Abstract
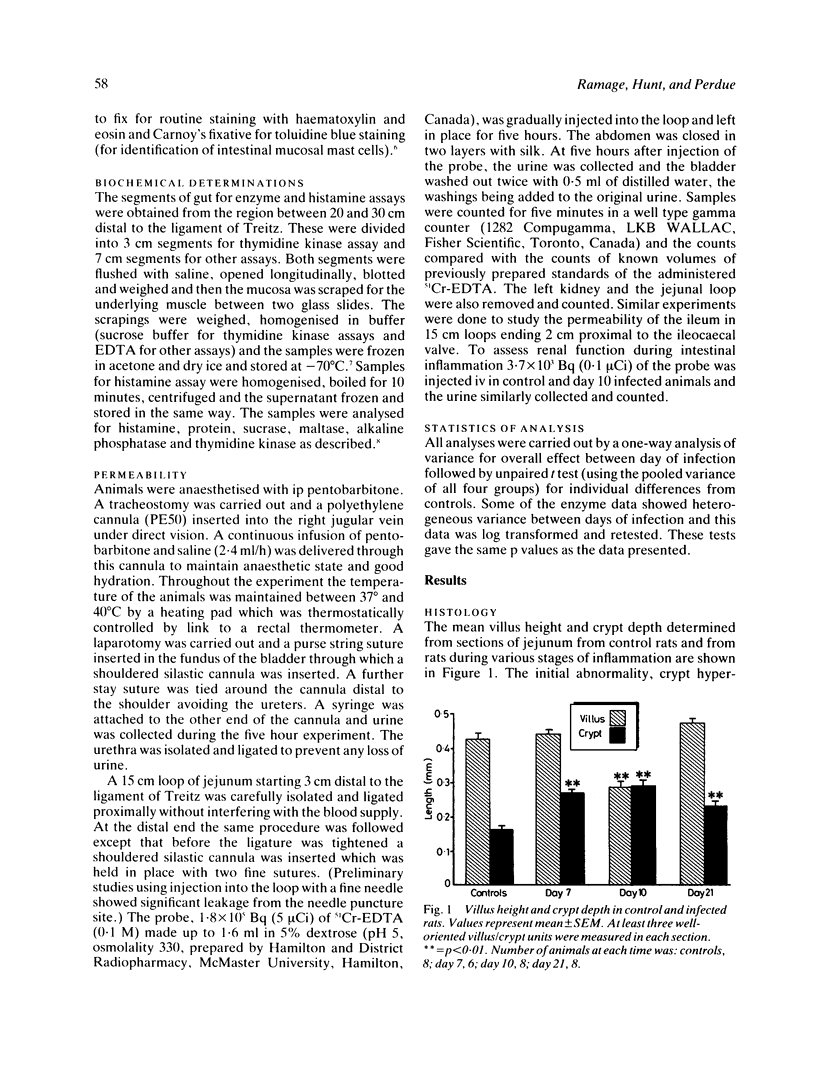
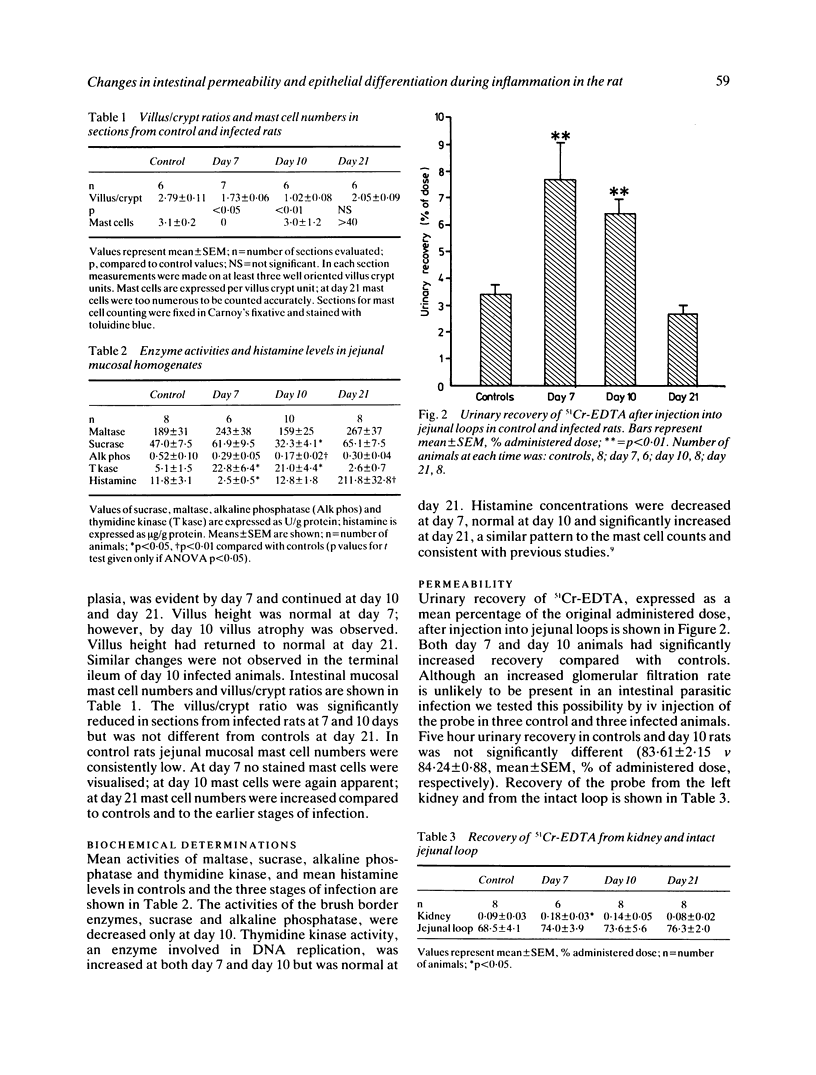
We examined changes in gut permeability in a controlled model of inflammation produced in rats after infection with the nematode parasite, Nippostrongylus brasiliensis. The probe, 51Cr-EDTA, was injected into ligated loops of jejunum in vivo and recovery of radioactivity was measured in urine, kidney, and intact loop at five hours. Urinary recovery was significantly increased during the early (day 7) and acute (day 10) stages of the infection compared with values in control rats but subsequently returned to normal. Urinary clearance of the probe after iv injection was unaltered during infection. Villus atrophy occurred only at the stage, whereas crypt hyperplasia was evident at both the early and acute stages. The terminal ileum appeared normal and showed normal permeability when compared with controls. We conclude that permeability changes are local to the site of inflammation, are reversible after healing and may be related to an increase in the proportion of relatively undifferentiated epithelium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Befus A. D., Johnston N., Bienenstock J. Nippostrongylus brasiliensis: mast cells and histamine levels in tissues of infected and normal rats. Exp Parasitol. 1979 Aug;48(1):1–8. doi: 10.1016/0014-4894(79)90048-1. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Marsh M. N., Price A., Levi A. J., Peters T. J. Intestinal permeability in patients with coeliac disease and dermatitis herpetiformis. Gut. 1985 Nov;26(11):1214–1219. doi: 10.1136/gut.26.11.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., O'Morain C., Levi A. J., Peters T. J. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983 Aug;85(2):318–322. [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Levi A. J., Peters T. J. Intestinal permeability to 51Cr-EDTA in rats with experimentally induced enteropathy. Gut. 1985 Jun;26(6):579–585. doi: 10.1136/gut.26.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecknauer R., Buck B., Breitig D. An experimental model for measuring intestinal permeability. Digestion. 1983;26(1):24–32. doi: 10.1159/000198865. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Jarrett E. E. Hypersensitivity reactions in small intestine. I Thymus dependence of experimental 'partial villous atrophy'. Gut. 1975 Feb;16(2):114–117. doi: 10.1136/gut.16.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford R. P., Menzies I. S., Phillips A. D., Walker-Smith J. A., Turner M. W. Intestinal sugar permeability: relationship to diarrhoeal disease and small bowel morphology. J Pediatr Gastroenterol Nutr. 1985 Aug;4(4):568–574. [PubMed] [Google Scholar]
- Forget P., Sodoyez-Goffaux F., Zappitelli A. Permeability of the small intestine to [51Cr]EDTA in children with acute gastroenteritis or eczema. J Pediatr Gastroenterol Nutr. 1985 Jun;4(3):393–396. doi: 10.1097/00005176-198506000-00012. [DOI] [PubMed] [Google Scholar]
- Kelly M., Butler D. G., Hamilton J. R. Transmissible gastroenteritis in piglets: a model of infantile viral diarrhea. J Pediatr. 1972 Jun;80(6):925–931. doi: 10.1016/S0022-3476(72)80003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. D., Swieter M., Befus A. D. Mast cell responses to helminth infection. Parasitol Today. 1986 Jul;2(7):186–191. doi: 10.1016/0169-4758(86)90190-0. [DOI] [PubMed] [Google Scholar]
- Marcial M. A., Carlson S. L., Madara J. L. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80(1):59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- Murray M., Jarrett W. F., Jennings F. W. Mast cells and macromolecular leak in intestinal immunological reactions. The influence of sex of rats infected with Nippostrongylus brasiliensis. Immunology. 1971 Jul;21(1):17–31. [PMC free article] [PubMed] [Google Scholar]
- Ogilvie B. M., Jones V. E. Parasitological review. Nippostrongylus brasiliensis: a review of immunity and host-parasite relationship in the rat. Exp Parasitol. 1971 Feb;29(1):138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Chung M., Gall D. G. Effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology. 1984 Mar;86(3):391–397. [PubMed] [Google Scholar]
- SYMONS L. E. KINETICS OF THE EPITHELIAL CELLS, AND MORPHOLOGY OF VILLI AND CRYPTS IN THE JEJUNUM OF THE RAT INFECTED BY THE NEMATODE NIPPOSTRONGYLUS BRASILIENSIS. Gastroenterology. 1965 Aug;49:158–168. [PubMed] [Google Scholar]
- Smith M. W. Expression of digestive and absorptive function in differentiating enterocytes. Annu Rev Physiol. 1985;47:247–260. doi: 10.1146/annurev.ph.47.030185.001335. [DOI] [PubMed] [Google Scholar]


