Abstract
Upon oral infection, Trypanosoma cruzi metacyclic trypomastigotes invade and replicate in the gastric mucosal epithelium, being apparently uniquely specialized for adhesion to mucin and mucosal invasion. Here we investigated the involvement of gp82, the metacyclic-stage-specific surface glycoprotein implicated in host cell entry, in both adhesion to gastric mucin and invasion of the mucosal epithelium upon oral challenge. Metacyclic forms, preincubated with a control monoclonal antibody (MAb) or with MAb 3F6 directed to gp82, were administered orally to BALB/c mice, and parasitemia was monitored. Mice that received parasites treated with MAb 3F6 had greatly reduced parasitemia, displaying at the peak a mean number of blood parasites more than 100-fold lower than that of the control group. MAbs directed to other T. cruzi surface glycoproteins had no such effect. gp82, as either a native or a recombinant molecule, but not the metacyclic trypomastigote surface molecule gp90 or gp35/50, bound to gastric mucin in enzyme-linked immunosorbent assays. MAb 3F6 significantly inhibited the penetration of cultured epithelial HeLa cells by metacyclic forms in the absence or in the presence of gastric mucin. Mucin alone did not affect parasite internalization. Parasite infectivity was not altered by treatment of metacyclic forms with pepsin, to which gp82 was resistant, or with proteinase K, which removed the N-terminal portion of gp82 but preserved its host cell binding site. Taken together, these findings suggest that gp82 mediates the interaction of metacyclic trypomastigotes with gastric mucin and the subsequent penetration of underlying epithelial cells.
Trypanosoma cruzi, the protozoan parasite that causes Chagas' disease and is transmitted by bloodsucking triatomine insects, can also infect people orally and by blood transfusion. More than half of the acute cases of Chagas' disease recorded between 1968 and 2000 in the Brazilian Amazon were attributable to microepidemics of orally transmitted infection from contaminated food (3). Potential sources of food contamination are whole triatomine insects or their feces containing infective metacyclic trypomastigotes and possibly the anal gland secretion of T. cruzi-infected opossums (3), which are important wild reservoirs of the parasite. Metacyclic trypomastigotes, typically present in the intestinal lumen of the insect vector, are also found in the lumen of the marsupial anal glands, where the parasite goes through an extracellular cycle corresponding to that in the triatomines (4). Oral transmission through contaminated food in a region with a high rate of T. cruzi-infected opossums has been reported (15).
Metacyclic trypomastigotes can invade and replicate in the gastric mucosal epithelium, and it appears that gastric mucosal invasion is the unique portal of entry for systemic T. cruzi infection after oral challenge (7). It has been shown that insect-derived metacyclic trypomastigotes delivered orally were sufficient for consistent infection of 100% of BALB/c mice, whereas blood form trypomastigotes were found to initiate mucosal infection rarely, suggesting that metacyclic forms have uniquely specialized functions for mucosal invasion (6). They may be resistant to proteolytic enzymes present in mucosal secretion, and the possibility has been raised that metacyclic forms express stage-specific surface molecules not present on blood trypomastigotes, which are required for adhesion to mucosal epithelial surface receptors or for the penetration of mucin, the protective secreted coat that lines mucosal surfaces (6).
A metacyclic-stage-specific surface molecule that could be involved in invasion of epithelial cells of the gastric mucosa is gp82. This glycoprotein, identified by MAb 3F6 in metacyclic trypomastigotes but not in blood trypomastigotes, epimastigotes, or amastigotes (18), has been implicated in T. cruzi penetration of cultured mammalian cells (10). gp82 is an adhesion molecule that binds to epithelial cells in a receptor-mediated manner and induces Ca2+ mobilization (11), which is an essential requirement for T. cruzi entry into host cells (5, 9, 17). In addition to the role of gp82 in mucosal infection upon oral challenge, it remains to be determined whether gp82 adheres to gastric mucin and to what extent the action of proteolytic enzymes digests gp82 and affects the infectivity of metacyclic forms. To address these questions, in this study, we performed in vivo and in vitro experiments with T. cruzi metacyclic trypomastigotes obtained in axenic cultures.
T. cruzi isolate CL, from the insect Triatoma infestans (2), was used. Parasites were maintained alternately in mice and in liver infusion tryptose medium. Grace's medium was used to obtain cultures enriched in metacyclic trypomastigotes, which were purified by passage through a DEAE-cellulose column as previously described (18). For oral infection, metacyclic forms were introduced by the intrapharyngeal route into 4- to 6-week-old female BALB/c mice through a plastic tube adapted to a 1-ml plastic syringe. Parasitemia was monitored by examining 5-μl peripheral blood samples under a phase-contrast microscope. HeLa cells, human carcinoma-derived epithelial cells, were grown at 37°C in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) in a humidified 5% CO2 atmosphere. Mammalian cell invasion assays were performed essentially as previously described (19), at a parasite-to-HeLa cell ratio of 10:1. Native gp82 was purified by antibody affinity Sepharose column chromatography from detergent-solubilized extracts of metacyclic trypomastigotes (20). Recombinant proteins J18, comprising the full-length gp82 sequence in frame with glutathione S-transferase, and J18b, which encodes 293 amino acids of the C-terminal domain, were generated in Escherichia coli and purified as previously detailed (12, 13). The purity of the isolated protein was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie blue, and its specificity was assessed by immunoblotting with MAb 3F6. An assay of gp82 binding to mucin from porcine stomach (type III; Sigma) was performed as follows. Microtiter plates (96 wells) coated with gastric mucin in phosphate-buffered saline (PBS; 10 μg/well) were blocked with PBS containing 10% fetal calf serum for 1 h at room temperature and then incubated sequentially, at 37°C for 1 h, with native or recombinant gp82, with MAb 3F6 (18) or polyclonal monospecific antibody to recombinant gp82 (13), and with peroxidase-conjugated anti-mouse IgG, in PBS containing 10% fetal calf serum. The final reaction was revealed by o-phenylenediamine as previously described (10). Binding of recombinant gp82 to HeLa cells was assayed by enzyme-linked immunosorbent assay (ELISA) as detailed elsewhere (10). For treatment with the proteolytic enzymes pepsin (Sigma) and proteinase K (Life Technologies), parasites were incubated at 37°C for 30 min with the enzymes, washed three times in PBS, and then used for experiments. Immunoblotting of parasite extracts was done as detailed elsewhere (19), and the final reaction was revealed by diaminobenzidine plus H2O2. To determine the levels of T. cruzi surface molecules that react with MAb 3F6, live parasites (3 × 107 cells) were incubated for 1 h on ice with MAb 3F6 or with unrelated isotype-matched MAb 1C3 directed to Leishmania amazonensis gp63 (1) and then processed for flow cytometry as previously described (11).
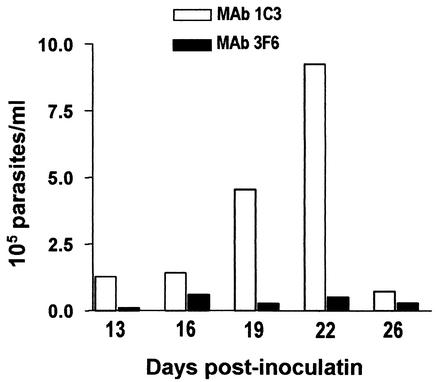
In preliminary experiments, we ascertained that oral administration of culture-derived T. cruzi metacyclic trypomastigotes to BALB/c mice leads to systemic infection. Next, we examined whether the metacyclic-stage surface molecule gp82 is implicated in mucosal infection. The parasites were preincubated with gp82-specific MAb 3F6 or with unrelated isotype-matched MAb 1C3 and then inoculated by the intrapharyngeal route into BALB/c mice. As the source of antibody, we used ascitic fluid, which was mixed with the parasite suspension (vol/vol) in a volume of 100 μl. After 30 min of incubation at room temperature, 1.2 ml of PBS was added and 0.2 ml of a parasite suspension containing 4 × 105 metacyclic forms was given to each mouse. Starting on day 13 postinoculation, blood samples were examined twice a week for the presence of parasites. As shown in Fig. 1, the parasitemia levels of mice that received metacyclic forms pretreated with MAb 3F6 were greatly reduced compared to those of the control group. At the peak of parasitemia, control animals suffered from cachexia and one died at day 25 postinfection. The experiment was repeated and gave similar results, confirming the infection-blocking activity of MAb 3F6. We presume that this antibody, which is devoid of any parasite-agglutinating or -immobilizing effect, acts by blocking gp82-mediated mucosal cell invasion, provided that the host cell binding site of gp82 is contiguous to and overlaps the epitope for MAb 3F6 (8). In addition, experiments were done to examine the effect of anti-T. cruzi MAbs other than MAb 3F6 by preincubating metacyclic trypomastigotes with MAb 1G7, which is directed to metacyclic-stage-specific surface glycoprotein gp90 (18), or with MAb 3C9, which recognizes a sialic acid-containing epitope in trypomastigote glycoproteins (14), before oral administration. We found no inhibitory effect of MAb 1G7 or MAb 3C9 on T. cruzi infection: both the control and experimental animals developed high parasitemias, and ∼20% mortality was observed in all groups.
FIG. 1.
Inhibitory effect of MAb 3F6 on oral T. cruzi infection by metacyclic trypomastigotes. Parasites pretreated with MAb 3F6 were given orally to a group of five BALB/c mice. The control group received parasites treated with unrelated MAb 1C3. Each datum point corresponds to the mean parasitemia of five animals in each group of mice.
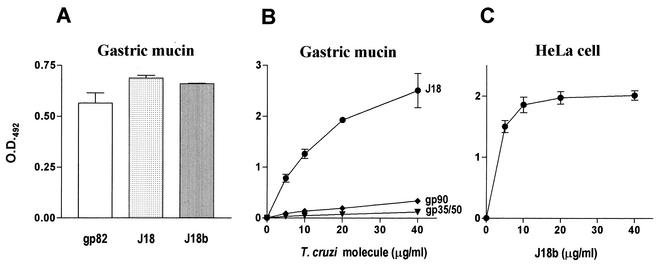
According to Hoft et al. (7), the gastric mucosal epithelium is the portal of T. cruzi entry upon oral challenge of mice with metacyclic trypomastigotes. By using an immunohistochemical staining technique specific for intracellular T. cruzi amastigotes, they found no evidence of parasite invasion within the oropharynx or esophagus, but in multiple experiments, they found pseudocysts of amastigotes within sections of gastric mucosa adjacent to the areas of inflammation, and by day 14 postchallenge, motile trypomastigotes were seen in the blood, indicating that T. cruzi can invade and replicate in the gastric mucosal epithelium. Adherence of parasites to gastric mucin could therefore be a step that precedes invasion. Shigella dysenteriae, for instance, whose pathogenic potential is correlated with its ability to invade and multiply within the cells of the colonic epithelium, preferentially adheres to colonic mucin (16). We examined the ability of gp82 to adhere to gastric mucin. An ELISA was performed by incubating mucin-coated microtiter plates with either native gp82 or corresponding recombinant protein J18. Both molecules bound to gastric mucin (Fig. 2A). Adhesion of J18b, a recombinant protein corresponding to the gp82 carboxy-terminal domain (amino acids 224 to 516), was also observed. Binding of gp82 to gastric mucin was dose dependent but not saturable (Fig. 2B), in contrast to adhesion to HeLa cells (Fig. 2C). To determine whether the gp82 mucin-binding site resides in the central region of the molecule, where the host cell binding site is located (8, 12), we tested a set of 20-mer synthetic peptides with 10 overlapping residues, spanning the domain comprising amino acids 224 to 363. Five of these, at 200 μg/ml and differing from the two peptides defined as part of the host cell binding site (8), exhibited some inhibitory effect on gp82 adhesion to mucin (data not shown). In addition to gp82, two other metacyclic surface glycoproteins, gp90 and gp35/50, were tested for binding activity toward gastric mucin. As shown in Fig. 2B, binding of these molecules to mucin was negligible.
FIG. 2.
Binding of T. cruzi metacyclic-stage surface molecule gp82 to gastric mucin. (A) gp82, either purified from parasite extracts or as a recombinant protein containing the entire gp82 peptide sequence (J18) or the C-terminal domain of gp82 (J18b), at 10 μg/ml, was added to microtiter plates coated with porcine gastric mucin. (B) Increasing concentrations of the T. cruzi surface molecule gp90, gp35/50, or gp82, as well as the recombinant protein J18, were added to wells in microtiter plates coated with gastric mucin. (C) Increasing doses of J18b were added to plates containing adherent HeLa cells. In all ELISAs, after washes, the plates were sequentially incubated with anti-gp82 antibody, anti-gp90 MAb 1G7, or anti-gp35/50 MAb 10D8 and anti-mouse immunoglobulin conjugated to peroxidase. The bound enzyme was revealed by using o-phenylenediamine. The values are the means ± standard deviations of triplicate samples. O.D.492, optical density at 492 nm.
We performed in vitro assays to confirm that gastric mucin does not interfere with penetration of epithelial cells by metacyclic forms and that MAb 3F6 inhibits parasite invasion in the presence of mucin. Parasites, either untreated or pretreated with MAb 3F6 or with control MAb 1C3 for 10 min at room temperature, were added to epithelial HeLa cells in the absence or in the presence of 500 μg of gastric mucin per ml. After 1 h of incubation at 37°C, the number of intracellular parasites in at least 500 Giemsa-stained cells was determined. Metacyclic forms entered HeLa cells at the same rate whether mucin was present or not, and pretreatment of parasites with MAb 3F6, but not with MAb 1C3, significantly inhibited cell invasion in the presence of gastric mucin (data not shown). MAbs 1G7 and 3C9, directed, respectively, to T. cruzi gp90 and mucin-like molecules, were devoid of an inhibitory effect.
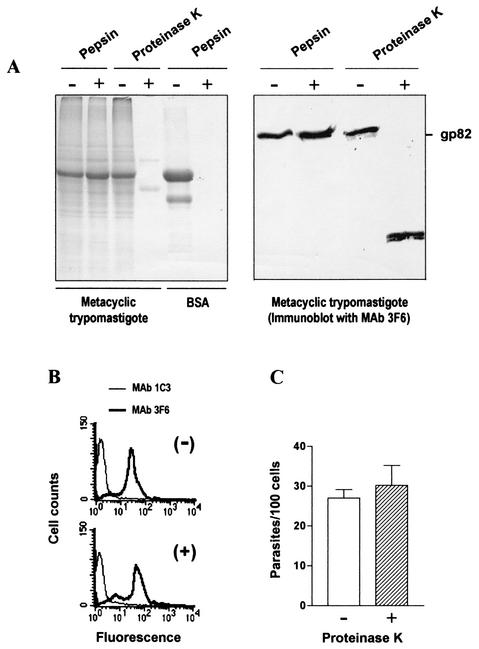
To successfully invade the mucosal epithelium, metacyclic trypomastigotes should resist the acidic pH within the host stomach and digestion by the proteolytic enzyme pepsin. To test their resistance to acidic conditions, we incubated parasites at different pHs lower than 7.0. At pH 3.5, in citrate buffer, metacyclic forms remained fully motile and morphologically indistinguishable from parasites at pH 7.0. When the pH was lowered to 3.0, some of the parasites lost motility. We examined their susceptibility to pepsin by treating parasites for 30 min at 37°C with 2 mg of pepsin per ml (optimal activity at pH 2.0 to 4.0) in citrate buffer at pH 3.5. After washings in PBS, the parasites were used for cell invasion assays and analysis by SDS-PAGE and immunoblotting. Pepsin-treated parasites showed the same protein profile as the untreated control and preserved intact the gp82 molecule (Fig. 3A), as well as the ability to enter HeLa cells (data not shown). Bovine serum albumin treated with pepsin at pH 3.5 was completely digested, showing that the enzyme was fully active. In addition to pepsin, we also tested the effects of other proteolytic enzymes, such as trypsin, papain, and proteinase K. The results obtained with trypsin and papain were similar to those obtained with pepsin. However, when metacyclic forms were treated with 1 mg of proteinase K per ml (optimal activity at pH 6.5 to 9.5) at pH 7.0 for 30 min at 37°C, we observed extensive protein digestion and conversion of gp82 to a smaller molecule (∼35 kDa) that was still recognized by MAb 3F6 (Fig. 3A). By analyzing the parasites by flow cytometry, we found that the levels of MAb 3F6-reactive surface molecules were comparable in proteinase K-treated and untreated samples (Fig. 3B). Recognition by MAb 3F6 upon proteinase K treatment indicated that the gp82 host cell binding site, which is located contiguous to and downstream of the epitope for MAb 3F6 (positions 244 to 263), was preserved and implied that the enzymatic treatment would not affect parasite infectivity. Accordingly, proteinase K-treated metacyclic trypomastigotes invaded HeLa cells at the same rate as untreated controls (Fig. 3C).
FIG. 3.
Effects of proteolytic enzymes on T. cruzi metacyclic-stage trypomastigotes. (A) Parasites, untreated or treated with pepsin or proteinase K, were analyzed by SDS-PAGE, staining with Coomassie blue, and immunoblotting with MAb 3F6. Note the pepsin digestion of bovine serum albumin, but not of metacyclic-form proteins, including gp82, which were susceptible to proteinase K digestion. (B) Live parasites, untreated (−) or treated with proteinase K (+), were reacted with MAb 3F6, fixed, and then analyzed by flow cytometry. (C) Control and proteinase K-treated metacyclic forms were used for HeLa cell invasion assay. Values represent the means ± standard deviation of three experiments.
In this study, for the first time, a chemically defined T. cruzi metacyclic-stage surface molecule was associated with in vivo infection and invasion of the mucosal epithelium. The finding that MAb 3F6, which specifically recognizes metacyclic-stage surface glycoprotein gp82, greatly reduces the systemic T. cruzi infection resulting from an oral challenge with metacyclic-stage trypomastigotes (Fig. 1) and also significantly inhibits parasite entry into epithelial HeLa cells in the presence of gastric mucin, suggests that gp82 is implicated in invasion of the mucosal epithelium. Before penetrating epithelial cells, metacyclic forms may engage gp82 to adhere to and traverse the mucin that lines the gastric mucosal surfaces. Several observations reinforce that notion. The presence of mucin did not affect the invasion of HeLa cells by metacyclic forms. gp82 bound to gastric mucin (Fig. 2), possibly with low affinity and through a recognition site different from the host cell binding site, so that adhesion to mucin did not interfere with parasite invasion. Metacyclic forms resisted an acidic pH and pepsin digestion. All of these observations are consistent with the ability of metacyclic trypomastigotes to invade the gastric mucosal epithelium efficiently by engaging the stage-specific surface molecule gp82.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
We thank Daniele Ferreira for help with assays of gp82 binding to gastric mucin and Sergio Schenkman for reading the manuscript and for suggestions.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Barbiéri, C. L., S. Giorgio, A. J. C. Merjan, and E. N. Figueiredo. 1993. Glycosphingolipid antigens of Leishmania (Leishmania) amazonensis amastigotes identified by use of a monoclonal antibody. Infect. Immun. 61:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brener, Z., and E. Chiari. 1963. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 5:220-224. [PubMed] [Google Scholar]
- 3.Coura, J. R., A. C. V. Junqueira, O. Fernandes, S. A. S. Valente, and M. A. Miles. 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18:171-176. [DOI] [PubMed] [Google Scholar]
- 4.Deane, M. P., H. L. Lenzi, and A. Jansen. 1984. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem. Inst. Oswaldo Cruz 79:513-515. [DOI] [PubMed] [Google Scholar]
- 5.Dorta, M. L., A. T. Ferreira, M. E. M. Oshiro, and N. Yoshida. 1995. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol. Biochem. Parasitol. 73:285-289. [DOI] [PubMed] [Google Scholar]
- 6.Hoft, D. F. 1996. Differential mucosal infectivity of different life stages of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 55:360-364. [DOI] [PubMed] [Google Scholar]
- 7.Hoft, D. F., P. L. Farrar, K. Kratz-Owens, and D. Shaffer. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 64:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manque, P. M., D. Eichinger, M. A. A. Juliano, L. Juliano, J. E. Araya, and N. Yoshida. 2000. Characterization of the cell adhesion site of Trypanosoma cruzi metacyclic stage surface glycoprotein gp82. Infect. Immun. 68:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno, S. N. J., J. Silva, A. E. Vercesi, and R. Docampo. 1994. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 180:1535-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez, M. I., R. C. Ruiz, J. E. Araya., J. Franco da Silveira, and N. Yoshida. 1993. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect. Immun. 61:3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz, R. C., S. Favoreto, Jr., M. L. Dorta, M. E. M. Oshiro, A. T. Ferreira, P. M. Manque, and N. Yoshida. 1998. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signaling activity. Biochem. J. 330:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santori, F. R., M. L. Dorta, L. Juliano, M. A. Juliano, J. Franco da Silveira, R. C. Ruiz, and N. Yoshida. 1996. Identification of a domain of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 required for attachment and invasion of mammalian cells. Mol. Biochem. Parasitol. 78:209-216. [DOI] [PubMed] [Google Scholar]
- 13.Santori, F. R., G. S. Paranhos-Bacalla, J. Franco da Silveira, L. M. Yamauchi, J. E. Araya, and N. Yoshida. 1996. A recombinant protein based on the Trypanosoma cruzi metacyclic trypomastigotes 82-kilodalton antigen that induces an effective immune response to acute infection. Infect. Immun. 64:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenkman, S., M. Jiang, G. W. Hart, and V. Nussenzweig. 1991. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell 65:1117-1125. [DOI] [PubMed] [Google Scholar]
- 15.Shikanai-Yasuda, M. A., C. B. Marcondes, L. A. Guedes, G. S. Siqueira, A. A. Barone, J. C. P. Dias, V. Amato Neto, J. E. Tolezano, B. A. Peres, E. B. Arruda, Jr., M. H. Lopes, M. Shiroma, and E. Chapadeiro. 1991. Possible oral transmission of acute Chagas disease in Brazil. Rev. Inst. Med. Trop. São Paulo 33:351-357. [DOI] [PubMed] [Google Scholar]
- 16.Sudha, P. S., H. Devaraj, and N. Devaraj. 2001. Adherence of Shigella dysenteriae 1 to human colonic mucin. Curr. Microbiol. 42:381-387. [DOI] [PubMed] [Google Scholar]
- 17.Tardieux, I., M. H. Nathanson, and N. W. Andrews. 1994. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic free Ca2+ transients. J. Exp. Med. 179:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira, M. M. G., and N. Yoshida. 1986. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol. Biochem. Parasitol. 18:271-282. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida, N., R. A. Mortara, M. F. Araguth, J. C. Gonzalez, and M. Russo. 1989. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35 and 50 kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun. 57:1663-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida, N., J. E. Araya, J. Franco da Silveira, and S. Giorgio. 1993. Trypanosoma cruzi: antibody production and T cell response induced by stage-specific surface glycoproteins purified from metacyclic trypomastigotes. Exp. Parasitol. 77:405-413. [DOI] [PubMed] [Google Scholar]