Abstract
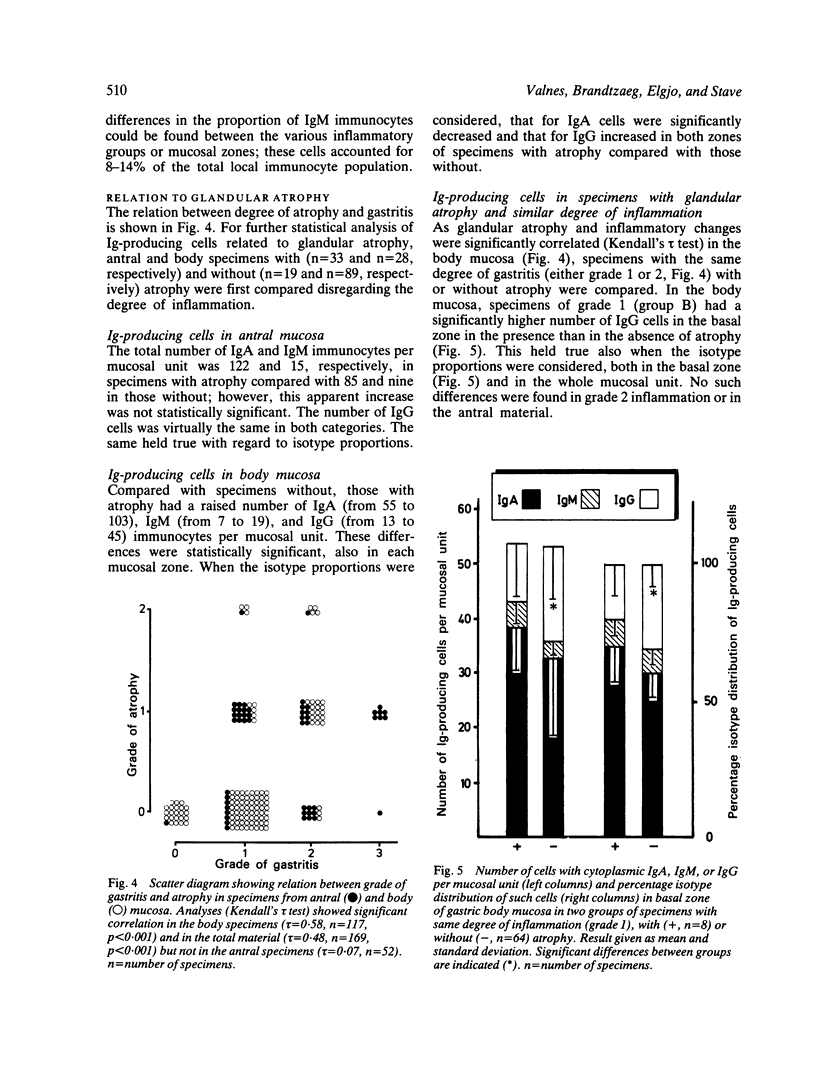
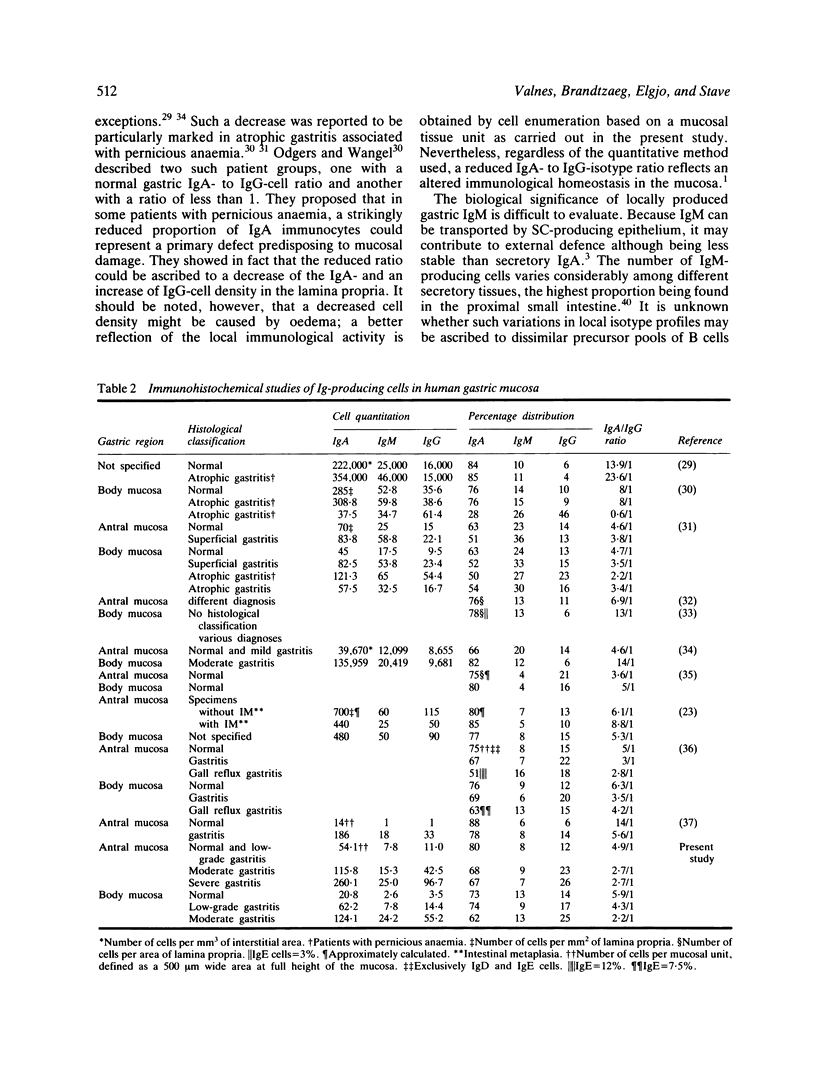
Immunoglobulin (Ig)-producing immunocytes were quantified by paired immunofluorescence staining in specimens of gastric antral (n = 52) and body (n = 117) mucosa obtained from 45 patients with various gastrointestinal disorders. Enumerations were carried out in a 500 micron wide zone from the muscularis mucosae to the lumen ('tissue unit'). The specimens were divided into three categories according to the degree of inflammation, and each specimen received a grade for atrophy (0-2). The total number of IgA-, IgM- and IgG-producing cells per tissue unit increased strikingly with increasing degree of inflammation, both in antral and body mucosa. IgA immunocytes predominated (61-91%) in all specimens, but the IgG isotype showed the largest relative increase (four to 17-fold), particularly in the basal part of the mucosa. In this layer of the gastric body the proportion of IgG cells was also significantly raised in association with atrophy, irrespective of degree of inflammation. Locally produced IgG may be of protective significance in terms of internal (or 'second line') defence but may at the same time maintain immunopathological mechanisms contributing to the chronicity of gastritis.
Full text
PDF

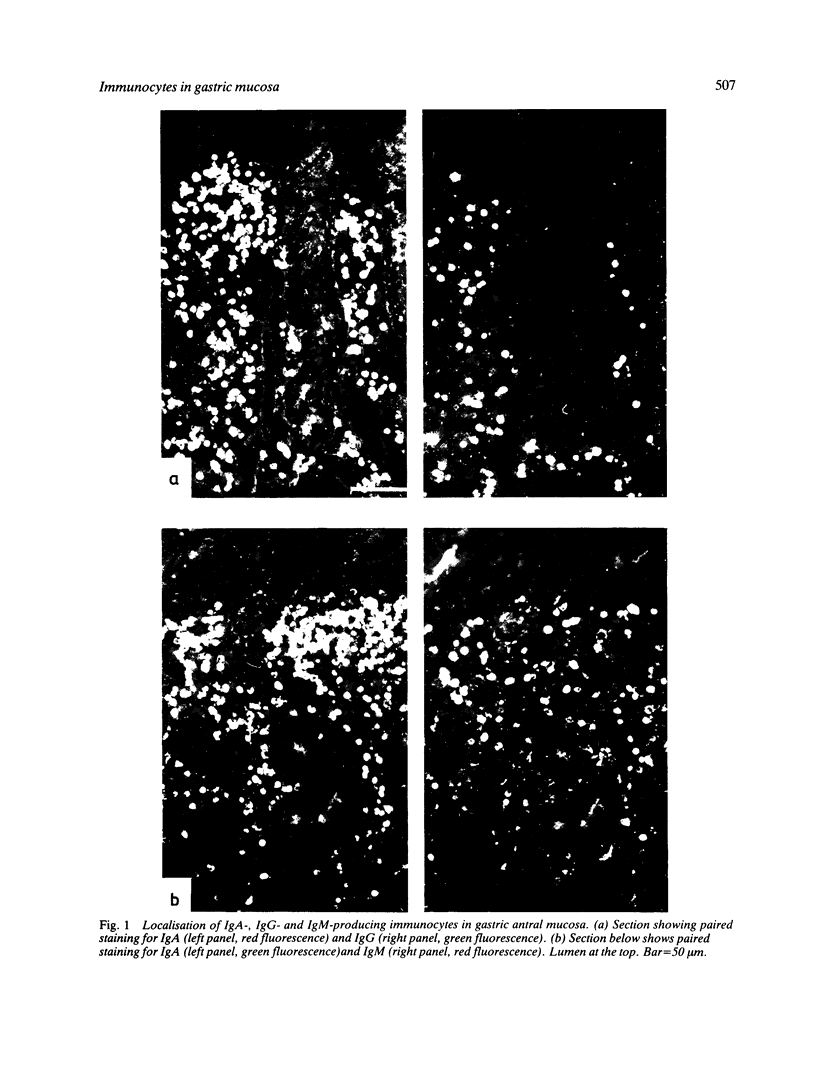
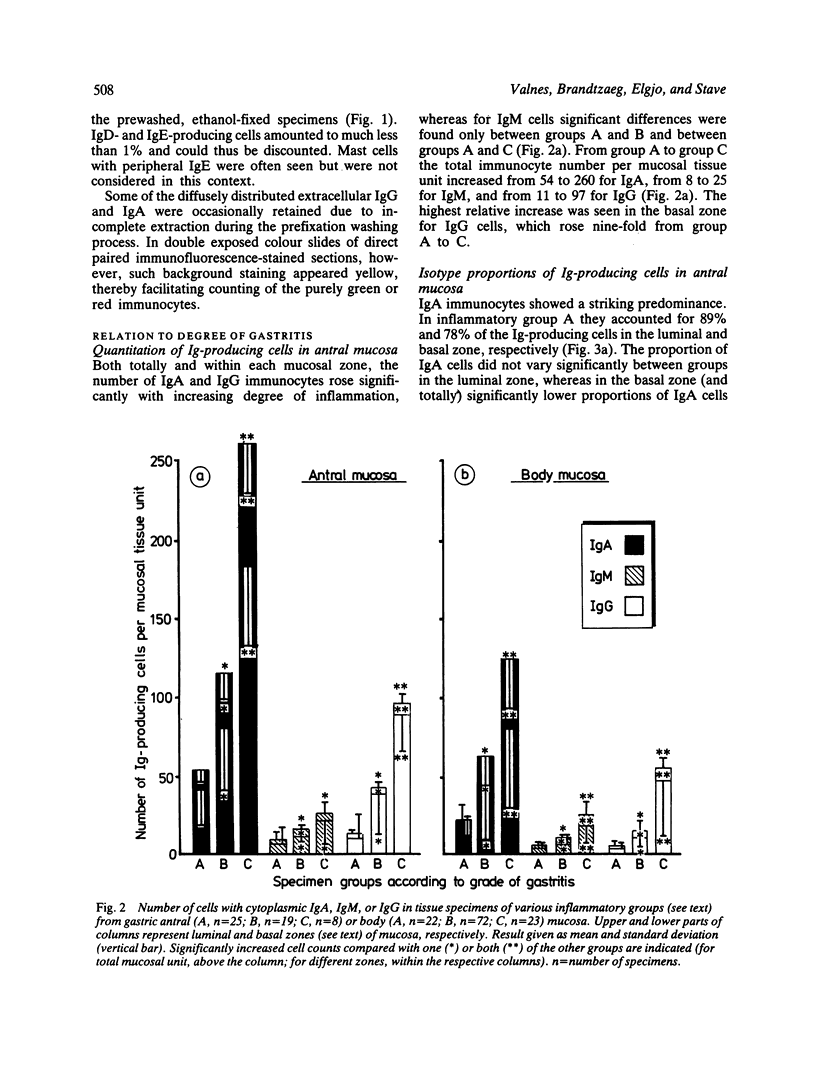
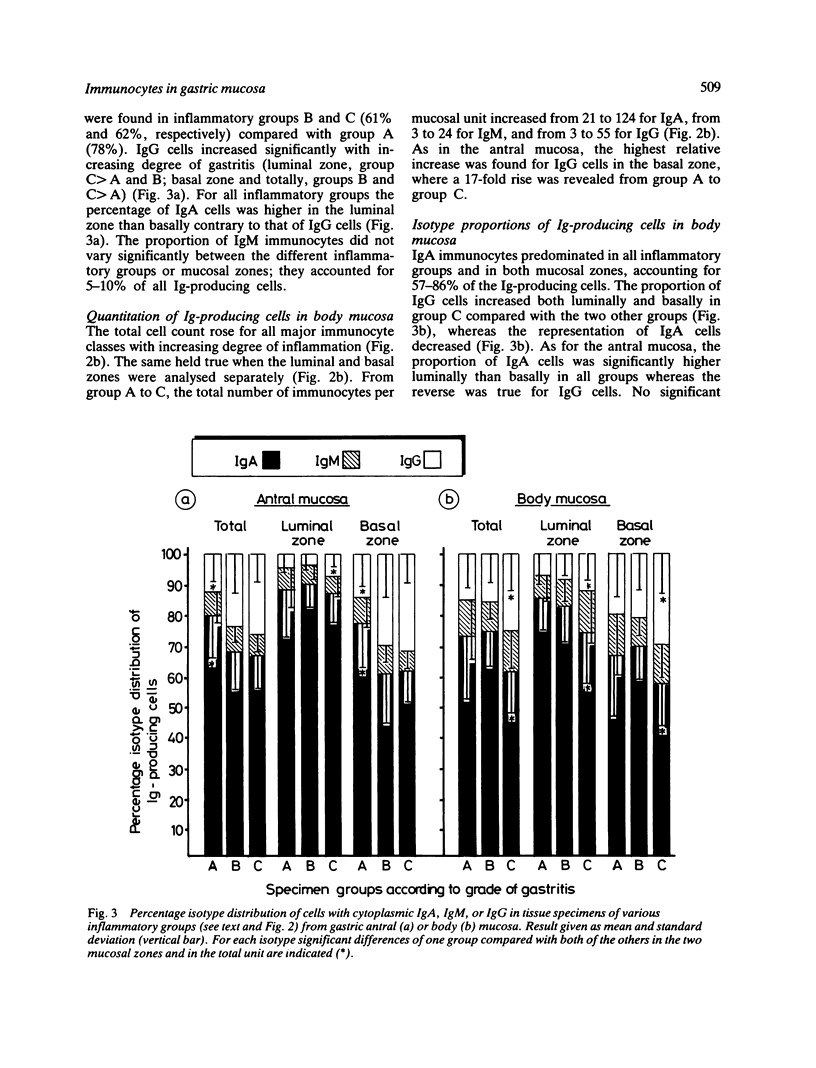



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baklien K., Brandtzaeg P. Immunohistochemical characterization of local immunoglobulin formation in Crohn's disease of the ileum. Scand J Gastroenterol. 1976;11(5):447–457. [PubMed] [Google Scholar]
- Brandtzaeg P., Baklien K., Fausa O., Hoel P. S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology. 1974 Jun;66(6):1123–1136. [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. II. Specific and non-specific binding properties. Scand J Immunol. 1973;2(4):333–348. doi: 10.1111/j.1365-3083.1973.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Prolonged incubation time in immunohistochemistry: effects on fluorescence staining of immunoglobulins and epithelial components in ethanol- and formaldehyde-fixed paraffin-embedded tissues. J Histochem Cytochem. 1981 Nov;29(11):1302–1315. doi: 10.1177/29.11.7033362. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984 Sep 6;311(5981):71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985 Aug;22(2):111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. The secretory immune system of lactating human mammary glands compared with other exocrine organs. Ann N Y Acad Sci. 1983 Jun 30;409:353–382. doi: 10.1111/j.1749-6632.1983.tb26883.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Tolo K. Mucosal penetrability enhanced by serum-derived antibodies. Nature. 1977 Mar 17;266(5599):262–263. doi: 10.1038/266262a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Valnes K., Scott H., Rognum T. O., Bjerke K., Baklien K. The human gastrointestinal secretory immune system in health and disease. Scand J Gastroenterol Suppl. 1985;114:17–38. doi: 10.3109/00365528509093765. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Borthistle B. K., Chen S. T. Immunoglobulin E (IgE) and IgE-containing cells in human gastrointestinal fluids and tissues. Clin Exp Immunol. 1975 May;20(2):227–237. [PMC free article] [PubMed] [Google Scholar]
- Brus I., Siegel H., Yamaguchi N., Jerzy Glass G. B. Immunoglobulins IgA and IgG in gastric mucosa of patients with atrophic gastritis and pernicious anemia. Scand J Gastroenterol. 1968;3(1):43–57. doi: 10.3109/00365526809180099. [DOI] [PubMed] [Google Scholar]
- Camilleri J. P., Bérault J., Picker M., Dièbold J. Distribution des cellules immunosécr-57etrices dans la muqueuse gastrique humaine, à l'état normal et au cours des gastrites chroniques. A propos de 46 gastro-biopsies dirigées. Biol Gastroenterol (Paris) 1973;6(3):231–241. [PubMed] [Google Scholar]
- Chapel H. M., Hoare A. M. A study of the aetiology of gastritis following gastric surgery. I. Immunofluorescent studies of the gastric mucosa. Clin Exp Immunol. 1979 Sep;37(3):441–444. [PMC free article] [PubMed] [Google Scholar]
- Chen S., Tobe T. Cellular sites of immunoglobulins. IV. Studies of antral mucosa of human stomachs. Digestion. 1974;10(3):177–183. doi: 10.1159/000197537. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Heremans J. F. The distribution of immunoglobulin-containing cells along the human gastrointestinal tract. Gastroenterology. 1966 Sep;51(3):305–316. [PubMed] [Google Scholar]
- Isaacson P. Immunoperoxidase study of the secretory immunoglobulin system and lysozyme in normal and diseased gastric mucosa. Gut. 1982 Jul;23(7):578–588. doi: 10.1136/gut.23.7.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuning J., Bosman F. T., Kuiper G., Wal A. M., Lindeman J. Gastric and duodenal mucosa in 'healthy' individuals. An endoscopic and histopathological study of 50 volunteers. J Clin Pathol. 1978 Jan;31(1):69–77. doi: 10.1136/jcp.31.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. L., Rowley D. The effect of antibody on the intestinal absorption of macromolecules and on intestinal permeability in adult mice. Int Arch Allergy Appl Immunol. 1982;68(1):41–46. doi: 10.1159/000233065. [DOI] [PubMed] [Google Scholar]
- Odgers R. J., Wangel A. G. Abnormalities in IgA-containing mononuclear cells in the gastric lesion of pernicious anaemia. Lancet. 1968 Oct 19;2(7573):846–849. doi: 10.1016/s0140-6736(68)91001-5. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Kino I., Kato Y., Aoyama Y. The distribution and localization of immunoglobulin in the gastric mucosa and gastric cancer. Acta Pathol Jpn. 1979 Jul;29(4):523–531. doi: 10.1111/j.1440-1827.1979.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Ploem J. S. The use of a vertical illuminator with interchangeable dichroic mirrors for fluorescence microscopy with incidental light. Z Wiss Mikrosk. 1967 Nov;68(3):129–142. [PubMed] [Google Scholar]
- Rao S. S., Krasner N., Thomson T. J. Chronic gastritis--a simple classification. J Pathol. 1975 Oct;117(2):93–96. doi: 10.1002/path.1711170206. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Baklien K., Hognestad J. Immunoglobulin-producing cells in the "transitional" mucosa adjacent to adenocarcinomas of the human large bowel. Int J Cancer. 1979 Feb;23(2):165–173. doi: 10.1002/ijc.2910230205. [DOI] [PubMed] [Google Scholar]
- Rosekrans P. C., Meijer C. J., van der Wal A. M., Cornelisse C. J., Lindeman J. Immunoglobulin containing cells in inflammatory bowel disease of the colon: a morphometric and immunohistochemical study. Gut. 1980 Nov;21(11):941–947. doi: 10.1136/gut.21.11.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Ek J., Baklien K., Brandtzaeg P. Immunoglobulin-producing cells in jejunal mucosa of children with coeliac disease on a gluten-free diet and after gluten challenge. Scand J Gastroenterol. 1980;15(1):81–88. doi: 10.3109/00365528009181436. [DOI] [PubMed] [Google Scholar]
- Slaoui H., André C., Dechavanne M., Tolot F. Immunofluorescence study of mucosal B lymphocytes in bile reflux gastritis. Digestion. 1979;19(2):131–133. doi: 10.1159/000198333. [DOI] [PubMed] [Google Scholar]
- Stave R., Brandtzaeg P. Fluorescence staining of gastric mucosa. A study with special reference to parietal cells. Scand J Gastroenterol. 1977;12(7):885–891. doi: 10.3109/00365527709181735. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P., Elgjo K., Stave R. Specific and nonspecific humoral defense factors in the epithelium of normal and inflamed gastric mucosa. Immunohistochemical localization of immunoglobulins, secretory component, lysozyme, and lactoferrin. Gastroenterology. 1984 Mar;86(3):402–412. [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P., Hanssen L. E., Stave R., Larsen S., Londong W. Quantitation of immunoglobulin- and peptide hormone-producing cells in gastrointestinal mucosa. Comparison of direct immunofluorescence and the unlabelled antibody peroxidase--antiperoxidase method. Histochem J. 1983 Oct;15(10):1011–1020. doi: 10.1007/BF01002496. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P. Retardation of immunofluorescence fading during microscopy. J Histochem Cytochem. 1985 Aug;33(8):755–761. doi: 10.1177/33.8.3926864. [DOI] [PubMed] [Google Scholar]
- Van Spreeuwel J. P., Lindeman J., Van der Wal A., Weterman I., Kreuning J., Meijer C. Morphological and immunohistochemical findings in upper gastrointestinal biopsies of patients with Crohn's disease of the ileum and colon. J Clin Pathol. 1982 Sep;35(9):934–940. doi: 10.1136/jcp.35.9.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandelli C., Bottazzo G. F., Doniach D., Franceschi F. Autoantibodies to gastrin-producing cells in antral (type B) chronic gastritis. N Engl J Med. 1979 Jun 21;300(25):1406–1410. doi: 10.1056/NEJM197906213002502. [DOI] [PubMed] [Google Scholar]



