Abstract
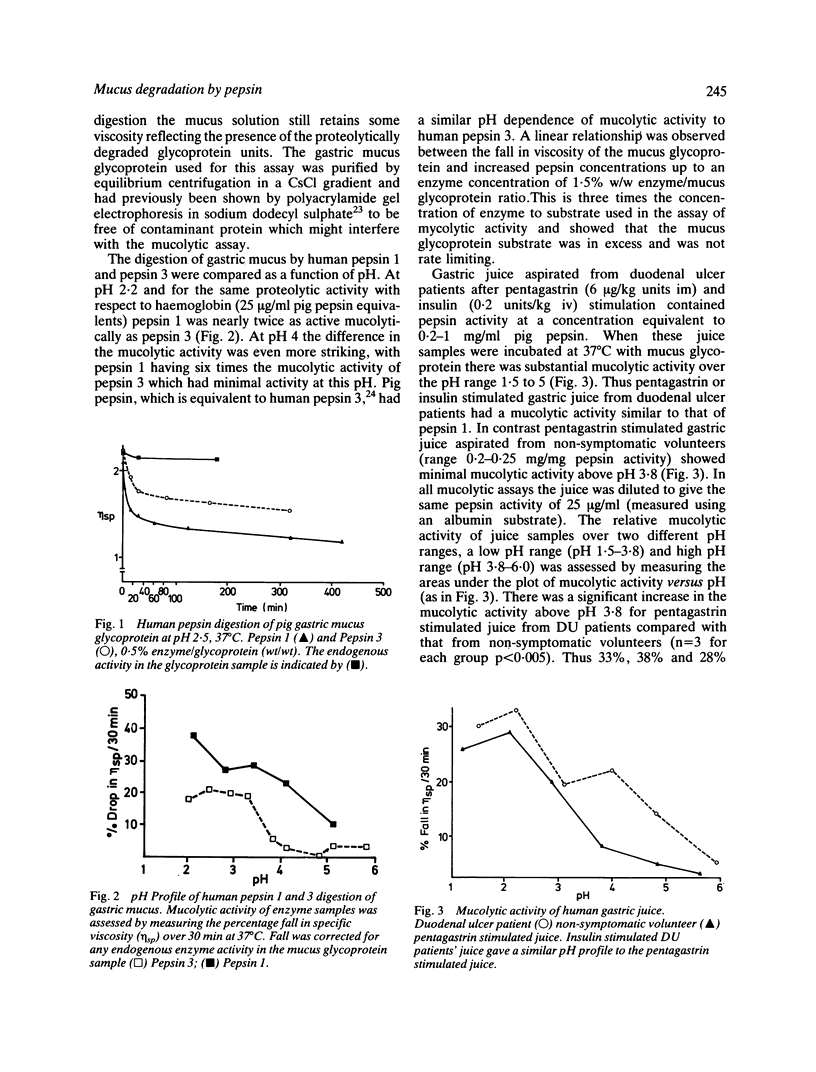
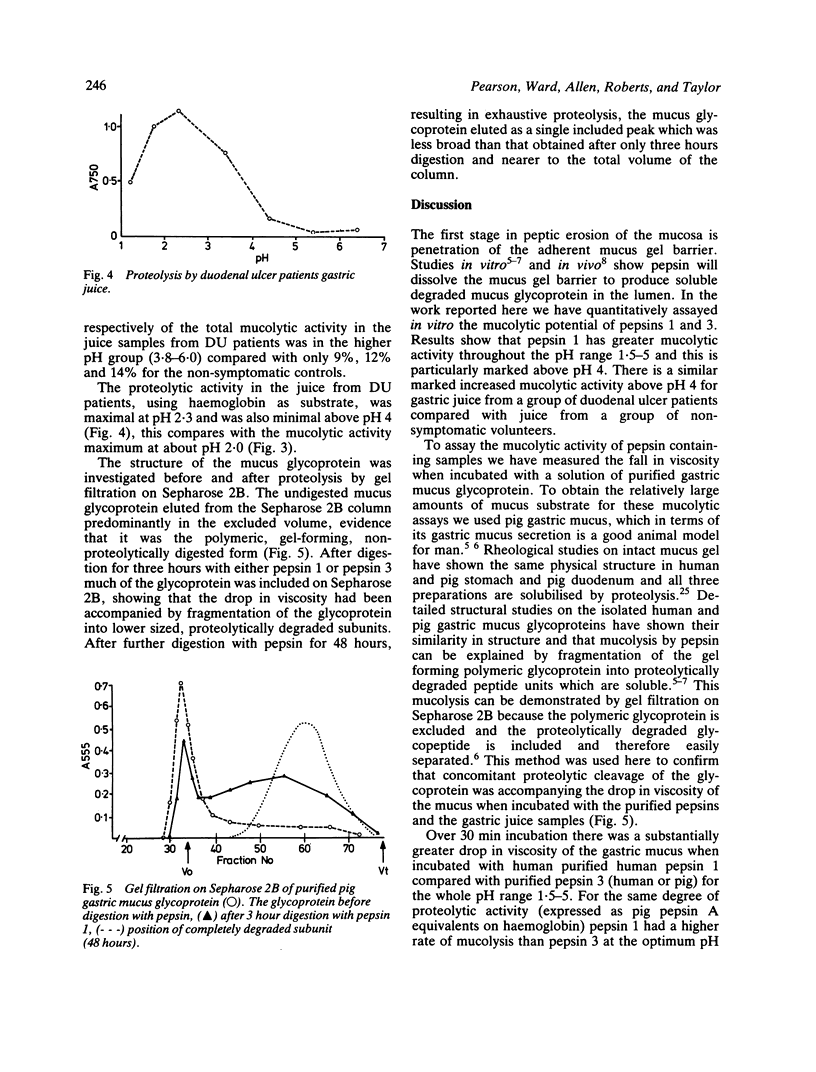
The ability to digest mucus, mucolytic activity of isolated pepsins and samples of human gastric juice has been assayed by measuring the fall in viscosity when incubated with purified pig gastric mucus glycoprotein. Pure human pepsin 1, the peptic ulcer associated pepsin, digested gastric mucus glycoprotein at a faster rate than did pure human pepsin 3 (the principal human pepsin), or the equivalent pig pepsin (pepsin A). At pH 2.0 pepsin 1 had twice the mucolytic activity of pepsin 3. Above pH 3.8 this difference became more marked and whereas pepsin 1 caused substantial mucolysis up to and including pH 5.1, pepsin 3 had minimal activity. At pH 4.0 pepsin 1 had six times the mucolytic activity of pepsin 3. Gastric juices from patients with duodenal ulcer each exhibited substantial mucolytic activity between pH 2 to 5, similar to that of pepsin 1. In contrast, gastric juice from non-symptomatic volunteers exhibited little mucolytic activity above pH 4. Analysis of the mucus glycoprotein by gel filtration showed that an increase in lower molecular weight, pepsin degraded, glycoprotein was associated with the fall in mucus viscosity for all enzyme preparations. These results showed that pepsin 1 can digest the mucus more effectively than pepsin 3 and at higher pH values. The raised concentrations of pepsin 1 in the juice of peptic ulcer patients may thus promote the ulcerative process by increased erosion of the mucus barrier under conditions likely to pertain in the duodenal bulb as well as the stomach.
Full text
PDF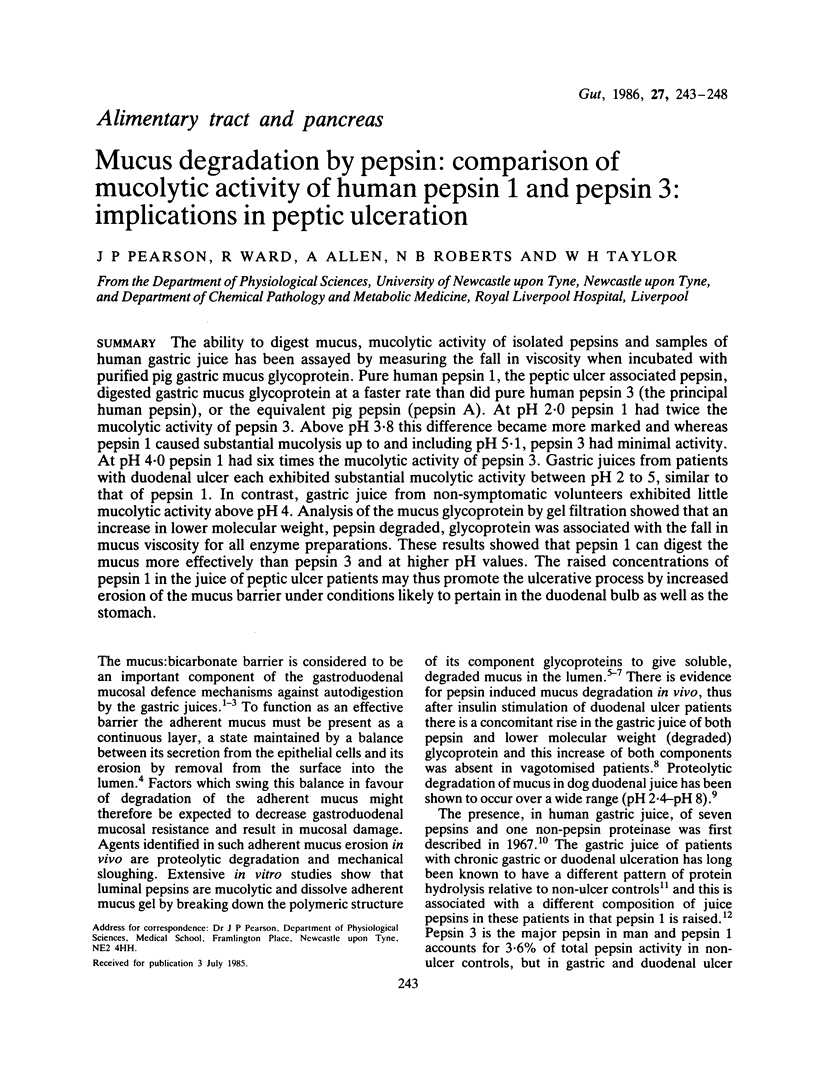



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980 Mar;21(3):249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Bell A. E., Sellers L. A., Allen A., Cunliffe W. J., Morris E. R., Ross-Murphy S. B. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985 Jan;88(1 Pt 2):269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Taylor W. H. Nomenclature of the pepsins. Nature. 1967 Oct 21;216(5112):279–280. doi: 10.1038/216279a0. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Taylor W. H. The pepsins from human gastric mucosal extracts. Biochem J. 1970 Jul;118(4):587–594. doi: 10.1042/bj1180587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemstrom G., Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982 Mar;242(3):G183–G193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- HARTIALA K., GROSSMAN M. I. Studies on chemical and physical changes in duodenal mucus. J Biol Chem. 1952 Mar;195(1):251–256. [PubMed] [Google Scholar]
- LENNARD-JONES J. E., BABOURIS N. EFFECT OF DIFFERENT FOODS ON THE ACIDITY OF THE GASTRIC CONTENTS IN PATIENTS WITH DUODENAL ULCER. I. A COMPARISON BETWEEN TWO 'THERAPEUTIC' DIETS AND FREELY-CHOSEN MEALS. Gut. 1965 Apr;6:113–117. doi: 10.1136/gut.6.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Rees W. D., Turnberg L. A. Mechanisms of gastric mucosal protection: a role for the 'mucus-bicarbonate' barrier. Clin Sci (Lond) 1982 Apr;62(4):343–348. doi: 10.1042/cs0620343. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Prestwich C. J. Acidity at different sites in the proximal duodenum of normal subjects and patients with duodenal ulcer. Gut. 1966 Oct;7(5):509–514. doi: 10.1136/gut.7.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. B., Taylor W. H. The preparation and purification of individual human pepsins by using diethylaminoethyl-cellulose. Biochem J. 1978 Mar 1;169(3):607–615. doi: 10.1042/bj1690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter J. I., Sones J. Q., Samloff I. M., Richardson C. T., Gursky J. M., Walsh J. H., Rimoin D. L. Duodenal-ulcer disease associated with elevated serum pepsinogen I: an inherited autosomal dominant disorder. N Engl J Med. 1979 Jan 11;300(2):63–66. doi: 10.1056/NEJM197901113000203. [DOI] [PubMed] [Google Scholar]
- Samloff I. M., Townes P. L. Electrophoretic heterogeneity and relationships of pepsinogens in human urine, serum, and gastric mucosa. Gastroenterology. 1970 Apr;58(4):462–469. [PubMed] [Google Scholar]
- Scawen M., Allen A. The action of proteolytic enzymes on the glycoprotein from pig gastric mucus. Biochem J. 1977 May 1;163(2):363–368. doi: 10.1042/bj1630363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H. Gastric proteolysis in disease. 2. The proteolytic activity of gastric juice and gastric mucosal extracts from patients with chronic gastric and duodenal ulcer. J Clin Pathol. 1959 Jul;12:338–343. doi: 10.1136/jcp.12.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H. Pepsins of patients with peptic ulcer. Nature. 1970 Jul 4;227(5253):76–77. doi: 10.1038/227076a0. [DOI] [PubMed] [Google Scholar]
- Venables C. W. An assessment of the value of measuring uncollected gastric secretion during routine secretion studies in man. Br J Surg. 1972 Jun;59(6):473–477. doi: 10.1002/bjs.1800590616. [DOI] [PubMed] [Google Scholar]
- Walker V., Taylor W. H. Pepsin 1 secretion in chronic peptic ulceration. Gut. 1980 Sep;21(9):766–771. doi: 10.1136/gut.21.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. L., Shaw B., Sanders D. J., Reed J. D. Variation in the proportions of individual pepsins secreted by the cat in response to vagal stimulation and hypoglycaemia. Clin Sci Mol Med. 1975 Apr;48(4):297–305. doi: 10.1042/cs0480297. [DOI] [PubMed] [Google Scholar]
- Younan F., Pearson J. P., Allen A., Venables C. W. Gastric mucus degradation in vivo in peptic ulcer patients and the effects of vagotomy. Adv Exp Med Biol. 1982;144:235–237. doi: 10.1007/978-1-4615-9254-9_35. [DOI] [PubMed] [Google Scholar]
- Younan F., Pearson J., Allen A., Venables C. Changes in the structure of the mucous gel on the mucosal surface of the stomach in association with peptic ulcer disease. Gastroenterology. 1982 May;82(5 Pt 1):827–831. [PubMed] [Google Scholar]


