPS-binding region of SP-C, thus explaining LPS inhibition of this interaction.
Another important observation of this study is that in the presence of SP-C, the interaction of LPS with soluble CD14 (Fig. 7) or with cells expressing membrane CD14 (Fig. 1D) is enhanced. This may indicate that the interaction between SP-C and CD14 modifies the conformation of the latter or dissociates LPS-nonreactive homodimers of CD14 and thus allows LPS-CD14 interactions. This is reminiscent of the effect of LBP, which helps LPS to bind to CD14. However, the two mechanisms are likely different because LBP acts in a catalytic fashion as a shuttle which transfers LPS to CD14 (41), whereas the result of Fig. 7 suggest that this is not the case with SP-C which must be present in noncatalytic amounts. This also suggests a ternary interaction of SP-C with CD14 and LPS and is thus reminiscent of the effect of SP-A, which has been shown to associate with LPS and CD14 (36).
A second analogy between the effects of SP-C and SP-A is that these proteins enhance the binding of a rough-type LPS to CD14, whereas SP-D inhibits this binding (36). In addition to SP-C, SP-A, and SP-D, the existence of other molecules able to bind both LPS and CD14 has been reported. In particular, human lactoferrin, an iron-binding glycoprotein released from neutrophil granules during inflammation, binds to the lipid A region of LPS (2) and to the membrane and soluble forms of CD14 (10, 5). Therefore, proteins belonging to structurally different families can, in addition to their normal function, bind LPS and CD14, which is likely relevant in innate immunity.
Because CD14 is a pattern recognition molecule which plays a central role in inflammation, our observation of an interaction between SP-C and CD14 opens the way to investigate the immunological role of SP-C during lung inflammatory processes induced by a panel of microorganisms including bacteria other than gram-negative bacteria.
Acknowledgments
This work was supported in part by grants from the Direction des Systèmes de Forces et de la Prospective (contract 99.34.033), the Pasteur Institute (grant 3540), the Swedish Research Council (project 10371), and the Anérs Foundation.
Editor: J. D. Clements
REFERENCES
- 1.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., Y. Q. An, M. Geerts, B. G. Thijs, H. A. de Boer, D. M. MacLaren, J. de Graaff, and J. H. Nuijens. 1994. Lactoferrin is a lipid A-binding protein. Infect. Immun. 62:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augusto, L., K. Le Blay, G. Auger, D. Blanot, and R. Chaby. 2001. Interaction of bacterial lipopolysaccharide with mouse surfactant protein C inserted into lipid vesicles. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L776-L785. [DOI] [PubMed] [Google Scholar]
- 4.Augusto, L. A., J. Li, M. Synguelakis, J. Johansson, and R. Chaby. 2002. Structural basis for interactions between lung surfactant protein C and bacterial lipopolysaccharide. J. Biol. Chem. 277:23484-23492. [DOI] [PubMed] [Google Scholar]
- 5.Baveye, S., E. Elass, D. G. Fernig, C. Blanquart, J. Mazurier, and D. Legrand. 2000. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 68:6519-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beers, M. F., S. R. Bates, and A. B. Fisher. 1992. Differential extraction for the rapid purification of bovine surfactant protein B. Am. J. Physiol. 262:L773-L778. [DOI] [PubMed] [Google Scholar]
- 7.Bone, R. C. 1991. The pathogenesis of sepsis. Ann. Intern. Med. 115:457-469. [DOI] [PubMed] [Google Scholar]
- 8.Curstedt, T., J. Johansson, P. Persson, A. Eklund, B. Robertson, B. Lowenadler, and H. Jornvall. 1990. Hydrophobic surfactant-associated polypeptides: SP-C is a lipopeptide with two palmitoylated cysteine residues, whereas SP-B lacks covalently linked fatty acyl groups. Proc. Natl. Acad. Sci. USA 87:2985-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, A. J. 1994. The C-type carbohydrate recognition domain (CRD) superfamily. Biochem. Soc. Trans. 22:83-88. [DOI] [PubMed] [Google Scholar]
- 10.Elass-Rochard, E., D. Legrand, V. Salmon, A. Roseanu, M. Trif, P. S. Tobias, J. Mazurier, and G. Spik. 1998. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 66:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrero, E., C. L. Hsieh, U. Francke, and S. M. Goyert. 1990. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J. Immunol. 145:331-336. [PubMed] [Google Scholar]
- 12.Girard, R., T. Pedron, and R. Chaby. 1993. Endotoxin-induced expression of endotoxin binding sites on murine bone marrow cells. J. Immunol. 150:4504-4513. [PubMed] [Google Scholar]
- 13.Greenwood, F. C., W. Hunter, and J. Glover. 1963. The preparation of 131I-labeled human growth hormone of high specific radioactivity. Biochem. J. 89:114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, D., T. N. Kirkland, S. Viriyakosol, and R. Dziarski. 1996. CD14 is a cell-activating receptor for bacterial peptidoglycan. J. Biol. Chem. 271:23310-23316. [DOI] [PubMed] [Google Scholar]
- 15.Haagsman, H. P., and L. M. van Golde. 1991. Synthesis and assembly of lung surfactant. Annu. Rev. Physiol. 53:441-464. [DOI] [PubMed] [Google Scholar]
- 16.Hattor, Y., K. Kasai, K. Akimoto, and C. Thiemermann. 1997. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem. Biophys. Res. Commun. 233:375-379. [DOI] [PubMed] [Google Scholar]
- 17.Jahr, T. G., L. Ryan, A. Sundan, H. S. Lichenstein, G. Skjak-Braek, and T. Espevik. 1997. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect. Immun. 65:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson, J., and T. Curstedt. 1997. Molecular structures and interactions of pulmonary surfactant components. Eur. J. Biochem. 244:675-693. [DOI] [PubMed] [Google Scholar]
- 19.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalina, M., H. Blau, S. Riklis, and V. Kravtsov. 1995. Interaction of surfactant protein A with bacterial lipopolysaccharide may affect some biological functions. Am. J. Physiol. 268:L144-L151. [DOI] [PubMed] [Google Scholar]
- 21.Katyal, S. L., L. W. Estes, and B. Lombardi. 1977. Method for the isolation of surfactant from homogenates and lavages of lung of adult, newborn, and fetal rats. Lab. Investig. 36:585-592. [PubMed] [Google Scholar]
- 22.King, R. J., D. J. Klass, E. G. Gikas, and J. A. Clements. 1973. Isolation of apoproteins from canine surface active material. Am. J. Physiol. 224:788-795. [DOI] [PubMed] [Google Scholar]
- 23.Kobe, B., and J. Deisenhofer. 1994. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19:415-421. [DOI] [PubMed] [Google Scholar]
- 24.Labeta, M. O., K. Vidal, J. E. Nores, M. Arias, N. Vita, B. P. Morgan, J. C. Guillemot, D. Loyaux, P. Ferrara, D. Schmid, M. Affolter, L. K. Borysiewicz, A. Donnet-Hughes, and E. J. Schiffrin. 2000. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J. Exp. Med. 191:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, B. L., J. Y. Wang, U. Holmskov, H. J. Hoppe, and K. B. Reid. 1994. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem. Biophys. Res. Commun. 202:1674-1680. [DOI] [PubMed] [Google Scholar]
- 26.McCray, P. B., Jr., and L. Bentley. 1997. Human airway epithelia express a beta-defensin. Am. J. Respir. Cell Mol. Biol. 16:343-349. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, D. C., and J. L. Ryan. 1979. Bacterial endotoxins and host immune responses. Adv. Immunol. 28:293-450. [DOI] [PubMed] [Google Scholar]
- 28.Nathan, C. F. 1987. Secretory products of macrophages. J. Clin. Investig. 79:319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedron, T., R. Girard, S. J. Turco, and R. Chaby. 1994. Phosphatidylinositol-anchored molecules and inducible lipopolysaccharide binding sites of human and mouse bone marrow cells. J. Biol. Chem. 269:2426-2432. [PubMed] [Google Scholar]
- 30.Pedron, T., R. Girard, K. Inoue, D. Charon, and R. Chaby. 1997. Lipopolysaccharide and the glycoside ring of staurosporine induce CD14 expression on bone marrow granulocytes by different mechanisms. Mol. Pharmacol. 52:692-700. [DOI] [PubMed] [Google Scholar]
- 31.Persoons, J. H., K. Schornagel, J. Breve, F. Berkenbosch, and G. Kraal. 1995. Acute stress affects cytokines and nitric oxide production by alveolar macrophages differently. Am. J. Respir. Crit. Care Med. 152:619-624. [DOI] [PubMed] [Google Scholar]
- 32.Pison, U., M. Max, A. Neuendank, S. Weissbach, and S. Pietschmann. 1994. Host defence capacities of pulmonary surfactant: evidence for “non-surfactant” functions of the surfactant system. Eur. J. Clin. Investig. 24:586-599. [DOI] [PubMed] [Google Scholar]
- 33.Pugin, J., I. D. Heumann, A. Tomasz, V. V. Kravchenko, Y. Akamatsu, M. Nishijima, M. P. Glauser, P. S. Tobias, and R. J. Ulevitch. 1994. CD14 is a pattern recognition receptor. Immunity 1:509-516. [DOI] [PubMed] [Google Scholar]
- 34.Raetz, C. R., R. J. Ulevitch, S. D. Wright, C. H. Sibley, A. Ding, and C. F. Nathan. 1991. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 5:2652-2660. [DOI] [PubMed] [Google Scholar]
- 35.Sano, H., H. Sohma, T. Muta, S. Nomura, D. R. Voelker, and Y. Kuroki. 1999. Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J. Immunol. 163:387-395. [PubMed] [Google Scholar]
- 36.Sano, H., H. Chiba, D. Iwaki, H. Sohma, D. R. Voelker, and Y. Kuroki. 2000. Surfactant proteins A and D bind CD14 by different mechanisms. J. Biol. Chem. 275:22442-22451. [DOI] [PubMed] [Google Scholar]
- 37.Soell, M., E. Lett, F. Holveck, M. Scholler, D. Wachsmann, and J. P. Klein. 1995. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-alpha release. J. Immunol. 154:851-860. [PubMed] [Google Scholar]
- 38.Watson, J., and R. Riblet. 1975. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipopolysaccharides. J. Immunol. 114:1462-1468. [PubMed] [Google Scholar]
- 39.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 40.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 41.Wurfel, M. M., E. Hailman, and S. D. Wright. 1995. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J. Exp. Med. 181:1743-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu, W., E. Soprana, G. Cosentino, M. Volta, H. S. Lichenstein, G. Viale, and D. Vercelli. 1998. Soluble CD14(1-152) confers responsiveness to both lipoarabinomannan and lipopolysaccharide in a novel HL-60 cell bioassay. J. Immunol. 161:4244-4251. [PubMed] [Google Scholar]