Abstract
We previously reported that Mycobacterium tuberculosis infection primes human alveolar macrophages (HAM) for tumor necrosis factor alpha (TNF-α)-mediated apoptosis and that macrophage apoptosis is associated with killing internalized bacilli. Virulent mycobacterial strains elicit much less apoptosis than attenuated strains, implying that apoptosis is a defense against intracellular infection. The present study evaluated the potential for phorbol myristate acetate-differentiated THP-1 cells to mimic this response of primary macrophages. Consistent with the behavior of alveolar macrophages, attenuated M. tuberculosis H37Ra and Mycobacterium bovis BCG strongly induce THP-1 apoptosis, which requires endogenous TNF. THP-1 apoptosis is associated with reduced viability of infecting BCG. In contrast, virulent wild-type M. tuberculosis H37Rv and M. bovis do not increase THP-1 apoptosis over baseline. BCG induced early activation of caspase 10 and 9, followed by caspase 3. In contrast, wild-type M. bovis infection failed to activate any caspases in THP-1 cells. BCG-induced THP-1 apoptosis is blocked by retroviral transduction with vectors expressing crmA but not bcl-2. We conclude that differentiated THP-1 cells faithfully model the apoptosis response of HAM. Analysis of the THP-1 cell response to infection with virulent mycobacteria suggests that TNF death signals are blocked proximal to initiator caspase activation, at the level of TNF receptor 1 or its associated intracytoplasmic adaptor complex. Interference with TNF death signaling may be a virulence mechanism that allows M. tuberculosis to circumvent innate defenses leading to apoptosis of infected host cells.
Alveolar macrophages infected with avirulent or attenuated strains of Mycobacterium tuberculosis undergo apoptosis in a tumor necrosis factor alpha (TNF-α)-dependent manner, in contrast to infection with virulent mycobacterial strains, which induce little or no apoptosis above background (1, 8, 9). Naive primary macrophages are resistant to TNF cytotoxicity but become primed for TNF death signals when infected with attenuated strains of M. tuberculosis and related mycobacteria. It is postulated that this apoptosis response represents an innate defense mechanism against intracellular infection. Alveolar macrophages constitute a critical growth niche for intracellular M. tuberculosis in the lung, as evidenced by the attenuation of disease after aerosol infection of mice whose macrophages were depleted by bisphosphonate liposome treatment (12). Host macrophage apoptosis, but not necrosis, is linked to killing of intracellular mycobacteria (4, 19). This suggests that programmed cell death of the host macrophage not only eliminates a preferred growth niche for M. tuberculosis but also activates a unique microbicidal mechanism.
M. tuberculosis-induced apoptosis in primary macrophages in vitro is mediated by TNF. Τhere is evidence for the involvement of caspase 9 and caspase 3 in this process (24), but little else is known about the signaling pathways that regulate the fate of infected macrophages. Mechanistic studies using primary human alveolar macrophages (HAM) are challenging because of the difficulty of obtaining these cells in large numbers and technical obstacles to genetic manipulation. In addition, primary alveolar macrophages are not a homogeneous population of cells. Cytokine production is highly variable among donors, and apoptosis does not proceed in a synchronous manner, making many experiments difficult to interpret. With this in mind, we evaluated the feasibility of using the monocytic THP-1 cells to study M. tuberculosis-induced apoptosis. Our data suggest that differentiated THP-1 cells closely model the behavior of primary HAM in this response. Experiments with M. tuberculosis-infected THP-1 cells provide evidence for activation of both caspase 10 and caspase 9 in this setting, but overexpression of bcl-2 failed to rescue infected cells from apoptosis.
MATERIALS AND METHODS
THP-1 cells.
THP-1 cells (American Type Culture Collection, Manassas, Va.) were grown in RPMI 1640 (Gibco BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, Utah), 1% HEPES, 1% l-glutamine, and 50 μg of cefotaxime (Sigma, St. Louis, Mo.)/ml. The cells were treated with 5 nM phorbol myristate acetate (PMA; Sigma) overnight and then washed three times. Cells were rested 3 days following chemical differentiation to ensure that they reverted to a resting phenotype before infection.
M. tuberculosis.
Cultures of wild-type Mycobacterium bovis, M. bovis bacillus Calmette-Guérin (BCG), M. tuberculosis H37Ra, and H37Rv were obtained from the American Type Culture Collection. Frozen cultures were grown to log phase in Middlebrook 7H9 broth (Becton Dickinson, Sparks, Md.). Prior to infection, 1-ml cultures of each mycobacterial strain were pelleted for 2 min, resuspended in RPMI 1640, vortexed for 2 min, and sonicated in a bath sonicator (Laboratory Supplies, Inc., Hicksville, N.Y.) for 5 min. Following sonication, dispersed bacterial suspensions were allowed to stand for 5 min, and the upper 500 μl was used in subsequent infections. To ensure an infection ratio of 5 to 10 bacilli per macrophage, multiplicities of infection (MOI) were determined by adding dilutions of prepared bacilli to 5 × 105 differentiated THP-1 cells, infecting for 4 h, and performing an acid-fast stain to count cell-associated mycobacteria by light microscopy (×100).
Cell death ELISA.
THP-1 cells differentiated with PMA were plated in 96-well flat-bottom plates (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) at 2 × 105 cells per well and allowed to adhere overnight. Dispersed bacilli were incubated with cells for 4 h (37°C, 5% CO2). The cells were then washed thoroughly with phosphate-buffered saline (PBS) and then incubated for 1 to 5 days as indicated in the figure legends. Where noted in Fig. 2, 5 μg of anti-TNF antibody (R&D Systems, Miles, Calif.)/ml or 100 μg of pentoxifylline (Sigma)/ml was added concurrently with M. tuberculosis to the cells. The cell death enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, Inc., Indianapolis, Ind.) was performed according to the manufacturer's protocol, and plates were analyzed with a MAXline microplate reader and SOFTmax system (Molecular Devices Corp., Menlo Park, Calif.). To compare individual experiments, data were analyzed by setting the basal level of apoptosis to 1.0, based on the optical density (OD) value for uninfected cells. All other OD values within an experiment were divided by the uninfected-cell OD to provide a relative apoptosis value over baseline in arbitrary units. The means and standard deviations were determined for multiple experiments, and differences between conditions were analyzed by using Student's t test. P values <0.05 were considered significant.
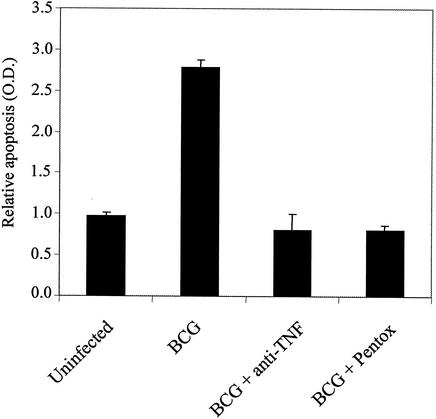
FIG. 2.
Nuclear fragmentation ELISA of THP-1 cells uninfected or infected with BCG alone, BCG plus neutralizing monoclonal anti-TNF antibodies (anti-TNF), or BCG plus the TNF inhibitor pentoxifylline (pentox). Data are means plus standard deviations.
DAPI staining.
THP-1 cells differentiated with PMA were plated into two-chamber LabTec slides (Nunc, Inc., Naperville, Ill.) at 5 × 105 cells per well and allowed to adhere overnight. Medium was changed daily until the cultures were harvested at the times noted in figure legends. Where noted in Fig. 3, 100 U of recombinant TNF (rTNF; R&D Systems)/ml was added. Cells were then fixed in 4% paraformaldehyde (Sigma) for 5 min at room temperature and incubated with 50 μg of DAPI (4,6-diamidino-2-phenylindole) nuclear stain (gift from Z. J. Xiao)/ml for 30 min at room temperature in the dark. Slides were subsequently washed twice in PBS and twice in sterile water, and then the chambers were removed and coverslips were added. Slides were analyzed by fluorescence microscopy, and digital images were obtained with OpenLab software (Improvision, Inc., Lexington, Mass.).
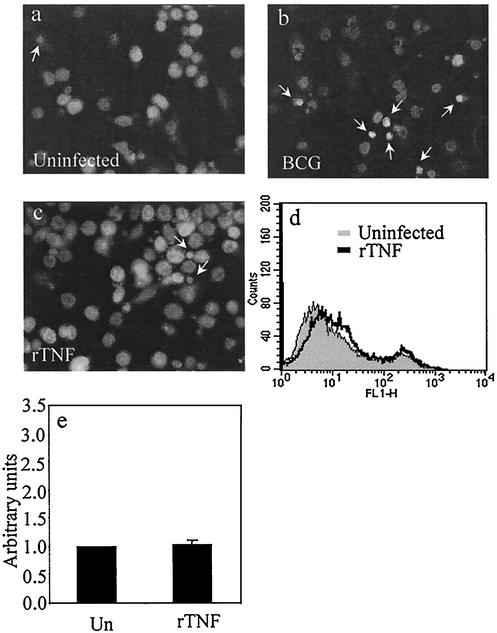
FIG. 3.
DAPI nuclear staining of uninfected THP-1 cells (a), THP-1 cells infected with BCG for 5 days (b), and uninfected THP-1 cells treated with 100 U of rTNF/ml (c). Arrows, condensed nuclei of apoptotic cells. (d) Annexin V-FITC staining of uninfected (gray) and rTNF-treated (black overlay) THP-1 cells. (e) Nuclear fragmentation ELISA of THP-1 cells unstimulated (Un) or treated with 100 U of rTNF/ml for 3 days. Data are means plus standard errors of four experiments (P = 0.530 by Student's t test).
Annexin V staining.
THP-1 cells differentiated with PMA were plated into 24-well flat-bottom plates (Falcon) at 5 × 105 cells per well, incubated 4 h with BCG (MOI of 5 to 10), and then washed thoroughly. Cells were harvested on successive days and then washed twice with PBS and resuspended in 400 μl of annexin V staining buffer (BD PharMingen, Los Angeles, Calif.). One hundred microliters of cell suspension was transferred to new tubes, and 5 μl of fluorescein isothiocyanate (FITC)-conjugated annexin V and 2 μl of propidium iodide were added. Green fluorescent protein (GFP)-positive PINCO-infected cells (see below) were stained with 5 μl of phycoerythrin (PE)-conjugated annexin V. Cells were incubated in the dark for 15 min, and then 400 μl of staining buffer was added. Stained cells were immediately analyzed with a Becton Dickinson FACScan and further analyzed with CellQuest software (Becton Dickinson).
Colony counting.
To quantify M. tuberculosis CFU, THP-1 cells were plated and infected as described above. At days 0, 3, and 5, infected cells were trypsinized and washed with PBS, homogenized in a Dounce homogenizer, and then plated on Middlebrook 7H11 agar plates supplemented with 100 μg of cycloheximide (Sigma)/ml. Cycloheximide was added to inhibit growth of possible contaminants during the extended incubation necessary to achieve mycobacterial colony growth (7). Colonies were counted after 2 weeks of growth at 37°C.
Retroviral vectors.
The PINCO retroviral vector (gift from G. Nolan, Stanford University) was used for transduction of THP-1 cells to express CrmA or Bcl-2. The crmA cDNA (gift from D. J. Pickup, Duke University) and the bcl-2 cDNA (gift from D. Hockenbery, University of Washington) were subcloned into the PINCO vector, and the constructs were confirmed by DNA sequence analysis. Phoenix cells (gift from G. Nolan) were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine. At 18 to 24 h prior to transfection, Phoenix cells were plated at 1.5 × 106 cells per 6-cm-diameter plate (Falcon). Cells were transfected with 5 μg of PINCO-crmA, PINCO-bcl-2, or PINCO vector alone by the calcium phosphate method described by Grignani et al. (6). At 24 h after transfection, media were changed, and plates were incubated at 32°C for an additional 24 h. At 48 h posttransfection, supernatants containing virus were harvested and filtered through 0.45-μm-pore-size filters and frozen in 5-ml aliquots at −75°C.
PINCO viral infection.
Undifferentiated THP-1 cells (106) were plated in 48-well plates (Falcon) and incubated with 1 ml of thawed viral supernatants supplemented with 4 μl of 4-mg/ml Polybrene. Plates were centrifuged twice (300 × g, 45 min) to increase infection efficiency (as indicated in optimization protocols provided by the Nolan laboratory), incubated at 32°C overnight, and then placed at 37°C. At 72 h postinfection, THP-1 cells were visualized by fluorescence microscopy for GFP expression and differentiated with PMA for experiments. For data presentation, results were graphed only for GFP+ cells gated “on” prior to annexin V-PE analysis.
Caspase activation.
PMA-differentiated THP-1 cells (2 × 106) were plated in six-well dishes (Corning) and incubated with BCG, M. bovis, H37Ra, or H37Rv to achieve an MOI of 5. Cells were washed at 4 h, and cell lysates were harvested at 12 h, after infection with Triton X lysis buffer (MBL, Watertown, Mass.). Caspase activation was measured by using para-nitroanilide (pNA)-conjugated specific substrates for caspase 3 (DEVD-pNA), caspase 8 (IETD-pNA), caspase 9 (LEHD-pNA), and caspase 10 (AEVD-pNA) (MBL) in accordance with the manufacturer's instructions. Cleaved substrates were quantified by reading OD at 405 nm on a MAXline microplate reader and analyzed by the SOFTmax system (Molecular Devices Corp.).
RESULTS
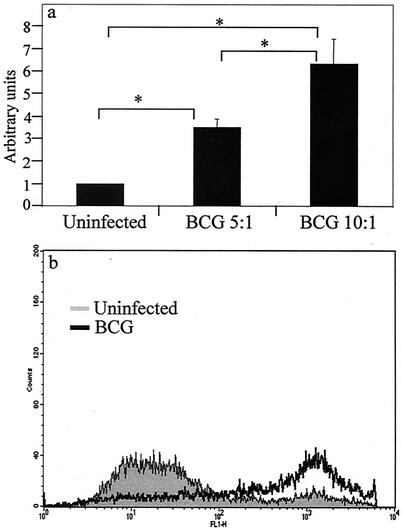
Initial experiments tested the feasibility of using THP-1 cells as an in vitro model of mycobacterium-mediated macrophage apoptosis. Previously published data from our laboratory showed that attenuated strains including BCG induce apoptosis in primary HAM (9). THP-1 cells differentiated by treatment with 5 nM PMA for 18 h were washed and rested for 3 days prior to infection with BCG. The number of cell-associated bacilli was determined by acid-fast staining 3 h after infection. The amount of THP-1 cell apoptosis, as measured by nucleosomal-particle ELISA, increased in a dose-dependent manner with increasing MOI (Fig. 1a). BCG-induced apoptosis was confirmed by annexin V staining of infected THP-1 cells, with results qualitatively similar to those obtained with the nucleosomal-particle ELISA (Fig. 1b).
FIG. 1.
(a) Nuclear fragmentation ELISA from THP-1 cells 3 days after infection with avirulent BCG at an infectious dose of 5 or 10 bacilli per macrophage. Data are means plus standard errors of four experiments. ∗, statistically significant difference between groups (P < 0.05). (b) Annexin V-FITC staining of THP-1 cells uninfected (gray) or infected with BCG (black overlay). At 5 days postinfection, 72% of cells were apoptotic.
Infection-induced apoptosis in primary macrophages is mediated by TNF (8). In order for THP-1 cells to model this response of alveolar macrophages, it was necessary to determine whether TNF is responsible for THP-1 cell cytotoxicity after mycobacterial infection. PMA-differentiated THP-1 cells were infected with BCG in the presence or absence of neutralizing anti-TNF monoclonal antibodies (MAb) or the TNF inhibitor pentoxifylline. These reagents were previously reported by our laboratory to block M. tuberculosis-mediated apoptosis in primary macrophages (8). As shown in Fig. 2, the addition of either anti-TNF MAb or pentoxifylline at the time of infection reduced the occurrence of THP-1 apoptosis. The inhibition appeared to be complete, suggesting that endogenous TNF is both necessary and sufficient for induction of apoptosis in response to M. tuberculosis.
We previously reported that uninfected primary HAM are resistant to TNF cytotoxicity but may become primed for this response after infection with viable mycobacteria (9). The addition of exogenous TNF to naive alveolar macrophages did not significantly increase the low level of spontaneous apoptosis, whereas macrophage apoptosis was potently augmented when TNF was added to cells infected with M. tuberculosis H37Ra. To test this model by using THP-1 cells, cells were plated on slides and treated with rTNF and then analyzed for apoptosis by DAPI staining. Healthy nuclei appear large and round, while apoptotic nuclei appear small and condensed and often exhibit a halo effect as chromosomes fragment and migrate to the periphery of the nucleus. As shown in Fig. 3a to c, TNF treatment of naive THP-1 cells did not cause an increase in nuclear condensation over background, as was observed in BCG-treated cells. This result was confirmed by analyzing both untreated and TNF-stimulated cells via flow cytometry for annexin V surface expression. The overlaid line in Fig. 3d represents annexin V-positive cells treated with rTNF alone. Furthermore, nucleosomal-particle ELISA data in Fig. 3e indicate that there was no statistical difference between untreated cells and those treated with exogenous rTNF. These experiments confirm that PMA-differentiated THP-1 cells are insensitive to TNF death signaling but become primed for TNF-mediated apoptosis after infection with mycobacteria. In this way, THP-1 cells faithfully model the response of primary HAM.
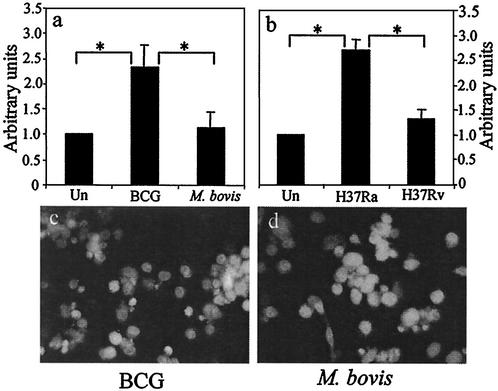
Our laboratory also reported evidence of a relationship between microbial virulence and the apoptosis response of infected macrophages. In HAM, avirulent M. tuberculosis strains induce a high level of apoptosis while more-virulent strains do not. We tested the ability of differentiated THP-1 cells to model this behavior by comparing apoptosis of cells infected with virulent wild-type M. bovis to that of cells infected with its attenuated counterpart, BCG. Apoptosis was measured by DNA fragmentation ELISA. Consistent with the response of primary HAM, we found that BCG (at an MOI of ∼5) induces a high level of nucleosomal fragmentation in THP-1 cells while wild-type M. bovis does not (Fig. 4a). Identical results were seen when THP-1 cells were infected with the avirulent M. tuberculosis strain H37Ra and its isogenic virulent counterpart, H37Rv (Fig. 4b). This result was confirmed by DAPI staining as shown in Fig. 4c and d.
FIG. 4.
(a) Nucleosomal-fragmentation ELISA of THP-1 cells uninfected (Un) or infected with avirulent BCG or virulent wild-type M. bovis. Data are means plus standard errors of three experiments. ∗, statistically significant difference between groups (P < 0.05). (b) Same experiment as in panel a with attenuated H37Ra and virulent H37Rv. Data are means plus standard errors of five experiments. (c and d) DAPI nuclear staining of THP-1 cells treated with BCG (c) or M. bovis (d). The BCG-treated cells show condensed DNA consistent with apoptosis, while the M. bovis-treated cells retain large intact nuclei.
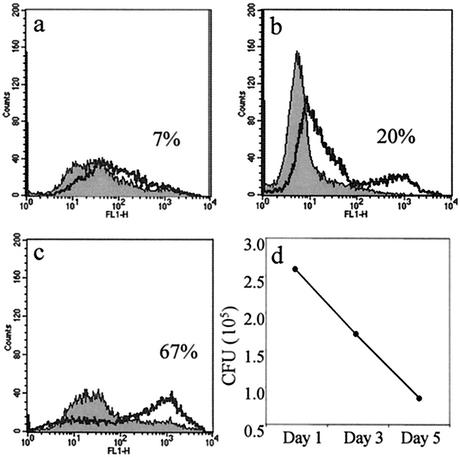
Apoptosis of primary macrophages has been linked to microbicidal activity against intracellular bacteria. This phenomenon was observed when macrophages were treated with exogenous apoptosis-inducing agents and in the context of infection-induced apoptosis (9, 19). We investigated this in PMA-differentiated THP-1 cells infected with BCG. Infected-cell cultures were harvested at 0, 3, and 5 days postinfection for annexin V staining, while parallel wells were harvested for plating on Middlebrook agar and colony counting. Figure 5 shows that as BCG-induced apoptosis in THP-1 cells increased the number of viable bacilli decreased. After 4 h of BCG infection, only 7% of the THP-1 cells were apoptotic and there were 2.6 × 105 CFU of viable bacilli present in the cells. By day 3, 20% of the cells were apoptotic and CFU were reduced to1.7 × 105. And finally, by 5 days postinfection, 67% of the THP-1 cells were apoptotic and CFU were further reduced to 8.6 × 104. Host macrophage apoptosis does not simply remove a supportive growth environment for BCG, since CFU declined below the initial infecting dose as THP-1 apoptosis proceeded.
FIG. 5.
Annexin V-FITC staining of uninfected (gray) or BCG-infected (black overlay) THP-1 cells at day 1 (a), day 3 (b), and day 5 (c) postinfection shows an increase in apoptosis from 7 to 67% through the course of the infection. (d) CFU of viable BCG decrease through the course of the infection. Colonies were counted 2 weeks after plating THP-1 lysates on 7H11 agar.
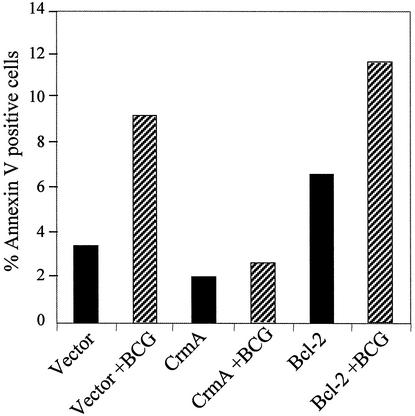
The PINCO retrovirus has been shown to be a potent initiator of exogenous gene expression in rapidly dividing cells (5, 6). We used this vector to introduce cowpox virus crmA and human bcl-2 into THP-1 cells. CrmA is known to be a nonspecific caspase inhibitor, while Bcl-2 has been shown to inhibit apoptosis in some cell systems by blocking the release of cytochrome c from the mitochondria. CrmA was reported to block TNF-α-mediated apoptosis (22), and infection with the strong-apoptosis-inducing strain BCG or H37Ra was shown to result in down-regulation of Bcl-2 expression in monocyte-derived macrophages (10). To test the role of caspase activation and Bcl-2 in our system, PINCO-transduced THP-1 cells were differentiated with PMA and infected with BCG and then analyzed by flow cytometry for annexin V staining. As shown in Fig. 6, cells transduced with a negative-control PINCO vector lacking a cDNA insert exhibited BCG-induced apoptosis as expected, while the expression of CrmA in THP-1 cells completely blocked apoptosis in response to BCG infection. In contrast, the expression of Bcl-2 in THP-1 cells did not block BCG-induced apoptosis (Fig. 6) but did inhibit apoptosis induced by staurosporine (data not shown).
FIG. 6.
Annexin V-PE staining of THP-1 cells infected with PINCO retroviral vector alone or vectors containing CrmA or Bcl-2 cDNA. The data were gated on PINCO-positive (GFP+) cells prior to annexin V analysis. Solid bars, uninfected cells; hatched bars, BCG-infected cells. The data are representative of three separate experiments.
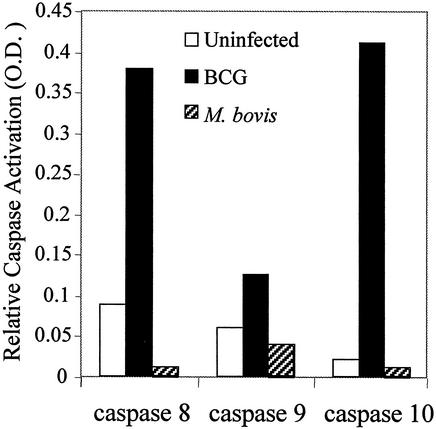
We next attempted to determine the specific caspases involved in M. tuberculosis-induced apoptosis in THP-1 cells. Caspase enzymatic activity was measured in a colorimetric substrate assay. At 12 h postinfection, BCG induced the activation of caspases 8, 9, and 10 (Fig. 7). At later time points, caspase 3 was also activated (data not shown). Interestingly, we saw a high level of activation of caspases 8, 9, and 10 by avirulent BCG but saw little or no caspase activation with the same infectious dose of virulent M. bovis. Similar results were seen when M. tuberculosis strains H37Ra and H37Rv were used (data not shown). These data suggest that the mechanism whereby virulent mycobacteria avoid triggering apoptosis is linked to early events, before the activation of the initiator caspases.
FIG. 7.
Colorimetric caspase activity assay 12 h postinfection shows an increase in protease activity of caspases 8, 9, and 10 due to BCG (solid bars) but not to M. bovis (hatched bars).
DISCUSSION
We previously reported that primary HAM can respond to intracellular invasion with pathogenic mycobacteria by undergoing apoptosis (9). This response is TNF dependent and requires a priming step, since naive macrophages are insensitive to TNF cytotoxicity. Infection-induced apoptosis may be an innate defense mechanism against intracellular infection. Our finding that virulent strains of pathogenic mycobacteria evade apoptosis is consistent with this model (8, 9). The mechanism of priming for TNF death signaling and the mechanism which permits virulent bacilli to avoid this response are presently unknown. The potential relevance of this phenomenon to human tuberculosis is supported by its occurrence in primary HAM. However, these terminally differentiated cells present several barriers to investigation, including their scarcity, heterogeneity, and resistance to gene transduction. The present study was undertaken to develop a suitable cell line model for M. tuberculosis-induced apoptosis and to use this model to elucidate the death signaling pathways activated after exposure to virulent or attenuated mycobacteria.
Cell line models of macrophage-M. tuberculosis interactions have been reported previously (20). Those studies used immortalized continuously cycling cells, whereas primary alveolar macrophages are terminally differentiated and may be expected to exhibit significant differences in the regulation of cell fate (3). We therefore chose to study human monocytic THP-1 cells, which can be differentiated into mature macrophage-like cells by using PMA. By activating protein kinase C, PMA mimics the effect of diacylglycerol, which mediates natural macrophage differentiation. PMA-differentiated THP-1 cells down-regulate DNA synthesis, adhere to plastic, and resemble primary macrophages in morphology. Our data presented here show that PMA-differentiated THP-1 cells undergo apoptosis in response to BCG infection in a dose-dependent manner. This apoptosis is mediated by endogenous TNF and requires priming by infection since the viability of uninfected THP-1 cells was not reduced when exogenous TNF was added. Furthermore, the association between mycobacterial virulence and host cell apoptosis is confirmed in THP-1 cells. Avirulent strains induce high levels of apoptosis, while a comparable infectious dose of virulent bacilli does not. Finally, we show that, as THP-1 cell apoptosis increases, the viability of intracellular BCG is reduced. Together these data demonstrate that differentiated THP-1 cells are a valid model system for M. tuberculosis-induced apoptosis in macrophages.
Although there is a large body of data detailing the signaling events initiated at the TNF receptor in diverse cell types, the specific pathway activated by M. tuberculosis infection of macrophages has not been described. It is generally understood that TNF-mediated apoptosis is activated when a TNF trimer binds and cross-links TNFR1, recruiting TNFR1-associated death domain (DD) protein (TRADD) to the intracytoplasmic tail of TNFR1. TRADD then binds the Fas-associated DD protein (FADD) via their DDs, which in turn recruits procaspase 8 (or procaspase 10) via its death effector domain. The multimolecular complex of TRADD, FADD, and procaspase 8 on the intracytoplasmic tail of TNFR1 comprises a death-inducing signal complex (DISC). The intracytoplasmic tail of TNFR2 lacks a DD and does not bind TRADD, yet it appears to contribute to TNFR1-mediated death signals (14). The requirement for TNFR2 in death signaling may be auxiliary rather than essential (23). The generation of caspase 8 by the DISC initiates a cascade sequentially activating downstream caspases that carry out the effector functions of apoptotic death by cleaving their various substrates.
Caspases are essential for TNF-mediated apoptosis. The cowpox virus product CrmA is a pancaspase inhibitor that can block TNF death signals (18). We show here that retroviral expression of CrmA completely blocks M. tuberculosis-induced apoptosis in THP-1 cells, indicating that this pathway is caspase dependent. Published data on monocyte-derived macrophages have shown that caspases 9 and 3 are activated in M. tuberculosis-induced apoptosis, indicating a role for mitochondrial death signals (2). Here we show for the first time that the initiator caspases 8 and 10 are also activated in M. tuberculosis-infected THP-1 cells (Fig. 7). Importantly, we found that low-dose infection with virulent mycobacteria does not activate the initiator caspases in THP-1 cells. Therefore, the ability of virulent bacilli to avoid triggering apoptosis appears to involve the DISC itself rather than the activation of downstream apoptosis inhibitors, which is a typical antiapoptotic strategy for viruses.
The possible mechanisms whereby death receptor signals are blocked in cells infected with virulent M. tuberculosis are numerous and will be the focus of future studies. An initial prospect is the surface expression level of the TNF receptors themselves. A second possibility is differential recruitment of signaling adaptors to TNFR1. The intracytoplasmic tail of TNFR1 is multifunctional and capable of binding signaling adaptors other than those of the DISC. Both TNFR1 and TNFR2 can bind TRAF2 and receptor interacting-protein, which activate the cJun N-terminal kinase and NF-κB pathways (21). There is strong evidence that NF-κB can induce the expression of gene products that protect cells from TNF-mediated apoptosis (17, 25). Differences in the relative levels of expression and recruitment of signaling mediators, either for cell death or survival, may exist. It is possible that avirulent M. tuberculosis tips the balance in the infected cell toward death while virulent M. tuberculosis manipulates TNF signaling to promote cell survival. There are also a number of receptor-associated inhibitors of apoptosis that could be differentially regulated depending on whether cells are infected with virulent or avirulent M. tuberculosis. FADD-like interleukin-1β-converting enzyme (FLICE) inhibitory protein is an NF-κB-regulated inhibitor of apoptosis that binds to the TNFR signaling complex and inhibits processing and activation of the initiator caspases 8 and 10 (11, 17). The NF-κB-inducible death effector domain-containing protein (NDED) is also regulated by NF-κB and has been shown to inhibit the activity, but not the processing, of procaspase 8 (25). These are possible candidates for the blockade of membrane-proximal death signals that we observe in virulent M. tuberculosis-infected THP-1 cells.
It is known that caspase 8 cleaves and activates the Bcl-2 family member Bid (13, 15). This provides a direct link between the death receptor and the mitochondrial pathway of apoptosis. Our data show that retroviral expression of Bcl-2 cannot block M. tuberculosis-induced apoptosis in THP-1 cells. However, the activation of caspase 9 suggests that mitochondria play a role in M. tuberculosis-induced THP-1 cell death. It remains possible that a distinct member of the Bcl-2 family is necessary to inhibit mitochondrial participation during M. tuberculosis-mediated apoptosis. Furthermore, recent reports showed that purified mannosylated lipoarabinomannan derived from a virulent strain of M. tuberculosis induces phosphorylation of the proapoptotic Bcl-2 member Bad in a phosphatidylinositol-3 kinase- and Akt-dependent manner (16). In its unphosphorylated state, Bad binds and sequesters Bcl-2, thus promoting cell death. However, when Bad is phosphorylated by Akt, Bcl-2 is released and acts to inhibit mitochondrion-associated apoptosis. This is a potential mechanism by which virulent strains of M. tuberculosis might inhibit activation of caspase 9 in infected macrophages. It is interesting to consider that the virulence-associated blockade of apoptosis may have evolved to disrupt more than a single death signaling event, perhaps events both upstream, at the level of the TNF receptor, and further downstream, at the mitochondrion. The opportunity to model infection-induced macrophage apoptosis with THP-1 cells will facilitate the investigation of these questions.
Acknowledgments
This study was supported in part by NIH grants HL-64517 and HL-64884.
Editor: B. B. Finlay
REFERENCES
- 1.Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161:2636-2641. [PubMed] [Google Scholar]
- 2.Duan, L., H. Gan, J. Arm, and H. G. Remold. 2001. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J. Immunol. 166:7469-7476. [DOI] [PubMed] [Google Scholar]
- 3.Durrbaum-Landmann, I., J. Gercken, H. D. Flad, and M. Ernst. 1996. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect. Immun. 64:5384-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fratazzi, C., R. D. Arbeit, C. Carini, and H. G. Remold. 1997. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 158:4320-4327. [PubMed] [Google Scholar]
- 5.Gasperi, C., M. Rescigno, F. Granucci, S. Citterio, M. K. Matyszak, M. T. Sciurpi, L. Lanfrancone, and P. Ricciardi-Gastagnoli. 1999. Retroviral gene transfer, rapid selection, and maintenance of the immature phenotype in mouse dendritic cells. J. Leukoc. Biol. 66:263-267. [DOI] [PubMed] [Google Scholar]
- 6.Grignani, F., T. Kinsella, A. Mencarelli, M. Valtieri, D. Riganelli, F. Grignani, L. Lanfrancone, C. Peschle, G. P. Nolan, and P. G. Pelicci. 1998. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 58:14-19. [PubMed] [Google Scholar]
- 7.Iivanainen, E., J. Northrup, R. D. Arbeit, M. Ristola, M. L. Katila, and C. F. von Reyn. 1999. Isolation of mycobacteria from indoor swimming pools in Finland. APMIS 107:193-200. [DOI] [PubMed] [Google Scholar]
- 8.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane, J., H. G. Remold, and H. Kornfeld. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164:2016-2020. [DOI] [PubMed] [Google Scholar]
- 10.Klingler, K., K. M. Tchou-Wong, O. Brandli, C. Aston, R. Kim, C. Chi, and W. N. Rom. 1997. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect. Immun. 65:5272-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger, A., S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 13.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 14.Li, X., Y. Yang, and J. D. Ashwell. 2002. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416:345-347. [DOI] [PubMed] [Google Scholar]
- 15.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 16.Maiti, D., A. Bhattacharyya, and J. Basu. 2001. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 276:329-333. [DOI] [PubMed] [Google Scholar]
- 17.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura, M., R. M. Friedlander, and J. Yuan. 1995. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc. Natl. Acad. Sci. USA 92:8318-8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molloy, A., P. Laochumroonvorapong, and G. Kaplan. 1994. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 180:1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas, M., L. F. Barrera, G. Puzo, and L. F. Garcia. 1997. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J. Immunol. 159:1352-1361. [PubMed] [Google Scholar]
- 21.Strasser, A., L. O'Connor, and V. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 22.Tewari, M., and V. M. Dixit. 1995. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J. Biol. Chem. 270:3255-3260. [DOI] [PubMed] [Google Scholar]
- 23.Ware, C. F., S. VanArsdale, and T. L. VanArsdale. 1996. Apoptosis mediated by the TNF-related cytokine and receptor families. J. Cell. Biochem. 60:47-55. [DOI] [PubMed] [Google Scholar]
- 24.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 25.You, Z., H. Ouyang, D. Lopatin, P. J. Polver, and C. Y. Wang. 2001. Nuclear factor-kappa B-inducible death effector domain-containing protein suppresses tumor necrosis factor-mediated apoptosis by inhibiting caspase-8 activity. J. Biol. Chem. 276:26398-26404. [DOI] [PubMed] [Google Scholar]