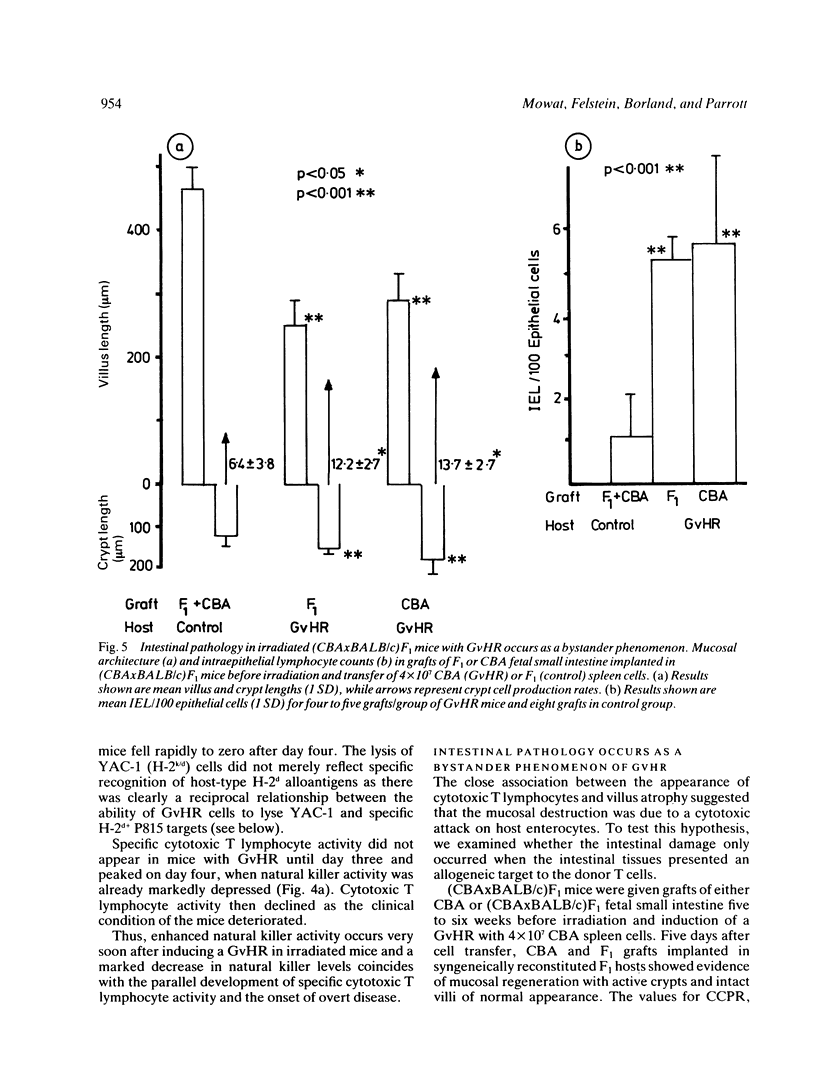
Abstract
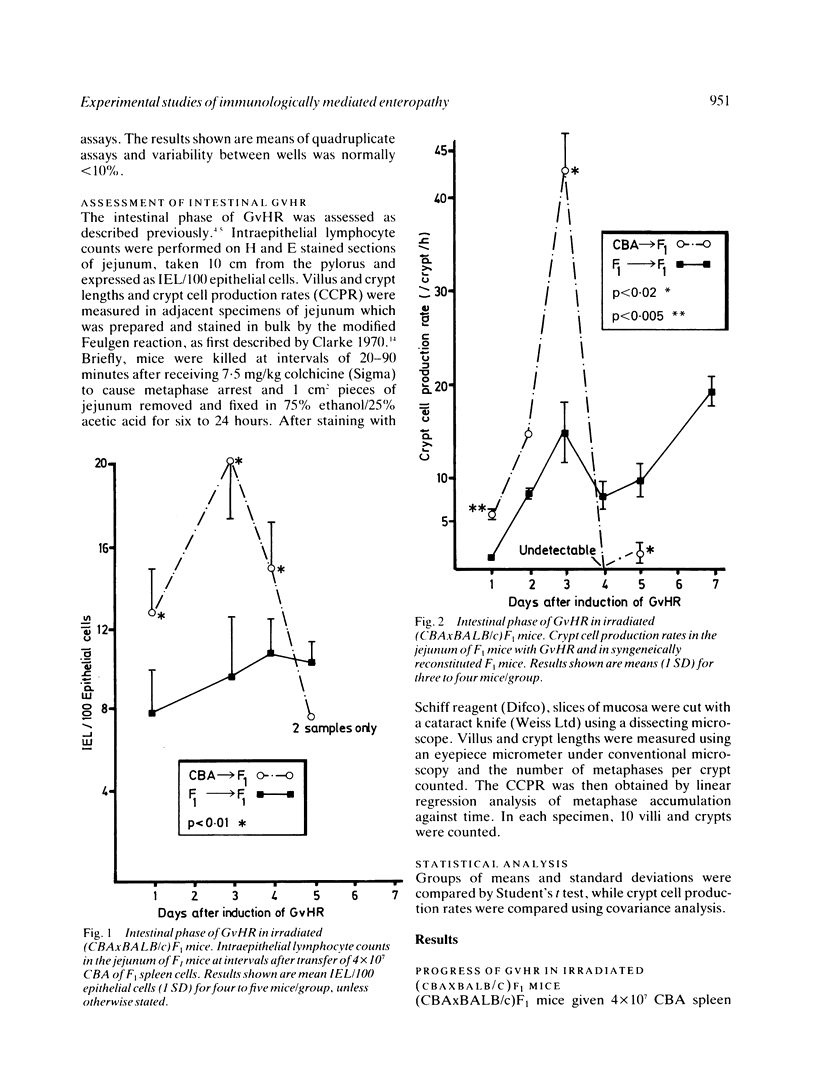
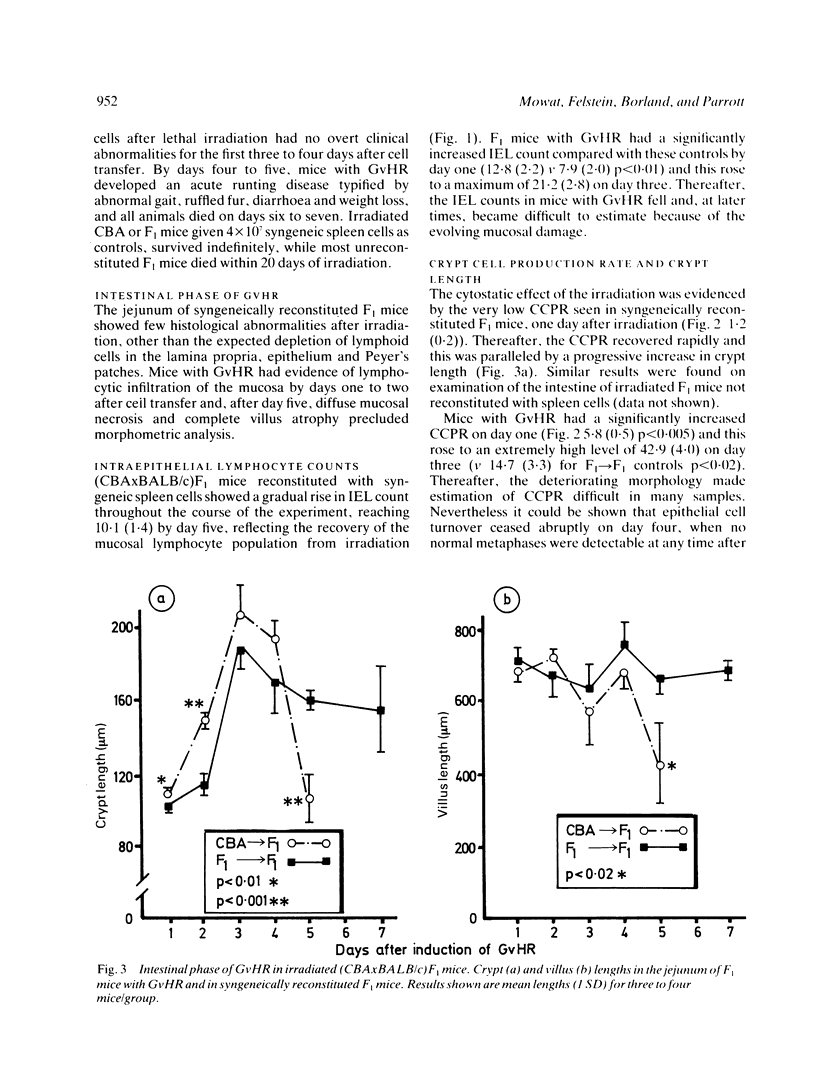
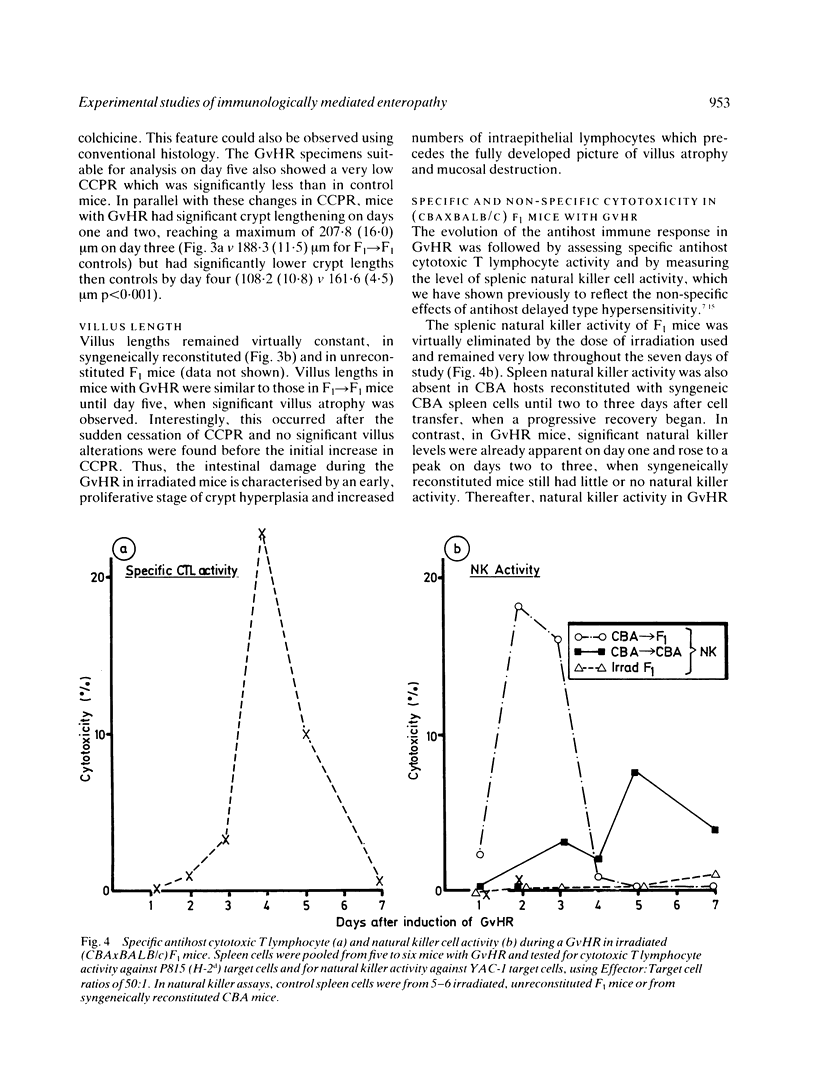
The intestinal component of a graft-versus-host reaction (GvHR) provides a useful experimental model to elucidate the pathogenesis of clinical enteropathies which cause villus atrophy and crypt hyperplasia and which are associated with a local immune response. One to three days after induction of GvHR in heavily irradiated (CBAxBALB/c)F1 mice, a proliferative form of enteropathy developed. Compared with controls, these mice had increased counts of jejunal intraepithelial lymphocytes and had a four-fold increase in crypt cell production rate as well as an increase in crypt length. These changes were accompanied by a marked enhancement of splenic natural killer cell activity. After day three, the crypt cell production rate fell to zero and cytotoxic T lymphocytes (CTL) which could lyse targets of host origin appeared. In parallel, mice with GvHR developed significant villus shortening and their clinical condition deteriorated. Further experiments showed that increased counts of intraepithelial lymphocytes, villus atrophy and crypt hyperplasia also occurred in grafts of fetal CBA intestine implanted under the kidney capsule of (CBAxBALB/c)F1 mice with GvHR. As these grafts are syngeneic to the injected CBA spleen cells, they should not be attacked by anti-host cytotoxic T lymphocytes. We suggest that the proliferative and destructive components of enteropathy in GvHR are caused by lymphokines released by an anti-host delayed type hypersensitivity reaction.
Full text
PDF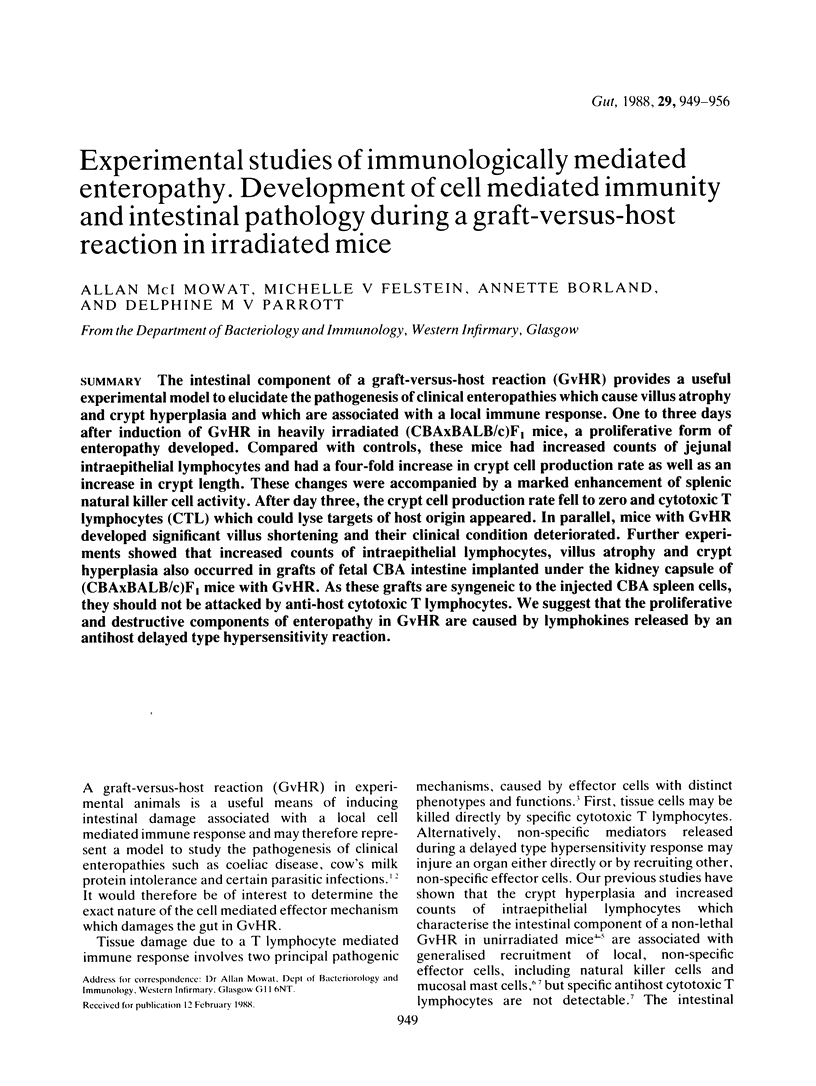



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borland A., Mowat A. M., Parrott D. M. Augmentation of intestinal and peripheral natural killer cell activity during the graft-versus-host reaction in mice. Transplantation. 1983 Nov;36(5):513–519. doi: 10.1097/00007890-198311000-00009. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Cornelius E. A. Protein-losing enteropathy in the graft-versus-host reaction. Transplantation. 1970 Mar;9(3):247–252. doi: 10.1097/00007890-197003000-00008. [DOI] [PubMed] [Google Scholar]
- Ferguson A., MacDonald T. T. Effects of local delayed hypersensitivity on the small intestine. Ciba Found Symp. 1977 Apr 26;(46):305–327. doi: 10.1002/9780470720288.ch15. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. Growth and development of "antigen-free" grafts of foetal mouse intestine. J Pathol. 1972 Feb;106(2):95–101. doi: 10.1002/path.1711060205. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Aggarwal B. B., Rinderknecht E., Assisi F., Chiu H. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083–1086. [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Hypersensitivity reactions in the small intestine. III. The effects of allograft rejection and of graft-versus-host disease on epithelial cell kinetics. Cell Tissue Kinet. 1977 Jul;10(4):301–312. [PubMed] [Google Scholar]
- Mowat A. M., Borland A., Parrott D. M. Augmentation of natural killer cell activity by anti-host delayed-type hypersensitivity during the graft-versus-host reaction in mice. Scand J Immunol. 1985 Oct;22(4):389–399. doi: 10.1111/j.1365-3083.1985.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Borland A., Parrott D. M. Hypersensitivity reactions in the small intestine. VII. Induction of the intestinal phase of murine graft-versus-host-reaction by Lyt 2- T cells activated by I-A alloantigens. Transplantation. 1986 Feb;41(2):192–198. [PubMed] [Google Scholar]
- Mowat A. M. Evidence that Ia+ bone-marrow-derived cells are the stimulus for the intestinal phase of the murine graft-versus-host reaction. Transplantation. 1986 Aug;42(2):141–144. doi: 10.1097/00007890-198608000-00007. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity reactions in the small intestine. 6. Pathogenesis of the graft-versus-host reaction in the small intestinal mucosa of the mouse. Transplantation. 1981 Sep;32(3):238–243. doi: 10.1097/00007890-198109000-00011. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982 Aug;83(2):417–423. [PubMed] [Google Scholar]
- Piguet P. F. GVHR elicited by products of class I or class II loci of the MHC: analysis of the response of mouse T lymphocytes to products of class I and class II loci of the MHC in correlation with GVHR-induced mortality, medullary aplasia, and enteropathy. J Immunol. 1985 Sep;135(3):1637–1643. [PubMed] [Google Scholar]
- REILLY R. W., KIRSNER J. B. RUNT INTESTINAL DISEASE. Lab Invest. 1965 Jan;14:102–107. [PubMed] [Google Scholar]
- Woodruff J. M., Hansen J. A., Good R. A., Santos G. W., Slavin R. E. The pathology of the graft-versus-host reaction (GVHR) in adults receiving bone marrow transplants. Transplant Proc. 1976 Dec;8(4):675–684. [PubMed] [Google Scholar]
- van Bekkum D. W., Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst. 1977 Mar;58(3):787–790. doi: 10.1093/jnci/58.3.787. [DOI] [PubMed] [Google Scholar]



