Abstract
Chlamydiae are bacterial pathogens which develop strictly inside the epithelial cells of their hosts. The mechanism used by chlamydiae to enter cells is not well characterized; however, it is thought to consist of a receptor-mediated process. In addition, the formation of clathrin-coated pits appears to be dispensable for chlamydiae to be internalized by host cells. Clathrin-independent endocytosis has recently been shown to occur through cholesterol-rich lipid microdomains, which are characterized by detergent insolubility. In the present study, we investigated whether these lipid domains play a role in Chlamydia trachomatis serovar L2 internalization by host cells. Our results show that after binding to HeLa cells, chlamydiae are associated with detergent-resistant lipid microdomains (DRMs), which can be isolated by fractionation of infected HeLa cells and flotation on a sucrose gradient. After internalization by HeLa cells, chlamydiae were still found in DRMs. In addition, extraction of plasma membrane cholesterol inhibited infection of HeLa cells by C. trachomatis. Many of the proteins associated with DRMs are glycosylphosphatidylinositol (GPI)-anchored proteins; however, our results could not identify a role for GPI-anchored proteins in the entry process. The same results were obtained for Chlamydia psittaci strain GPIC. We propose that cholesterol-rich domains participate in the entry of chlamydiae into host cells. Chlamydia binding to cholesterol-rich domains may lead to coalescence of the bacterial cells, which could trigger internalization by host cells.
Chlamydiae are gram-negative bacterial pathogens which proliferate strictly inside host cells and are responsible for a wide range of diseases in animals (33). In humans, various strains of Chlamydia trachomatis are the causative agents of prevalent sexually transmitted bacterial diseases and are the main causes of preventable blindness worldwide. Chlamydiae are characterized by two distinct morphological forms: the small metabolically inactive elementary body (EB) (diameter, 0.3 μm) and the larger metabolically active reticulate body (RB) (diameter, 1 μm). EBs initiate infection by binding to host epithelial cells. Several hours following their internalization, EBs differentiate into noninfectious replicative RBs, which proliferate within a membrane-bound vacuole termed the inclusion. Within 2 days of infection, newly produced RBs have differentiated into EBs, which are released from the cell and can begin a new cycle of infection.
The identity of the host cell receptor which binds EBs has remained elusive. Heparan sulfate glycosaminoglycans appear to be involved in the attachment process (42, 48), although the dependence on glycosaminoglycans for attachment may vary among chlamydial species and strains (12). Other putative chlamydial ligands used for attachment to host cells include the major outer membrane protein (MOMP) (43), the outer membrane protein OmcB (45), a heat-labile chlamydial cytadhesin (18), and heat shock proteins (29). Internalization of chlamydiae by epithelial cells has been reported to be a slow process, occurring within 2 h following attachment (24). Although C. trachomatis cells bound to host cells have been observed in electron-dense structures having the appearance of clathrin-coated pits by electron microscopy (47), the internalization process does not require formation of the clathrin endocytic machinery. Indeed, the entry of both C. trachomatis and Chlamydia psittaci into host cells was not altered by specific inhibition of clathrin-dependent endocytosis through overexpression of dominant-negative mutations of Eps15, a constitutive component of the AP-2 clathrin adapter protein complex (4). It is noteworthy that differences have been observed in the attachment to host cells between Chlamydia strains, as well as between different serovars of C. trachomatis, suggesting that the entry mechanisms may be different even in different serovars.
Once inside a host cell, chlamydiae do not leave their internalization vacuole, which avoids fusion with late endocytic compartments and eventually localizes to a perinuclear location in close apposition to the Golgi apparatus (12). Recent evidence suggests that the mechanism of entry of pathogens could be decisive for their intracellular fate. For instance, Escherichia coli expressing the FimH adhesin appears to be internalized by a mechanism distinct from that of opsonized E. coli in macrophages, and whereas FimH-mediated internalization leads to survival of E. coli within macrophages, opsonized E. coli is killed following phagocytosis (3). Like FimH-expressing E. coli, mycobacteria are not delivered to lysosomes following phagocytosis by macrophages. Instead, they are internalized by binding to complement receptor 3 (28) and remain within a phagosome through a mechanism which has been shown to require the recruitment and retention of the tryptophan-aspartate-containing coat protein (9). A common feature of the entry processes leading to survival of both FimH-expressing E. coli and mycobacteria is the involvement of cholesterol-rich lipid microdomains, where the receptor for FimH (CD48) (37), a population of complement receptor 3, and the tryptophan-aspartate-containing coat protein localize (28). In fact, cholesterol depletion from the plasma membrane has been shown to inhibit the phagocytosis of both FimH-expressing E. coli and mycobacteria by macrophages (11, 37).
Lipid microdomains enriched in cholesterol and sphingolipids are characterized by their insolubility in cold Triton X-100 and have thus been termed detergent-resistant membrane domains (DRMs) or detergent-insoluble glycolipid-rich domains, often referred to as lipid rafts (14). The biochemical feature of lipid microdomains results from the organization of cholesterol and sphingolipids, which are thought to form a liquid-ordered (lo) phase due to tight packing of unsaturated acyl chains (5). In many cell types, expression of caveolin and its association with lipid microdomains lead to the formation of specialized DRMs known as caveolae, which consist of 50- to 100-nm flask-shaped invaginations located at the plasma membrane (38). The enrichment of DRMs for molecules involved in signal transduction events, including G protein-coupled receptors, receptor tyrosine kinases, and mitogen-activated protein kinases, suggests that localization to lipid microdomains plays an important role in signaling mechanisms (40). DRMs have also been involved in polarized secretion and transcytosis in epithelial cells (38). Importantly, internalization mediated by DRMs and by caveolae has been shown to be distinct from clathrin-dependent endocytosis (19, 25, 27, 32, 41).
In the present study, we investigated the role of cholesterol-rich lipid microdomains in Chlamydia entry. Here we provide evidence that C. trachomatis associates with DRMs early in the infection process, while cholesterol depletion leads to inhibition of Chlamydia internalization by host cells.
MATERIALS AND METHODS
Cells and bacterial strains.
HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) (Life Technologies) containing 10% fetal calf serum, 2 mM l-glutamine, and 4.5 g of glucose per liter at 37°C in a 5% CO2 atmosphere. Both wild-type Chinese hamster ovary (CHO) cells and mutagenized CHO-LA1 cells were cultured in F-12 medium (Life Technologies) containing 10% fetal calf serum and 2 mM l-glutamine at 37°C in a 5% CO2 atmosphere (1). The lymphogranuloma venereum serovar L2 strain of C. trachomatis (from the American Type Culture Collection) was cultured in HeLa cells and purified as described previously (4). E. coli MC4100 harboring plasmid pRI203 (a gift from Guy Tran Van Nhieu, Institut Pasteur) was grown in L broth containing 100 μg of ampicillin per ml as described previously (16).
Antibodies and other reagents.
Nonlabeled and fluorescein isothiocyanate (FITC)-labeled mouse anti-Chlamydia antibodies and the monoclonal antibody specific for MOMP of C. trachomatis were obtained from Argene, Biosoft. Tetramethyl rhodamine isothiocyanate-labeled anti-mouse antibody was obtained from Dako. Mouse anti-human transferrin receptor antibody H68.4 was a gift from I. Trowbridge. Mouse anti-human Rab5 antibody was obtained from BD Transduction Laboratories. Horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G antibody and HRP-enhanced chemiluminescence reagent (ECL) were obtained from Amersham Pharmacia Biotech. HRP-conjugated cholera toxin subunit B, methyl-β-cyclodextrin (MβCD), soluble cholesterol (complexed with MβCD), a protease inhibitor cocktail, and a Bradford protein assay kit were obtained from Sigma.
MβCD treatment assays.
For Chlamydia internalization assays, subconfluent HeLa cells growing on glass coverslips in 24-well plates were incubated with 0, 1, 5, or 10 mM MβCD in serum-free DMEM for 30 min at 37°C. For cholesterol repletion assays, the cells were preincubated with 10 mM MβCD in serum-free DMEM for 30 min at 37°C and then incubated for 12 min at 37°C in serum-free DMEM alone or DMEM containing 800 μg of soluble cholesterol per ml. The cells were infected with C. trachomatis at a multiplicity of infection (MOI) of approximately 3 for 90 min at room temperature in serum-free DMEM, rinsed once with serum-free DMEM, and then incubated in complete DMEM for either 3 or 16 h at 37°C, as indicated below. Cell-associated bacteria and inclusions were immunofluorescently labeled as described below.
An E. coli MC4100/pRI203 internalization assay was performed as described previously (16). Subconfluent HeLa cells growing in 12-well plates were incubated with 10 mM MβCD in serum-free DMEM for 30 min at 37°C. The cells were infected with approximately 106 bacteria per well for 2 h at room temperature in serum-free DMEM, rinsed with serum-free DMEM, and incubated in complete DMEM for 90 min at 37°C. The cells were rinsed with complete DMEM and then incubated in complete DMEM containing 50 μg of gentamicin per ml for 90 min at 37°C to kill extracellular bacteria. Then the cells were rinsed twice with phosphate-buffered saline (PBS) and lysed with 0.1% Triton X-100 in PBS. Released bacteria were titrated on L agar containing 100 μg of ampicillin per ml, and bacterial internalization was calculated by determining the percentage of total counts remaining after incubation in the presence of gentamicin.
Infection of CHO cells.
Subconfluent wild-type CHO cells or CHO-LA1 cells growing on glass coverslips in 24-well dishes were incubated with C. trachomatis at an MOI of approximately 3 for 90 min at 37°C. The bacterial suspension was removed, and the cells were incubated for 6 h at 37°C prior to immunofluorescent labeling as described below.
Immunofluorescent labeling and measurement of internalized Chlamydia cells.
For internalization assays with HeLa or CHO cells, the infected cells were rinsed twice with PBS and fixed for 30 min with 4% paraformaldehyde (24). Noninternalized bacteria were fluorescently labeled under nonpermeabilizing conditions (1 mg of bovine serum albumin per ml in PBS) with a mouse anti-Chlamydia antibody and a tetramethyl rhodamine isothiocyanate-labeled anti-mouse antibody, and internalized bacteria were subsequently labeled under permeabilizing conditions (0.5% saponin and 1 mg of bovine serum albumin per ml in PBS) with an FITC-labeled anti-Chlamydia antibody, as described previously (24). For overnight infections, the cells were rinsed twice with PBS and fixed for 30 min with 4% paraformaldehyde, and the inclusions were labeled with an FITC-labeled anti-Chlamydia antibody under permeabilizing conditions (0.5% saponin and 1 mg of bovine serum albumin per ml in PBS). The number of bacteria (adhered or internalized) or the number of inclusions and the number of cells were counted in three or four different fields per coverslip. For each type of experimental conditions, a minimum of 30 different fields in three independent experiments were analyzed. Data from different experiments were combined to calculate the average and the standard error. Samples were examined with a Zeiss Axiophot fluorescence microscope (Zeiss, Jena, Germany) attached to a cooled charge-coupled device camera (Photometrics, Tucson, Ariz.) by using a 63× Apochromat lens. Images were acquired by using IPlab software and were analyzed by using Adobe Photoshop software.
Cell fractionation and isolation of DRMs.
HeLa cells or CHO cells (30 × 106 cells) growing in 10-cm dishes were incubated with approximately 5 × 106 inclusion-forming units of C. trachomatis for 90 min at 4°C or for 5 h at 37°C, as indicated below. The cells were rinsed twice with cold PBS and scraped from the dishes into PBS. Each cell pellet was resuspended in ice-cold Tris-buffered saline (TBS) (20 mM Tris [pH 7.4], 1 mM EDTA, 140 mM NaCl) containing 1% Triton X-100, 1 mM phenylmethylsulfoxide, and 1% protease inhibitor cocktail, and the cells were passaged 10 times through a 26G3/8 needle. The cell lysate was incubated on ice for 15 min and centrifuged at 800 × g for 10 min at 4°C. The postnuclear supernatant was adjusted to 40% (wt/vol) sucrose in TBS, placed at the bottom of an SW41 centrifuge tube, and layered with 6 ml of 36% (wt/vol) sucrose in TBS and 3 ml of 5% (wt/vol) sucrose in TBS. After centrifugation at 38,000 rpm for 15 h at 4°C, 1-ml fractions were collected from the top of the gradient. Lipids were solubilized by incubating the fractions on ice for 15 min with 0.02% sodium deoxycholate, and the protein concentrations in the different fractions were measured by the Bradford protein assay. For detection of ganglioside GM1, 2 μg of proteins from each fraction was dot blotted on a nitrocellulose filter, which was incubated with 200 ng of HRP-cholera toxin subunit B per ml and treated with ECL. The remaining proteins in each fraction were precipitated with trichloroacetic acid and resuspended in 100 μl of Laemmli buffer (3% β-mercaptoethanol). The presence of the transferrin receptor, the presence of Rab5, and the presence of MOMP were revealed by resolving 50 μg of proteins from each fraction by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and performing Western blotting with the H68.4 antibody, the anti-Rab5 antibody, and the anti-MOMP antibody, respectively, followed by HRP-anti-mouse immunoglobulin G blotting and ECL analysis.
To test for the presence of infectious chlamydiae in the sucrose gradient, DRMs (fractions 3 to 5) prepared from C. trachomatis-infected cells were pooled, and the bacteria were pelleted by centrifugation with a Microfuge at 13,000 rpm for 20 min at 4°C and washed once in ice-cold PBS. The recovered chlamydiae were incubated with HeLa cells growing on glass coverslips in 24-well plates for 20 h at 37°C. Infected cells were fixed, and the inclusions were immunofluorescently labeled as described above.
To analyze the fractionation behavior of chlamydiae treated with Triton X-100, 5 × 106 inclusion-forming units of C. trachomatis was resuspended in ice-cold TBS containing 1% Triton X-100 and protease inhibitors and then subjected to the DRM isolation protocol as described above. Fractions were collected from the top of the gradient, and the total protein from each fraction was precipitated with trichloroacetic acid, resuspended in Laemmli buffer (3% β-mercaptoethanol), resolved by SDS-PAGE, and Western blotted against MOMP.
RESULTS
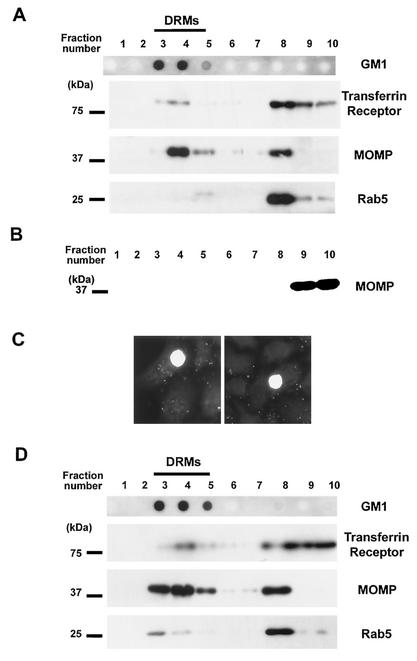
Chlamydia entry into host epithelial cells has been shown to occur in the absence of functional clathrin-coated pits (4). Recently, characterization of cholesterol-rich microdomains at the plasma membrane has allowed workers to define an internalization pathway which is distinct from the clathrin-dependent pathway (19). We therefore investigated the possible involvement of cholesterol-rich microdomains in the entry of chlamydiae into host cells. If these membrane domains participate in the internalization of chlamydiae by host cells, chlamydiae bound to the surfaces of HeLa cells should be found associated with DRMs after subcellular fractionation in Triton X-100 at 4°C (39). Thus, HeLa cells were incubated with C. trachomatis for 90 min at 4°C and subsequently fractionated by lysis in 1% Triton X-100 on ice, followed by flotation on a sucrose gradient. Fractions were collected from the top of the sucrose gradient, and equivalent amounts of proteins from each fraction were analyzed to assess the efficiency of separation of DRMs from the bulk of the solubilized membranes. Ganglioside GM1, a widely used marker for DRMs in many cell types, including HeLa cells (10), was localized in fractions 3 to 5 (Fig. 1A), which corresponded to the 5% sucrose-36% sucrose interface where DRMs are typically found. In contrast, the transferrin receptor and Rab5 were detected in fractions 8 to 10 (Fig. 1A), where 90% of the total protein content in the gradient was located. This result is in agreement with the known association of the transferrin receptor and Rab5 with the bulk of the detergent-soluble membrane (8, 19). In order to detect the presence of chlamydiae in the different fractions forming the sucrose gradient, Western blotting with an antibody specific for MOMP was used. MOMP is an abundant chlamydial protein, which can readily reveal low levels of chlamydiae. The proteins found in DRMs typically represented ∼5% of the total protein content in the gradient, yet the MOMP immunoreactivity detected in these fractions indicated that there was important enrichment of chlamydiae in DRMs relative to the transferrin receptor or to Rab5 (Fig. 1A). MOMP immunoreactivity was also important in fraction 8, possibly indicating that chlamydiae also bind to sites which are not located in cholesterol-rich domains. However, the distribution of MOMP in the different fractions and its relative enrichment in DRMs were comparable to the characteristics of proteins which have been shown to reside in cholesterol-rich domains (8). Based on the protein concentrations in each fraction, the MOMP immunoreactivity in DRMs was estimated to represent approximately 15% of the total MOMP immunoreactivity in the gradient. In contrast to Chlamydia-infected HeLa cells, when a suspension of Chlamydia cells alone was subjected to the DRM isolation protocol, proteins were not detected in fractions 1 to 8 and MOMP immunoreactivity was found only in the loading fractions of the sucrose gradient, indicating that the bacteria alone do not float at the 5% sucrose-36% sucrose interface (Fig. 1B). To verify that the MOMP immunoreactivity found in the DRM-containing fractions revealed the presence of EBs in these fractions, the bacteria were recovered from the isolated DRMs and used to infect HeLa cells. After a 20-h incubation period, large inclusions were observed in the infected cells, demonstrating that infectious chlamydiae were associated with the isolated DRMs (Fig. 1C). Thus, C. trachomatis can bind to cholesterol-rich domains at the plasma membrane of host cells. In order to determine if chlamydiae remained associated with cholesterol-rich domains after their internalization by the host cells, DRMs were prepared from HeLa cells infected with C. trachomatis for 5 h at 37°C. As shown in Fig. 1D, MOMP was enriched in DRM-containing fractions compared to the levels of the transferrin receptor or Rab5, indicating that internalized chlamydiae remained associated with cholesterol-rich domains.
FIG. 1.
Chlamydiae associate with DRMs during infection of HeLa cells. (A) HeLa cells were incubated with C. trachomatis for 90 min at 4°C prior to cellular fractionation. Ganglioside GM1 staining, detected by dot blotting 2 μg of each fraction with HRP-conjugated cholera toxin, indicated the low-density fractions containing DRMs (fractions 3 to 5). Equivalent amounts of proteins from each fraction were resolved by SDS-PAGE. The same membrane was Western blotted with antibodies against the transferrin receptor or Rab5 (found in fractions 8 to 10, which contained the bulk of the solubilized membrane proteins) or against C. trachomatis MOMP. (B) A suspension of C. trachomatis alone was incubated in 1% Triton X-100 and fractionated on a sucrose gradient. The total content of each fraction was resolved by SDS-PAGE and Western blotted against MOMP. (C) DRMs prepared from Chlamydia-infected cells as described above for panel A were pooled, and the bacteria were pelleted. The recovered bacteria were washed in PBS and incubated with HeLa cells for 20 h at 37°C. Infected cells were fixed and immunofluorescently labeled with an anti-Chlamydia antibody to reveal the intracellular inclusions. (D) HeLa cells were incubated with C. trachomatis for 5 h at 37°C prior to fractionation. Ganglioside GM1, the transferrin receptor, Rab5, and MOMP were detected as described above for panel A.
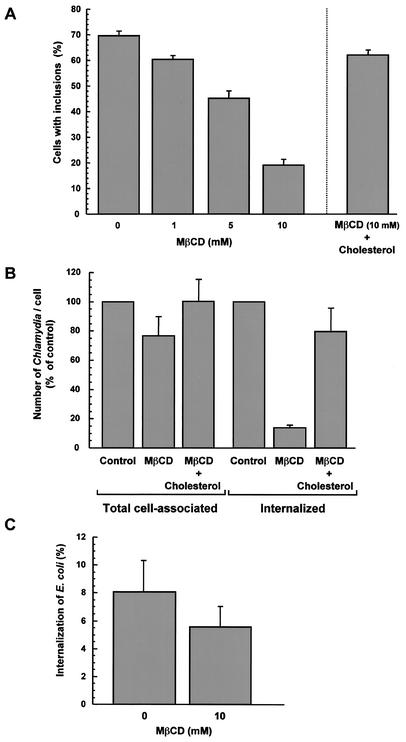
Removal of cholesterol from the plasma membrane disrupts the organization of sphingolipids and proteins in cholesterol-rich membrane domains and inhibits internalization through these domains. However, the proteins which normally reside in DRMs can remain accessible at the cell surface in cholesterol-depleted cells (17). Since chlamydiae associate with cholesterol-rich domains during the infection process, we next determined if cholesterol had functional importance in the internalization of chlamydiae by host cells. Cholesterol was extracted from HeLa cells by using MβCD, which has been extensively used to assess the role of cholesterol in various internalization events, and the cells were infected with C. trachomatis for 16 h. As shown in Fig. 2A, increasing concentrations of MβCD led to a gradual diminution in the percentage of infected HeLa cells; approximately 20% of cells treated with 10 mM MβCD were infected, compared to 70% of control cells. However, when MβCD-treated HeLa cells were incubated with cholesterol prior to infection, the percentage of infected cells returned to a value comparable to the value for nontreated cells (Fig. 2A), suggesting that the effect of MβCD was an effect on the efficiency of Chlamydia entry into host cells. MβCD-treated HeLa cells were then infected for 4 h with C. trachomatis, and the number of internalized chlamydiae was determined by immunofluorescent labeling of the bacteria. Within 4 h, approximately 50% of the total number of C. trachomatis cells found associated with control cells had been internalized. In contrast, following extraction of cholesterol with 10 mM MβCD, internalization of bacteria was strongly inhibited (Fig. 2B). Internalized chlamydiae represented only around 10% of the total number of cell-associated bacteria in MβCD-treated cells. Although MβCD-treated HeLa cells displayed an approximately 20% decrease in the total number of cell-associated chlamydiae (Fig. 2B), the reduction in the number of bound chlamydiae could not account for the observed 90% inhibition of internalized chlamydiae (Fig. 2B). Part of the inhibitory effect of MβCD might simply result from delayed Chlamydia entry; however, the nearly fourfold inhibition of infection in 10 mM MβCD-treated cells observed 16 h after infection (Fig. 2A) indicates that fewer bacteria were indeed internalized when cholesterol was extracted. Figure 2B also demonstrates that the inhibitory effect of MβCD treatment on Chlamydia internalization could be ascribed to cholesterol removal, as the effect could be reversed by adding membrane cholesterol prior to infection. Importantly, the internalization of E. coli expressing the Yersinia pseudotuberculosis invasin protein (E. coli MC4100/pRI203) was not significantly impaired by extraction of cholesterol with 10 mM MβCD (Fig. 2C).
FIG. 2.
Extraction of membrane cholesterol inhibits Chlamydia entry. (A) HeLa cells were incubated in the absence of MβCD or in the presence of different concentrations of MβCD for 30 min at 37°C. The cells either were immediately incubated with C. trachomatis at an MOI of 3 for 90 min at room temperature or were treated with 800 μg of cholesterol per ml for 12 min at 37°C prior to infection (right bar). The cells were washed to remove unbound bacteria and were incubated for an additional 16 h at 37°C. The cells were fixed, and Chlamydia inclusions were immunofluorescently labeled. (B) HeLa cells were incubated in the absence of MβCD or in the presence of 10 mM MβCD for 30 min at 37°C. MβCD was removed, and the cells were not treated or treated with 800 μg of cholesterol per ml for 12 min at 37°C. The cells were then infected with C. trachomatis at an MOI of 3 for 90 min at room temperature, washed, and incubated for an additional 3 h at 37°C. The cells were fixed and immunofluorescently labeled to visualize cell-associated and internalized bacteria. (C) HeLa cells were incubated in the absence of MβCD or in the presence of 10 mM MβCD for 30 min at 37°C and infected with E. coli MC4100/pRI203 for 2 h at room temperature. Unbound bacteria were removed, and the cells were incubated for an additional 90 min at 37°C. Extracellular bacteria were killed by incubating the cells in the presence of 50 μg of gentamicin per ml for 90 min at 37°C. The cells were rinsed with PBS and lysed in 0.1% Triton X-100. Released bacteria were titrated, and bacterial internalization was calculated by determining the percentage of viable counts.
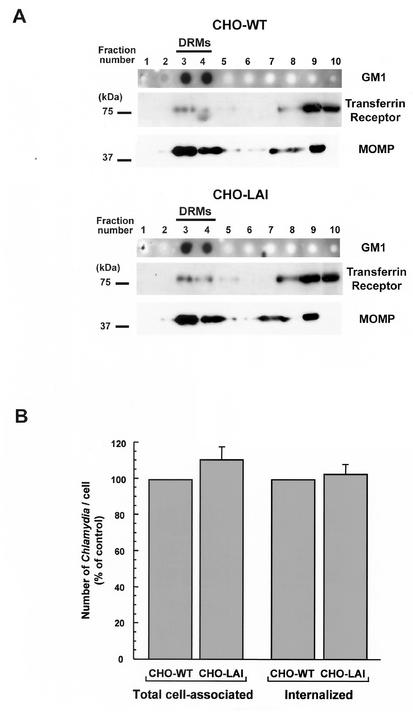
The involvement of cholesterol-rich domains in Chlamydia attachment and entry into host cells suggested that a receptor for chlamydiae could be enriched in these membrane domains. Proteins attached to membranes by a glycosylphosphatidylinositol (GPI) anchor are known to localize mainly at the plasma membrane, where they partition predominantly in DRMs (14). CHO cells deficient in GPI-anchored proteins (CHO-LA1 cells) have recently been isolated by incubating wild-type CHO cells with aerolysin, a lethal pore-forming toxin that binds the GPI anchor (1). Since GPI-anchored proteins are important components of DRMs, we investigated whether such a protein could play a role in Chlamydia entry into cells by using CHO-LA1 cells. Wild-type CHO cells or CHO-LAI cells were incubated with C. trachomatis for 90 min at 4°C and fractionated after lysis in 1% Triton X-100. As shown in Fig. 3A, for equivalent amounts of proteins loaded, the fractionation profiles for MOMP in the sucrose gradients from wild-type CHO and CHO-LAI cells were indistinguishable. In both cell types, MOMP was enriched in DRMs compared to the transferrin receptor, indicating that chlamydiae bound to the cholesterol-rich domains of CHO cells independent of GPI-anchored proteins. In addition, the capacities of the two cell types to internalize chlamydiae were compared by estimating the number of internalized bacteria 6 h after infection with C. trachomatis. The absence of GPI-anchored proteins at the plasma membrane of CHO-LA1 cells did not impair C. trachomatis internalization by these cells, which demonstrated that the susceptibility to infection was similar to that of wild-type CHO cells (Fig. 3C). Although we cannot rule out the possibility that other cell types, such as HeLa cells, express additional GPI-anchored proteins possibly involved in Chlamydia internalization, these results show that GPI-anchored proteins are dispensable for C. trachomatis entry into host cells.
FIG. 3.
(A) Chlamydiae associate with the DRMs of CHO cells deficient in GPI-anchored proteins. Wild-type CHO cells (CHO-WT) or GPI-anchored protein-deficient CHO cells (CHO-LAI) were incubated with C. trachomatis for 90 min at 4°C prior to isolation of DRMs. Ganglioside GM1 staining indicated the low-density fractions containing DRMs (fractions 3 and 4). Equivalent amounts of proteins from each fraction were resolved by SDS-PAGE. The same membrane was Western blotted with an antibody against the transferrin receptor or against C. trachomatis MOMP. (B) Chlamydiae efficiently enter CHO cells deficient in GPI-anchored proteins. Wild-type CHO or CHO-LAI cells were infected with C. trachomatis for 90 min at 37°C. Unbound chlamydiae were removed, and the cells were incubated for an additional 6 h at 37°C. The cells were fixed, and the chlamydiae were immunofluorescently labeled to visualize cell-associated or internalized bacteria.
DISCUSSION
Although a variety of mechanisms appear to have been developed by pathogens to establish proper intracellular niches, increasing evidence suggests that some of these pathogens may have a common dependence on cholesterol-rich lipid domains for invasion of host cells. In the present study, we found that C. trachomatis binds to cholesterol-rich domains at the plasma membrane and remains associated with these domains after internalization by the host cell. In addition, C. trachomatis can enter host cells in a cholesterol-dependent manner, a result that was also obtained with C. psittaci (data not shown). Our findings suggest that cholesterol-rich lipid domains could participate in Chlamydia infection and are in agreement with the recent finding that C. trachomatis entry into host cells exhibits notable sensitivity to cholesterol-binding drugs (23).
Many viral and bacterial pathogens exploit receptor-mediated endocytosis in order to be internalized by the appropriate host cells (30). While endocytosis through clathrin-coated vesicles has been well described, more recent studies have convincingly shown that some toxins and viruses are internalized through caveolae, independent of clathrin-coated pits. For instance, substantial evidence has established that caveolae mediate the internalization of cholera toxin and simian virus 40 (2, 26, 32). Moreover, clathrin-independent endocytosis clearly occurs in cells devoid of caveolae, a process shown to be nonetheless dependent on cholesterol-rich domains (19, 25). Importantly, however, our results do not exclude the possibility that chlamydiae can enter host cells by a pathway independent of cholesterol-rich domains, as chlamydiae may well have evolved to exploit different routes of entry into host cells. In fact, even proteins such as cholera toxin, which undergo internalization through cholesterol-rich domains, are partially endocytosed by the clathrin-dependent pathway (22). Furthermore, intracellular bacteria often have several binding sites on host cells and may use different internalization mechanisms (7, 20).
The mechanism responsible for Chlamydia internalization by host cells involves heparan sulfate glycosaminoglycans, which could either bridge a chlamydial ligand to a host cell receptor (48) or be host cell receptors for chlamydiae (42). Previous studies have indicated that there are saturable and trypsin-sensitive binding sites for chlamydiae on host cells (6, 46). Interestingly, most of the heparan sulfate proteoglycans on the cell surface are attached to the membrane by a GPI anchor (31). Our results indicate that a putative host cell receptor for chlamydiae may constitutively reside in DRMs or at least localize to DRMs following binding; however, we could not demonstrate a role for GPI-anchored protein in Chlamydia entry. Characterization of CHO-LA1 cells (which are deficient in GPI-anchored proteins) has shown that these cells exhibit up-regulation in caveolin-1 expression, leading to an increase in the number of caveolae at the plasma membrane (1). We have not observed that C. trachomatis entry is altered by this up-regulation, perhaps simply because the method used to assess Chlamydia internalization is not sensitive enough to reveal a significant, although moderate, effect. It is nonetheless probable that the number of caveolae is not limiting for the entry process under the conditions used. In addition, Chlamydia entry through DRMs could be independent of the caveolar structure, as shown for the internalization of cholera toxin (25). The large size of infectious EBs (300 nm) compared with the average size of caveolae (∼60 nm) is consistent with binding of an EB to multiple sites in DRMs, which could allow lipid microdomains to coalesce and thereby trigger the internalization process.
Considering the variety of pathogenic agents capable of entering through cholesterol-rich domains, it has recently been hypothesized that this internalization pathway could result in a common intracellular advantage, such as protecting pathogens from lysosomal degradation (36). According to this hypothesis, because cholesterol-rich domains have been involved in transcytosis and retrograde transport to the Golgi apparatus, pathogens entering through these lipid domains would be preferentially targeted to these transport routes rather than to the lysosomal pathway (36). Endocytosed simian virus 40 has recently been shown to localize for several hours in caveolin-1-containing organelles, which are devoid of endosomal markers and do not acidify, before it is transported to a smooth endoplasmic reticulum compartment, where it accumulates (27). Likewise, cholera toxin, en route from the plasma membrane to the Golgi apparatus, resides in endosomes which are devoid of markers for early and recycling endosomes under conditions which inhibit clathrin-dependent endocytosis (21). In the case of chlamydiae, the inclusions have been reported to be devoid of markers for early or late endosomes (15, 44) and to maintain a pH well above 6 (34). The inclusions eventually localize close to the Golgi apparatus and intercept host sphingolipids in transit from the Golgi apparatus to the plasma membrane (13). Chlamydia-containing inclusions appear to be competent for fusion with Golgi apparatus-derived vesicles as early as 2 h following internalization (13). Chlamydial protein synthesis appears to be crucial in avoiding fusion of the inclusions with lysosomes, probably in part by modifying the inclusion membrane (35). However, even when chlamydial protein synthesis is inhibited, fusion of the inclusions with lysosomes occurs slowly, and Chlamydia-containing vacuoles can remain within the host cell for several hours before colocalizing with lysosomal markers (35). Taken together, these findings are consistent with the hypothesis that internalization of Chlamydia occurs through cholesterol-rich domains, thereby directing internalized chlamydiae to a transport route capable of bypassing the classical endosomal pathway. In light of the role played by cholesterol-rich domains in signaling mechanisms (40), chlamydiae could also trigger specific signaling pathways by entering through these domains. Whether entry through cholesterol-rich domains plays a role in the intracellular transport of internalized chlamydiae remains to be investigated.
Acknowledgments
We are grateful to Christophe Lamaze for help with the DRM isolation protocol, Philippe Souque for technical assistance, and Gisou van der Goot for critical reading of the manuscript.
This work was supported by the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires 2000 (MENRT) and by the Association pour la Recherche sur le Cancer. I.J. is a recipient of postdoctoral fellowships from the Heart and Stroke Foundation of Canada and from the Fonds pour la Recherche en Santé du Québec.
Editor: D. L. Burns
REFERENCES
- 1.Abrami, L., M. Fivaz, T. Kobayashi, T. Kinoshita, R. G. Parton, and F. G. van der Goot. 2001. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276:30729-30736. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 4.Boleti, H., A. Benmerah, D. M. Ojcius, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J. Cell Sci. 112:1487-1496. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, G. I. 1976. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect. Immun. 14:645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dramsi, S., P. Dehoux, M. Lebrun, P. L. Goossens, and P. Cossart. 1997. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect. Immun. 65:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevot, P., C. Langlet, X. J. Guo, A. M. Bernard, O. Colard, J. P. Chauvin, R. Lasserre, and H. T. He. 2002. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 21:1899-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 10.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1994. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269:30745-30748. [PubMed] [Google Scholar]
- 11.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 12.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology Press, Washington, D.C.
- 13.Hackstadt, T., D. D. Rockey, R. A. Heinzen, and M. A. Scidmore. 1996. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 15:964-977. [PMC free article] [PubMed] [Google Scholar]
- 14.Harder, T., and K. Simons. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534-542. [DOI] [PubMed] [Google Scholar]
- 15.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 17.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph, T. D., and S. K. Bose. 1991. A heat-labile protein of Chlamydia trachomatis binds to HeLa cells and inhibits the adherence of chlamydiae. Proc. Natl. Acad. Sci. USA 88:4054-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamaze, C., A. Dujeancourt, T. Baba, C. G. Lo, A. Benmerah, and A. Dautry-Varsat. 2001. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7:661-671. [DOI] [PubMed] [Google Scholar]
- 20.Marra, A., and R. R. Isberg. 1997. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect. Immun. 65:3412-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols, B. J. 2002. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4:374-378. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norkin, L. C., S. A. Wolfrom, and E. S. Stuart. 2001. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 266:229-238. [DOI] [PubMed] [Google Scholar]
- 24.Ojcius, D. M., Y. Bravo de Alba, J. M. Kanellopoulos, R. A. Hawkins, K. A. Kelly, R. G. Rank, and A. Dautry-Varsat. 1998. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J. Immunol. 160:1297-1303. [PubMed] [Google Scholar]
- 25.Orlandi, P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 28.Peyron, P., C. Bordier, E. N. N′Diaye, and I. Maridonneau-Parini. 2000. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol. 165:5186-5191. [DOI] [PubMed] [Google Scholar]
- 29.Raulston, J. E., C. H. Davis, D. H. Schmiel, M. W. Morgan, and P. B. Wyrick. 1993. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J. Biol. Chem. 268:23139-23147. [PubMed] [Google Scholar]
- 30.Rosenberger, C. M., J. H. Brumell, and B. B. Finlay. 2000. Microbial pathogenesis: lipid rafts as pathogen portals. Curr. Biol. 10:R823-R825. [DOI] [PubMed] [Google Scholar]
- 31.Rostand, K. S., and J. D. Esko. 1997. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 33.Schachter, J. 1999. Infection and disease epidemiology. American Society for Microbiology Press, Washington, D.C.
- 34.Schramm, N., C. R. Bagnell, and P. B. Wyrick. 1996. Vesicles containing Chlamydia trachomatis serovar L2 remain above pH 6 within HEC-1B cells. Infect. Immun. 64:1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scidmore, M. A., D. D. Rockey, E. R. Fischer, R. A. Heinzen, and T. Hackstadt. 1996. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect. Immun. 64:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin, J. S., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 37.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 38.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 39.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 40.Smart, E. J., G. A. Graf, M. A. McNiven, W. C. Sessa, J. A. Engelman, P. E. Scherer, T. Okamoto, and M. P. Lisanti. 1999. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19:7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su, H., L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143-11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson, A. F., and C. C. Kuo. 1994. Binding of the glycan of the major outer membrane protein of Chlamydia trachomatis to HeLa cells. Infect. Immun. 62:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taraska, T., D. M. Ward, R. S. Ajioka, P. B. Wyrick, S. R. Davis-Kaplan, C. H. Davis, and J. Kaplan. 1996. The late chlamydial inclusion membrane is not derived from the endocytic pathway and is relatively deficient in host proteins. Infect. Immun. 64:3713-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ting, L. M., R. C. Hsia, C. G. Haidaris, and P. M. Bavoil. 1995. Interaction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surface. Infect. Immun. 63:3600-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vretou, E., P. C. Goswami, and S. K. Bose. 1989. Adherence of multiple serovars of Chlamydia trachomatis to a common receptor on HeLa and McCoy cells is mediated by thermolabile protein(s). J. Gen. Microbiol. 135:3229-3237. [DOI] [PubMed] [Google Scholar]
- 47.Wyrick, P. B., J. Choong, C. H. Davis, S. T. Knight, M. O. Royal, A. S. Maslow, and C. R. Bagnell. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 57:2378-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]